Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 7
Higher diastolic blood pressure at admission and antiedema therapy is associated with acute kidney injury in acute ischemic stroke patients
Authors Micozkadioglu H
Received 19 December 2013
Accepted for publication 9 January 2014
Published 20 February 2014 Volume 2014:7 Pages 101—105
DOI https://doi.org/10.2147/IJNRD.S59443
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Hasan Micozkadioglu
Department of Nephrology, Faculty of Medicine Hospital of Adana, Baskent University School of Medicine, Adana, Turkey
Abstract: Antiedema therapy with mannitol and furosemide is widely used for prevention and management of cerebral edema, elevated intracranial pressure, and cerebral hernia. There are some reports about mannitol and furosemide as risk factors of acute kidney injury (AKI). We investigated the risk factors for AKI including antiedema therapy in acute ischemic stroke patients. The subjects were 129 patients with acute ischemic stroke including 56 females and 73 males with a mean age 68.16±12.29 years. Patients were divided into two groups: patients with AKI and without AKI according to Acute Kidney Injury Network criteria. All patients had undergone cranial, carotid, and vertebral artery evaluation with magnetic resonance imaging. The number of patients with AKI was 14 (10.9%). Subjects experiencing atrial fibrillation (P=0.043) and higher diastolic blood pressure (DBP) (P=0.032) treated with mannitol (P=0.019) and furosemide (P=0.019) disclosed significant association with AKI. Regression analysis revealed that higher DBP (P=0.029) and management with mannitol (P=0.044) were the risk factors for AKI. Higher DBP at admission is the most important risk factor for AKI. However antiedema therapy should be used carefully in patients with acute ischemic stroke. Serum creatinine levels or estimated glomerular filtration rate should be watched frequently to prevent AKI.
Keywords: furosemide, mannitol, renal failure, cerebrovascular disease
Introduction
Antiedema therapy with mannitol and furosemide is widely used for prevention and management of encephaloedema, elevated intracranial pressure, and cerebral hernia.1 These drugs are also used to improve urine output, facilitate fluid management, and to take advantage of possible renoprotective properties in most intensive care units.2
Conversely, there are some reports about mannitol and furosemide as risk factors of acute kidney injury (AKI).3–5 The reason for AKI is the diuretic effect of the two drugs, which can induce hypovolemia.6 Mannitol can also cause AKI with proximal tubular injury, which is classified as osmotic nephrosis.7 In this pathological process, proximal tubule epithelial cells swell and tubular lumen obstruction can occur due to vacuolization. In advanced stages, tubular necrosis can be seen.7
There is no well-designed study to show the relation between antiedema therapy and AKI. Most of the studies are observational and case reports. The reason for this could be the variable definitions and diagnoses of AKI, and different management protocols.1 We investigated the risk factors for AKI including antiedema therapy in acute ischemic stroke patients.
Material and methods
The data from consecutive patients with acute ischemic stroke onset between January 2010 and July 2011 were analyzed. The patients had been admitted to Baskent University Adana Training and Research Hospital. Subjects had undergone magnetic resonance imaging for cranial, carotid and vertebral artery evaluation. Patients who had died or were discharged within 72 hours were excluded due to insufficient data. Subjects who experienced systemic infection, cardiopulmonary arrest, or hypotension during the hospitalization period were excluded. Patients having dialysis treatment or with transplantation history were excluded because of confounding factors.
Age, sex, history of diabetes mellitus, hypertension, coronary artery disease, peripheral artery disease, atrial fibrillation, antihypertensive drugs, anticoagulant drugs, and blood pressure at admission were recorded. Hypertension was defined according to the following criteria: previous hypertension; currently taking antihypertensive medication; or chronically high blood pressure exceeding 140/90 mmHg. Diabetes mellitus was defined as: fasting blood sugar ≥126 mg/dL; nonfasting blood sugar ≥200 mg/dL; or current use of insulin or oral hypoglycemic agent. Coronary artery disease was defined as prior myocardial infarction history and/or prior angiographic examination. As an antiedema therapy, mannitol was used 3 g/kg/day and furosemide 40 mg/day. The medications used from the time of admission to the time AKI occurred were recorded.
Identification of kidney damage was established based on the Kidney Disease Outcomes Quality Initiative of the National Kidney Foundation guidelines.8 Glomerular filtration rate (GFR) was estimated by the modification of diet in renal disease method.8 Serum creatinine levels at admission were used to calculate GFR. Low GFR was defined as lower than 60 mL/minute/1.73m2, which means moderate stage of chronic kidney disease. Patients with estimated GFR <60 mL/minute/1.73m2 were defined as having chronic kidney disease. Determination of AKI was established by Acute Kidney Injury Network criteria, which was based on the Risk, Injury, Failure, Loss, End-stage renal disease classification.9 AKI was defined as: an abrupt reduction in kidney function as evidenced by an increase in serum creatinine of more than or equal to 0.3 mg/dL, a percentage increase in serum creatinine of more than or equal to 50%, or a decrease in urine output.
All patients underwent routine T1 and T2 weighted brain magnetic resonance imaging (MRI), and 3D time-of-flight cerebral, carotid, and vertebral magnetic resonance angiography and diffusion MRI. MRI examinations were performed using a 1.5 tesla MRI unit (Siemens AG, Erlangen, Germany). All patients underwent routine T1-weighted spin echo, T2-weighted turbo spin echo, and fluid attenuated inversion recovery, as well as brain MRI and T1-weighted gradient echo 3D time-of-flight cerebral, carotid, and vertebral MR angiography. Additionally, single shot-echo planar diffusion weighted brain MRI and apparent diffusion coefficient maps were obtained. All patients were reviewed retrospectively based on the written reports.
A Vivid 7 color-Doppler ultrasound imager (General Electric, Fairfield, CT, USA) with a 2.5–5 MHz transducer was used for cardiac ultrasound. Tracings were recorded under 2-dimensional guidance, and measurements were taken at the tip of the mitral valve or just below that point. Left ventricular measurements were performed at end diastole and end systole according to the recommendations of the American Society of Echocardiography.
Serum was assessed using standard laboratory methods (Roche Hitachi analyzer 902; Hitachi Ltd, Tokyo, Japan).
Statistical analysis was performed using the statistical package SPSS version 17.0 (IBM Corporation, Armonk, NY, USA). For each continuous variable, normality was checked by Kolmogorov–Smirnov and Shapiro-Wilk tests. Comparisons between groups were applied using one-way Student’s t-test for normally distributed data and Mann–Whitney U-test was used for the data not normally distributed. The categorical variables between the groups were analyzed by using the chi-square test. A multiple logistic regression analysis was used to determine associations between AKI and other measurements, with AKI as dependent variable. Values of P<0.05 were considered as statistically significant.
Results
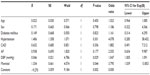
The subjects were 160 patients with acute ischemic stroke including 70 females and 90 males with a mean age 67.90±12.63 years (range =35–94 years). Only 129 subjects were selected for the study these subjects had results of serum creatinine levels at 72 hours of admission. These were 56 females and 73 males with a mean age 68.16±12.29 years. Mean mannitol dose was 247.47±23.35 (range =200–300) g/day. Furosemide dose was 80 mg/day. When these patients were divided into two groups – with AKI and without AKI – results revealed that there was no difference between these two groups regarding age, sex, hypertension, diabetes mellitus, coronary artery disease, chronic kidney disease, carotid artery stenosis, vertebral artery stenosis, left ventricular hypertrophy, and aortic valve calcification. Meanwhile, having atrial fibrillation, higher DPB at admission, and antiedema therapy with mannitol and furosemide was significantly associated with the development of AKI. No patient had been managed with nephrotoxic drugs like aminoglycosides or nonsteroidal anti-inflammatory drugs. The baseline characteristics of the subjects regarding AKI are outlined in Table 1.
Mannitol and furosemide were used together to manage cerebral edema. Because of this, only mannitol was used in regression analysis to represent antiedema therapy. Multiple logistic regression analysis was used to show associations between AKI and other factors in acute ischemic stroke patients. AKI was used as a dependent variable and results revealed that higher DBP (P=0.029) and management with mannitol (P=0.044) were the risk factors for AKI (Table 2).
Discussion
Mannitol is an osmotic diuretic agent widely used in perioperative settings because it is believed that mannitol increases renal blood flow and GFR, and decreases tubular obstruction rate and acute renal failure risk. However, Redfors et al showed that mannitol increases renal oxygen consumption and does not change renal blood flow.10 Meanwhile, some randomized controlled studies including cardiac surgery, traumatic rhabdomyolysis, and vascular and biliary tract surgery patients found no risk reduction in acute renal failure.11–13 On the contrary, mannitol-induced acute renal failure was described after treatment with more than 200 g/day, and accumulative doses of 750–1000 g in some studies.5,14,15
In this study, subjects were managed with a mannitol dose more than 200 g/day. Antiedema therapy consisted of mannitol and furosemide. Both of them were significantly associated with AKI, probably increasing nephrotoxicity of each other with their hypovolemic effects. Additionally, osmotic nephrosis caused by mannitol could result in AKI.1 Furosemide had a synergistic role in the development of osmotic nephrosis. Other predisposing factors were advanced age, diabetes mellitus, and preexisting renal insufficiency.7 However, results of this study revealed that there was no significant association between development of AKI and these factors. This could be attributed to the low number of patients in the study.
Having atrial fibrillation was another risk factor associated with AKI in acute ischemic stroke patients. Atrial fibrillation could promote systemic inflammation, induce fibrosis within the myocardium, and contribute to decline of left ventricular systolic and diastolic function over time.16 It was known that effective arterial volume could be low in patients with atrial fibrillation. This could explain the association of atrial fibrillation with AKI in this study.
Systolic and diastolic blood pressure at admission were both higher in patients who developed AKI. Meanwhile, only DBP revealed significant association with AKI. Regression analysis disclosed that higher DBP and management with mannitol were the significant risk factors for AKI in acute ischemic stroke patients. Hypertension history was found to be frequent among patients of this study. More severe hypertension or uncontrolled hypertension could explain the association between higher DBP and AKI.
Sympathetic nerve system activity was increased in rats with acute ischemic stroke.17 This activity could induce proinflammatory cytokine production such as tumor necrosis factor alpha and monocyte chemoattractant protein-1.17 Monocyte chemoattractant protein-1 and tumor necrosis factor alpha could mediate acute ischemic and toxic kidney injury.18,19 Higher systolic and diastolic blood pressure in patients developing AKI could be attributed to higher sympathetic nerve system activity.
The limitations of this study were the retrospective design of the study, low patient number, and lack of data about smoking history and antiplatelet drug medication.
In conclusion, higher DBP at admission is the most important risk factor for AKI. However antiedema therapy should be used carefully in patients with acute ischemic stroke. Serum creatinine levels or estimated GFR should be monitored frequently to prevent AKI.
Disclosure
This study is supported by Baskent University School of Medicine. Study was approved by Baskent University Ethics Committee. Ethics Committee Project no: KA12/261. The author did not receive financial support. There was no experimental investigation on human subjects. The author alone is responsible for the content and writing of the paper. The author reports no other conflicts of interest in this work.
References
Fang L, You H, Chen B, et al. Mannitol is an independent risk factor of acute kidney injury after cerebral trauma: a case-control study. Ren Fail. 2010;32:673–679. | |
Bragadottir G, Redfors B, Ricksten SE. Mannitol increases renal blood flow and maintains filtration fraction and oxygenation in postoperative acute kidney injury: a prospective interventional study. Crit Care. 2012;16:R159. | |
Goldwasser P, Fotino S. Acute renal failure following massive mannitol infusion. Appropriate response of tubuloglomerular feedback? Arch Intern Med. 1984;144:2214–2216. | |
Whelan TV, Bacon ME, Madden M, Patel TG, Handy R. Acute renal failure associated with mannitol intoxication. Report of a case. Arch Intern Med. 1984;144:2053–2055. | |
Pérez-Pérez AJ, Pazos B, Sobrado J, Gonzalez L, Gándara A. Acute renal failure following massive mannitol infusion. Am J Nephrol. 2002;22:573–575. | |
Girbes AR. Prevention of acute renal failure: role of vaso-active drugs, mannitol and diuretics. Int J Artif Organs. 2004;27:1049–1053. | |
Dickenmann M, Oetti T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis. 2008;51:491–503. | |
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39:S1–S266. | |
Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. | |
Redfors B, Swärd K, Sellgren J, Ricksten SE. Effects of mannitol alone and mannitol plus furosemide on renal oxygen consumption, blood flow and glomerular filtration after cardiac surgery. Intensive Care Med. 2009;35:115–122. | |
Fry AC, Farrington K. Management of Acute Renal Failure. Postgrad Med J. 2006;82:106–116. | |
Lameire NH, De Vriese AS, Vanholder R. Prevention and nondialytic treatment of acute renal failure. Curr Opin Crit Care. 2003;9:481–490. | |
Bellomo R, Bonventre J, Macias W, Pinsky M. Management of early acute renal failure: focus on post-injury prevention. Curr Opin Crit Care. 2005;11:542–547. | |
Dorman HR, Sondheimer JH, Cadnapaphornchai P. Mannitol-induced acute renal failure. Medicine (Baltimore). 1990;69:153–159. | |
Lin SL, Hung KY, Wu FL, Wei SC, Wu KD. Mannitol-induced acute renal failure. Nephrol Dial Transplant. 1995;10:120–122. | |
Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. 2013;127:569–574. | |
Wang YY, Lin SY, Chuang YH, Chen CJ, Tung KC, Sheu WF. Adipose proinflammatory cytokine expression through sympathetic system is associated with hyperglycemia and insulin resistance in a rat ischemic stroke model. Am J Physiol Endocrinol Metab. 2011;300:E155–E163. | |
Munshi R, Johnson A, Siew ED, et al. MCP-1 gene activation marks acute kidney injury. J Am Soc Nephrol. 2011;22:165–175. | |
Susantitaphong P, Perianayagam MC, Tighiouart H, Liangos O, Bonventre JV, Jaber BL. Tumor Necrosis Factor Alpha Promoter Polymorphism and Severity of Acute Kidney Injury. Nephron Clin Pract. 2013;123:67–73. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.