Back to Journals » Cancer Management and Research » Volume 11
High systemic immune–inflammation index represents an unfavorable prognosis of malignant pleural mesothelioma
Received 11 January 2019
Accepted for publication 14 March 2019
Published 2 May 2019 Volume 2019:11 Pages 3973—3979
DOI https://doi.org/10.2147/CMAR.S201269
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Ming Ma,1 Nina Yu,2 Bing Wu1
1Department of Radiation Oncology, Linyi People’s Hospital, Linyi, People’s Republic of China; 2Department of Gynecology and Obstetrics, Linyi People’s Hospital, Linyi, People’s Republic of China
Background: Malignant pleural mesothelioma (MPM) represents a fatal disease with high aggressiveness, and limited biomarkers have yet been identified for MPM. The present study aims to explore potential serum prognostic factors of MPM.
Materials and methods: A retrospective analysis of 97 pathologically diagnosed MPM was performed. The optimal cutoff value of pretreatment systemic immune–inflammation index (SII) was determined by receiver operating characteristic curve. Kaplan–Meier curves and Cox regression analysis were performed to assess the potential prognostic roles of parameters.
Results: A total of 59.8% (n=58) patients are male, with a median age of 56.0 years (range 18–77). The optimal cutoff value of SII was 988.6×109/L. High and low SII were found in 44 (45.4%) and 53 (54.6%) patients, respectively. Median survival time for total 97 cases was 18.5 months. The median overall survival for patients with low and high SII was 47.0 and 13.0 months, respectively. The 1-, 2- and 3-year survival rates for patients with low SII were 85.8%, 57.8% and 52.0% compared to that of 53.9%, 23.6% and 13.8% in patients with high SII. On univariate analysis, Eastern Cooperative Oncology Group performance status (ECOG PS)P<0.05) were found to be closely correlated with a better prognosis of MPM. Only ECOG PS (P=0.036) and SII (P=0.009) held statistical significance on multivariate analysis.
Conclusion: Pretreatment SII is easy to access to, and it represents an efficiency and noninvasive biomarker of MPM. High SII represents an unfavorable independent prognostic factor of MPM, and this needs to be validated in further studies.
Keywords: malignant pleural mesothelioma, systemic immune-inflammation index, prognosis
Introduction
Malignant mesothelioma represents a rare but fatal disease, and 80% mesothelioma cases have a pleural origin, which is called malignant pleural mesothelioma (MPM).1–3 In recent years, with the aggravation of environmental pollution, occupational exposure and long latency of 20–40 years between asbestos exposure and mesothelioma onset, the incidence of MPM will still be increasing.3,4 MPM shows an insidious characteristics and the diagnosis of MPM is hardly made due to the non-specific clinical presentations and noncontributory radiological characteristics in an early stage.
In addition to high aggressivity, MPM also shows a treatment-resistant characteristic, with a median overall survival (OS) time of 9–17 months regardless of tumor stage.3 With the development of diagnostic strategies and antitumor drugs, prognosis of some mesothelioma patients has been greatly improved. Recently, Serio et al report one case of malignant peritoneal mesothelioma with environmental asbestos exposure treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, who has survived over 17 years.5 Folate receptor 1 (FOLR1) and epidermal growth factor receptor (EGFR) have been demonstrated to be highly expressed in MPM. Preclinical investigation shows that folate-porphysomes-based photodynamic therapy (PDT) can induce selective destruction of MPM cells based on FOLR1 targeting, and the combination of pretreatment EGFR-TKI and PDT significantly enhance the therapeutic response, which provides a new treatment option for MPM.6 However, no uniform treatment strategy has been established for MPM at present, and combined modality treatment remains the recommended strategy for MPM.7–10 Surgery-based combined modality treatment seems to confer a prolonged survival for selected MPM patients.
A series of studies have been performed to evaluate the potential prognostic factors of MPM at present. Female gender, high estrogen receptor beta expression level, younger age at diagnosis, epithelial pathology, earlier tumor stage, low pretreatment platelet counts, and low soluble mesothelin-related peptide have been demonstrated to be positive prognostic factors of MPM in previous studies.11–13 However, the uniform established prognostic factors mainly involve tumor stage and epithelial histology.4 Given the negative prognosis of MPM, it is urgently needed to identify ideal prognostic factors of MPM, which is of great value for prognosis predicting and treatment decision-making.
Inflammation plays an important role in cancer development and progression.14,15 Serum samples are easy to access, and the identification of serum biomarkers as prognostic factors has more important meaning for clinicians. As systematic inflammatory response indicators, serum neutrophils, lymphocytes, monocytes and platelets, neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR) and platelet-to-lymphocyte ratio (PLR) have been demonstrated to be closely associated with prognosis of many malignancies.2,16–19 Systemic immune–-inflammation index (SII) (SII=N×P/L), known as systemic immune–inflammation index, which is calculated on neutrophil (N), platelet (P) and lymphocyte (L) counts, has been found to be a better prognosis predicting factor.20–23 However, the role of pretreatment SII in MPM remains poorly understood. The present study aims to explore the potential prognostic value of pretreatment SII in MPM.
Materials and methods
Patients
A retrospective analysis of 97 pathologically diagnosed MPM between April 2003 and December 2015 in Linyi People’s Hospital was performed. Our study was approved by the Ethics Committee of Linyi People’s Hospital. Prior to the present study, written informed consent was obtained from each patient. Our present study was conducted in accordance with the Declaration of Helsinki.
The eligibility criteria were (1) pathologically confirmed MPM; (2) age≥18 years; (3) available clinical and follow-up data; (4) no patient received the treatment of anti-tumor therapies before definitive diagnosis. Patients with inflammatory diseases or autoimmune disease were excluded. In detail, patients with any other virus infection (HBV, HCV, HIV, etc.), autoimmune diseases involving systemic lupus erythematosus, leukemia or any other blood system diseases or inflammatory diseases or organic disease (liver, spleen, etc.) were excluded, which is consistent with previous studies.24,25
Serum SII examinations
Serum samples were collected and examined before the definitive diagnosis of MPM. No patients received any anti-tumor activities before. Pretreatment SII was calculated as neutrophil (N) ×platelet (P)/lymphocyte (L) counts.
Data collection
Parameters were collected as potential prognostic factors including age (<65 or ≥65 years), gender (male or female), smoking history (never or ever), Eastern Cooperative Oncology Group performance status (ECOG PS) scores (<2 or ≥2 points), primary site of tumor (left or right), pleural effusion (yes or no), weight loss (yes or no), pretreatment SII (<988.6 or ≥988.6×109/L) and treatment strategies (best supportive care [BSC] or adjuvant therapies). Adjuvant therapies included chemotherapy, radiotherapy, surgery or the combination of any treatment strategies above.
Statistical analysis
Chi-squared test was performed to assess the relationships between pretreatment SII and clinicopathological characteristics enrolled in the present study. Survival outcomes were dichotomized by survival status in the ROC curve analysis. OS was defined from a definitive diagnosis to the date of death or the last follow-up. The survival curves were obtained using the Kaplan–Meier method, and survival difference in variable groups was evaluated by the log-rank test. Multivariate analysis was performed using the Cox proportional hazard model, and all variables assessed on univariate analysis were included in the last multivariate analysis. SPSS 20.0 statistical software was used for statistical analysis. A P-value<0.05 were considered statistically significant.
Results
Patient characteristics
Ninety-seven MPM patients were enrolled in our study, and the detailed information of MPM patients is shown in Table 1. Fifty-eight patients (59.8%) were male, and 39 patients were female (40.2%), with a male-to-female ratio of 1.49:1. The median age for total cases was 56.0 years (range 18–77 years). Among the cases, 33 (34.0%) patients had a positive smoking history. Patients with ECOG PS 0–1 and ≥2 points were seen in 91 (93.8%) and 6 (6.2%) cases, respectively. Median OS for all cases was 18.5 months. At the diagnosis of MPM, 83 (85.6%) and 18 (18.6%) patients showed positive pleural effusion and weight loss, respectively.
 | Table 1 Prognostic analysis of MPM patients |
Treatment strategy
After the definition of MPM diagnosis, 15 (15.5%) patients were treated with best supportive care and they did not receive any positive anti-tumor therapies. The rest 82 (84.5%) cases received the adjuvant treatment of single chemotherapy, radiotherapy, surgery or the combination of therapies above. The number of patients treated with single chemotherapy was 11 (11.3%): single pemeterexed (n=4, 4.1%) and the rest 7 patients were treated with platinum-based chemotherapy or other anti-tumor drugs. Another 3 (3.1%) patients were treated with the combined management of surgery, immunotherapy and radiotherapy with chemotherapeutic drugs. In addition, 67 (69.1%) patients were treated with pemeterexed and platinum-based chemotherapy. Eight patients (6.6%) received the treatment of surgery and adjuvant therapies. The number of patients treated with single radiotherapy or radiotherapy plus other treatment strategies were 1 (1.0%) and 8 (6.6%), respectively.
The optimal cutoff value of SII
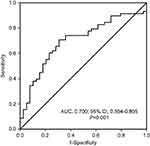
The mean pretreatment SII level of 97 cases was 1275.4±955.9×109/L (range 247.5–6725.1). According to the result of ROC analysis, the optimal cut-off value of pretreatment SII was 988.6×109/L (Figure 1, AUC, 0.700; 95% CI, 0.594–0.805; P=0.001). High and low pretreatment SII could be seen in 53 (54.6%) and 44 (45.4%) cases, respectively. The median OS for patients with low and high pretreatment SII was 47.0 and 13.0 months, respectively. The 1-, 2- and 3-year survival rates for patients with low SII were 85.8%, 57.8% and 52.0% compared to that of 53.9%, 23.6% and 13.8%, respectively, in patients with high SII (Figure 2B).
Univariate analysis
On univariate analysis, ECOG PS (Figure 2A; <2 vs ≥2 points; 21.5 vs 1.5 months; P<0.001), pretreatment SII (Figure 2B; <988.6 vs ≥988.6×109/L; 47.0 vs 13.0 months; P<0.001) and treatment strategy (Figure 2C; BSC vs adjuvant therapies; 2.3 vs 21.5 months; P=0.003) were shown to be closely correlated with the prognosis of MPM. No significant survival difference was found in age, gender, smoking history, primary site of tumor, pleural effusion and weight loss (P>0.05). ECOG PS 0–1 points, low pretreatment SII and adjuvant therapies were closely correlated with a prolonged survival.
Multivariate analysis
All variables enrolled were examined on multivariate analysis. The results showed ECOG PS scores (HR=3.573; 95% CI=1.085–11.765; P=0.036) and pretreatment SII (HR=2.298; 95% CI=1.232–4.289; P=0.009) were independent prognostic factors of MPM. Compared with ECOG PS score 0–1 point and low pretreatment SII, the risk of death for patients with ECGO PS ≥2 points and pretreatment SII≥988.6×109/L increased 257.3% and 129.8%, respectively. ECOG PS 0–1 point and low pretreatment SII were independent positive prognostic factors of MPM.
Relationship between pretreatment SII and clinicopathological characteristics
We also evaluated the relationships between pretreatment SII levels (high and low) and clinicopathological characteristics. Low SII was found to be closely correlated with weight loss (no vs yes, 40.5% vs 66.7%, P=0.044). However, no significant correlation was found in age, gender, smoking history, ECOG PS scores, primary site of tumor and pleural effusion (P>0.05, Table 2).
 | Table 2 Relationship between pretreatment SII and clinicopathological characteristics |
Discussion
Our present study shows that high pretreatment SII is easily accessible and is closely associated with a poorer prognosis of MPM. SII represents a powerful noninvasive, inexpensive and reproducible prognostic factor for MPM. These results are of great value for clinics, and it may help clinicians better understand the natural history of this lethal disease and make the best treatment decisions.
Inflammation plays a critical role in cancer development and progression. As key inflammatory factors, serum neutrophils, lymphocytes, monocytes and platelets, NLR, LMR and PLR have been demonstrated to be independent prognostic factors of many malignancies.2,16–19 However, the factors mentioned above are most calculated by one or two inflammatory cells. In some studies, these results cannot be repeated and their prognosis predicting ability may not be so powerful.17,26
Pretreatment SII is a novel systematic inflammatory response indicator, which is calculated by neutrophil (N)×platelet (P)/lymphocyte (L) counts. Pretreatment SII can better reflect body immune and inflammatory status. Previous studies have demonstrated that pretreatment SII can be a powerful prognosis predicting factor in a series of malignancies including lung, gastric, metastatic renal cell cancer, esophageal squamous cell carcinoma, etc.20,21,23,27 However, the prognostic role of pretreatment SII in MPM remains poorly understood. Fortunately, high pretreatment SII is also found to be closely correlated with a negative prognosis of MPM in our study.
High pretreatment SII always reflect a status of elevated neutrophil and platelet counts or decreased lymphocytes counts. Neutrophils play a key role in cancer progression by the secretion of proangiogenic factor vascular endothelial growth factor (VEGF).28 On one hand, by the secretion of VEGF, transforming growth factor beta and platelet-derived growth factor, platelets can facilitate cancer invasion, migration and metastasis.29,30 On the other hand, platelets can also promote angiogenesis via the secretion of cytokine VEGF.23 As key body immune cells, lymphocytes can induce direct and indirect anti-tumor activities, leading to the inhibition of tumor cells proliferation, invasion and migration.31 Low lymphocytes counts always reflect the impaired host immunosurveillance, leading to a poorer prognosis.
In our study, low SII was found to be closely correlated with weight loss, and no significant correlation was found between SII and age, gender, smoking history, ECOG PS scores, primary site of tumor and pleural effusion. This result is consistent with the previous study performed by Li et al32. The study involves patients with age≥70 years, and no correlation is found between SII and clinical characteristics. However, high SII is demonstrated to be a rough indicator of tumor differentiation. The potential relationship between pretreatment SII and clinical characteristics remains to be evaluated.
MPM represents a fatal, high aggressive and treatment-resistant disease, and no uniform treatment strategy has been established at present. Although pemetrexed and cisplatin-based chemotherapy has been approved for the first-line management of MPM, most patients died with 1 year.33 In our study, adjuvant therapies including single chemotherapy, radiotherapy, surgery or the combination of chemo-, radio- and surgery show a prolonged survival compared with patients treated with BSC. The best treatment strategies for MPM remain to be explored.
There are some limitations of our present study. Firstly, our study is a single institution retrospective study with limited numbers of patients enrolled. Secondly, due to the retrospective characteristics of our study and the long timespan of 2003–2015, some important feature information including protein c reactive erythrocyte sedimentation rate, histological type, asbestos exposure and TNM stage cannot be reached at this time. So we do not evaluate the histological data, asbestos exposure and TNM stage in this present study. In addition, the potential correlations between pretreatment SII and histological types and asbestos exposure were also not evaluated. We will pay more attention to these questions and we will present more detailed prognostic factor information of MPM in our further study.
In conclusion, the present study is performed in pathological proved MPM and we do not set a healthy control group. Pretreatment SII is easy to access, and it represents an efficiency and noninvasive biomarker of MPM. High SII represents an unfavorable independent prognostic factor of MPM, and the results need to be validated in further studies with a larger number of patients enrolled.
Acknowledgments
We thank all participants in this present study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cao S, Jin S, Cao J, et al. Malignant pericardial mesothelioma: a systematic review of current practice. Herz. 2018;43:61–68. doi:10.1007/s00059-016-4522-5
2. Zhang A, Cao S, Jin S, et al. Elevated aspartate aminotransferase and monocyte counts predict unfavorable prognosis in patients with malignant pleural mesothelioma. Neoplasma. 2017;64:114–122. doi:10.4149/neo_2017_114
3. Tsao AS, Wistuba I, Roth JA, Kindler HL. Malignant pleural mesothelioma. J Clin Oncol. 2009;27:2081–2090. doi:10.1200/JCO.2008.19.8523
4. Geltner C, Errhalt P, Baumgartner B, et al. Management of malignant pleural mesothelioma - part 1: epidemiology, diagnosis, and staging: consensus of the Austrian Mesothelioma Interest Group (AMIG). Wien Klin Wochenschr. 2016;128:611–617. doi:10.1007/s00508-016-1080-z
5. Serio G, Pezzuto F, Marzullo A, et al. Peritoneal mesothelioma with residential asbestos exposure. Report of a case with long survival (Seventeen years) Analyzed by Cgh-array. Int J Mol Sci. 2017;18(8).
6. Kato T, Jin CS, Lee D, et al. Preclinical investigation of folate receptor-targeted nanoparticles for photodynamic therapy of malignant pleural mesothelioma. Int J Oncol. 2018;53:2034–2046. doi:10.3892/ijo.2018.4555
7. De Bondt C, Psallidas I, Van Schil PEY, van Meerbeeck JP. Combined modality treatment in mesothelioma: a systemic literature review with treatment recommendations. Transl Lung Cancer Res. 2018;7:562–573. doi:10.21037/tlcr.2018.10.02
8. Frick AE, Nackaerts K, Moons J, et al. Combined modality treatment for malignant pleural mesothelioma: a single-centre long-term survival analysis using extrapleural pneumonectomy. Eur J Cardiothorac Surg. 2018. doi:10.1093/ejcts/ezy385
9. McCambridge AJ, Napolitano A, Mansfield AS, et al. Progress in the management of malignant pleural mesothelioma in 2017. J Thorac Oncol. 2018;13:606–623. doi:10.1016/j.jtho.2018.02.021
10. Yao ZH, Tian GY, Yang SX, et al. Serum albumin as a significant prognostic factor in patients with malignant pleural mesothelioma. Tumour Biol. 2014;35:6839–6845. doi:10.1007/s13277-014-1938-5
11. van Gerwen M, Alpert N, Wolf A, et al. Prognostic factors of survival in patients with malignant pleural mesothelioma: an analysis of the National Cancer Data Base. Carcinogenesis. 2019. doi:10.1093/carcin/bgz004
12. Zhuo Y, Lin L, Zhang M. Pretreatment thrombocytosis as a significant prognostic factor in malignant mesothelioma: a meta-analysis. Platelets. 2017;28:560–566. doi:10.1080/09537104.2016.1246712
13. Dipalma N, Luisi V, Di Serio F, et al. Biomarkers in malignant mesothelioma: diagnostic and prognostic role of soluble mesothelin-related peptide. Int J Biol Markers. 2011;26:160–165. doi:10.5301/JBM.2011.8614
14. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi:10.1016/j.cell.2010.01.025
15. Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125:3347–3355. doi:10.1172/JCI80007
16. Bernhardt D, Aufderstrasse S, Konig L, et al. Impact of inflammatory markers on survival in patients with limited disease small-cell lung cancer undergoing chemoradiotherapy. Cancer Manag Res. 2018;10:6563–6569. doi:10.2147/CMAR.S180990
17. Cao S, Jin S, Shen J, et al. Selected patients can benefit more from the management of etoposide and platinum-based chemotherapy and thoracic irradiation-a retrospective analysis of 707 small cell lung cancer patients. Oncotarget. 2017;8:8657–8669. doi:10.18632/oncotarget.14395
18. Shimizu K, Okita R, Saisho S, Maeda A, Nojima Y, Nakata M. Preoperative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non-small cell lung cancer. World J Surg Oncol. 2015;13:291. doi:10.1186/s12957-015-0710-7
19. Jin S, Cao S, Xu S, Wang C, Meng Q, Yu Y. Clinical impact of pretreatment prognostic nutritional index (PNI) in small cell lung cancer patients treated with platinum-based chemotherapy. Clin Respir J. 2018;12:2433–2440. doi:10.1111/crj.12925
20. Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q. Preoperative increased systemic immune-inflammation index predicts poor prognosis in patients with operable non-small cell lung cancer. Clin Chim Acta. 2018;484:272–277. doi:10.1016/j.cca.2018.05.059
21. Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. 2019;234:1794–1802. doi:10.1002/jcp.27052
22. Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. 2017;15:221. doi:10.1186/s12967-017-1326-1
23. Chen L, Yan Y, Zhu L, et al. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag Res. 2017;9:849–867. doi:10.2147/CMAR.S151026
24. Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CHJ, Stricker BH. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep. 2018;8:10566. doi:10.1038/s41598-018-28646-w
25. Meng X, Chang Q, Liu Y, et al. Determinant roles of gender and age on SII, PLR, NLR, LMR and MLR and their reference intervals defining in Henan, China: a posteriori and big-data-based. J Clin Lab Anal. 2018;32(2).
26. Ak G, Metintas S, Metintas M, et al. Prognostic factors according to the treatment schedule in malignant pleural mesothelioma. J Thorac Oncol. 2009;4:1425–1430. doi:10.1097/JTO.0b013e3181ba2033
27. Lolli C, Basso U, Derosa L, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget. 2016;7:54564–54571. doi:10.18632/oncotarget.10515
28. Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–287. doi:10.1023/B:AGEN.0000029415.62384.ba
29. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–249. doi:10.1111/j.1538-7836.2010.04131.x
30. Lim JU, Yeo CD, Kang HS, et al. Prognostic value of platelet count and lymphocyte to monocyte ratio combination in stage IV non-small cell lung cancer with malignant pleural effusion. PLoS One. 2018;13:e0200341. doi:10.1371/journal.pone.0200341
31. Shoji F, Morodomi Y, Akamine T, et al. Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non-small cell lung cancer. Lung Cancer. 2016;98:15–21. doi:10.1016/j.lungcan.2016.05.010
32. Li C, Tian W, Zhao F, et al. Systemic immune-inflammation index, SII, for prognosis of elderly patients with newly diagnosed tumors. Oncotarget. 2018;9:35293–35299. doi:10.18632/oncotarget.24293
33. Favoni RE, Florio T. Combined chemotherapy with cytotoxic and targeted compounds for the management of human malignant pleural mesothelioma. Trends Pharmacol Sci. 2011;32:463–479. doi:10.1016/j.tips.2011.03.011
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.