Back to Journals » Clinical Optometry » Volume 15
High Prevalence of Symptomatic Dry Eye Disease Among University Students During the COVID-19 Pandemic in University of West Indies, Trinidad and Tobago
Authors Ezinne N , Alemu HW , Cheklie T , Ekemiri K , Mohammed R, James S
Received 17 December 2022
Accepted for publication 22 February 2023
Published 3 March 2023 Volume 2023:15 Pages 37—43
DOI https://doi.org/10.2147/OPTO.S396135
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Mr Simon Berry
Ngozika Ezinne,1 Haile W Alemu,2 Tarekegn Cheklie,2 Kingsley Ekemiri,1 Ryan Mohammed,1 Sakeem James1
1Optometry Unit, Department of Clinical Surgical Sciences, University of the West Indies, Saint Augustine Campus, Trinidad and Tobago, West Indies; 2Department of Optometry, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Tarekegn Cheklie, Email [email protected]
Background: The Covid-19 pandemic lockdown obligated higher education students to attend online courses, leading to prolonged exposure to digital displays. Excessive time on digital devices could be a risk factor for ocular problems, including symptomatic dry eye. There are limited evidences to show the magnitude of symptomatic dry eye disease and its associated factors during COVID-19 pandemic. This study aimed to fill this gap, among university students in Trinidad and Tobago.
Methods: An institutional-based cross-sectional study was conducted among undergraduate students attending the University of West Indies, Saint Augustine Campus from October 2020 to April 2021. The standardized ocular surface disease index questionnaire, descriptive statics and binary logistic regression were used to assess the prevalence and associated factors of dry eye diseases. Variables with a p-value of less than 0.05 were considered to be statistically significant.
Results: Four hundred (96.3%) participants completed the questionnaire. Among all, 64.8% were female and 50.5% were east Indians. About 48% were using visual display units for average of 10– 15 hours/day. The prevalence of symptomatic dry eye disease was 84.3% (95% CI = 80.8– 87.5%) with OSDI score ≥ 13. Lack of education about dry eye 2.69 (95% CI: 1.41– 5.13), use of the reading mode of computer 3.92 (95% CI: 1.57– 9.80), refractive error 3.20 (95% CI: 1.66– 6.20), previous systemic medications 2.80 (95% CI: 1.15– 6.81), and average hours of visual display unit use/day (p< 0.001) were significantly associated with symptomatic dry eye disease.
Conclusion: Symptomatic dry eye disease was a prominent problem among students at the University of West Indies. Average of > 4 hours of visual display unit use/day, refractive error, positive history of systemic medication, lack of education about dry eye, and using computers in reading mode were associated factors.
Keywords: symptomatic dry eye disease, prevalence, associated factors, West Indies, Caribbean
Introduction
The ongoing COVID-19 pandemic and associated lockdowns required online university educations, which resulted in excessive use of electronic devices like phones, tablets, laptops, and computers. This excessive time spent using electronic devices led to an increased risk of ocular problems, including eye diseases like dry eye.1
Symptomatic dry eye disease (SDED) is a multi-factorial ocular surface condition that is manifested by symptoms of ocular pain, burning, visual disturbance, eye fatigue, dryness, grittiness, soreness, irritation, photophobia, and tearing.2–6 It occurs because of a disturbance in tear film layers (the inner mucous, middle aqueous, and outer lipid layer), especially in the outer lipid layer.7 SDED frequently affects quality of life by disturbing crucial daily activities like reading, using Visual Display Units (VDUs), watching television, recognizing friends, and driving.5,8–10 Studies confirm that the economic burden of SDED is very high.8,11–15 The United States healthcare system reported an overall annual burden of $59.24 billion due to SDED.9
Factors affecting SDED include age,3,16,17 sex,2,3,13,18,19 contact lens wear,18,20–22 VDU use,18–20,23 topical ophthalmic medication,2,22 allergies,2,24 current smoking,8,25 systemic health conditions,4,8,24,26 ocular surgery27 and systemic medications.26,28,29
The prevalence of SDED reported among students varies in different geographical locations. For example, it was estimated in Japan (21.6% to 32.3%),20 China (18.7% to 23.7%),23 Ghana (48.1%),2 Turkey (52.8%),19 Mexico (56%),30 Spain (51.8),31 and Saudi Arabia (62.4%).17 Since the COVID-19 pandemic, ophthalmic clinic visits due to SDED have increased.32 The prevalence of SDED increased from 48.1% before the COVID-19 pandemic to 62.4% during the pandemic, especially among those who are Visual Display Unit (VDU) users and other long-time near activity workers like students (high school and university), administrative and bank workers.30,33
Generally, although there are so many studies done about SDED in many parts of the world before and during the pandemic,2,13,20–22,34 due to the lack of evidence pertaining to the effect of COVID-19 epidemic on the population of Trinidad and Tobago, this study was aimed assessing the prevalence and associated factors of SDED among undergraduate students of the University of West Indies. This study also provides an insight into the effects of the COVID-19 pandemic on the Caribbean population.
Methods and Materials
An institutional-based cross-sectional study was conducted among undergraduate students attending the University of West Indies (UWI), Saint Augustine Campus, Trinidad and Tobago from October 2020 to April 2021. The university has approximately 17,035 students and eight faculties including Medical Sciences, Science and Technology, Engineering, Sports, Humanities and Education, Food and Agriculture, Social Sciences, and the Faculty of Law.35
All undergraduate students at UWI Saint Augustine campus made up the study population. All undergraduate students attending the UWI Saint Augustine campus who are between the ages of 18–35 years and gave their consent to take part were included as the study population. But the study excluded students who have not stayed up to a semester as UWI students.
Sample size calculated with Raosoft software considering a population of 17,035 and 95% confidence interval with a 5% margin of error using the formula below: x=Z(c/100)2r(100-r) n=N x/((N-1)E2 + x) E=Sqrt[(N - n) x/n(N-1)] Where n is the sample size, E is the margin of error, N is the population size, r is the fraction of responses that you are interested in, and Z(c/100) is the critical value for the confidence level c. Using the above formula, the calculated sample size was 376. By considering a 10% non-response rate, it increased the final sample size to 415.
The list of all registered students was picked up from the university database and labeled according to their faculties. Google random number generator (https://stattrek.com/statistics/random-number-generator.aspx), was used to select 415 study participants.
Though the gold standard diagnostic test is debatable, the symptom questionnaire is the most repeatable diagnostic test. There are various questionnaires for the diagnosis of SDED. But, the ocular surface disease index (OSDI) is the best-validated standard questionnaire with 12 questions, having high sensitivity (80%), specificity (79%), and reliability (>0.7 by Cronbach’s α) for discriminating patients with and without SDED.36 The standard OSDI questionnaire was adapted by incorporating factors that affect SDED after the literature review.3,37–42 The questionnaire was divided into three sections: the first evaluated the frequency of symptoms; the second evaluated the effect of symptoms on daily tasks; and the third evaluated the effect of environmental factors, such as windy conditions and air conditioning. The scores on the three sections were summed up to get the final OSDI score (sum of the 12 questions multiplied by 25 and divided by 12), which ranged from 0 to 100, with higher values indicating symptom severity. Symptomatic dry eye was defined as an OSDI score ≥13.43 Because of Covid-19 pandemic restrictions, online data collection was the method used to collect data. The student’s contact was obtained from the university database. Consent sheets and questionnaire forms were sent out to those students with their email. Following getting the consent email, research investigators sent the questionnaire link to each student.
The dependent variable was symptomatic dry eye disease and explanatory variables were age, sex, ethnicity, previous ocular surgery, refractive error, smoking, total VDU use hour per day, consistent near work, use of systemic medications, an omega-3 supplement, family history of dry eye, education about dry eye and protective cover of VDUs.
Collected data downloaded from browsers and checked for completeness and consistency before merging with Microsoft Excel 2010. Finally, merged data was exported to SPSS version 20.0, to clean, code, and analyzed. The prevalence of symptomatic dry eye with 95% confidence interval (CI) was determined by using the OSDI score. Descriptive components summarized were frequency, percent, and summary statistics. Binary logistic regression model was fitted and variables with a p-value less than 0.2 with bi-variable analysis were considered for the multivariable analysis. In multivariable logistic regression, explanatory variables with a value of p < 0.05 were significant. Odds ratios (OR) and 95% confidence intervals were calculated to determine the magnitude of association. Hosmer–Lemeshow test was used to assess the model fitness.
SDED was defined as symptomatic dry eye disease with OSDI score ≥13.42 VDU use hour was defined as the average continuous hours of visual display unit use like a computer, television, and smartphones per day.20,21 Current smoking was defined as a daily smoker who smokes any tobacco product at least once a day within the last week.39 Previous ocular surgery was defined as a history of ocular surgery within the last 1 year.44
Ethical approval was obtained from the University of the West Indies, Saint Augustine campus, Research and Ethics Committee (Registration number: CREC-SA.0721/01/2021). The study followed the tenets of the Declaration of Helsinki.
Results
In this study, 400 undergraduate students took part with a response rate of 96.4% (400/415). The participants’ age ranges from 18 to 35 years. The majority of participants (64.8%) were females and about half (50.5%) were of East Indian ethnicity. Nearly half (48%) of the participants used VDU for an average of 10–15 hours per day, whereas nearly 2/3rd (63%) performed near work consistently for 2–5 hours. Additionally, 2/3rd (67.5%) used reading mode (blue light filter or increase the warmth of the device’s screen display) when using their computers. About 1/4th (24.8%) of the study participants had history of systemic medication and 8.3% of them reported taking an Omega 3 supplements (Table 1).
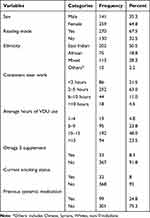 |
Table 1 Factors of SDED Among Undergraduate Students at the University of West Indies (n=400) |
The prevalence of SDED among the participants was 84.3% (95% CI = 80.8–87.5%). The bi-variable logistic regression model found an association (p<0.2) between SDED and, sex, family history of dry eye, refractive error, education about dry eye, average hours of VDU use per day, hours of consistent near activity, use of reading mode on the VDU and systemic medication history.
A multivariate logistic regression found an association between SDED and education about dry eye 2.69 (95% CI: 1.41–5.13) times, use of reading mode of computer 3.92 (95% CI: 1.57–9.80) times, average hours of VDU use per day (p<0.001), refractive error 3.20 (95% CI: 1.66–6.20) times and previous systemic medications 2.80 times (95% CI: 1.15–6.81) (Table 2).
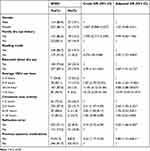 |
Table 2 Result of Binary Logistic Regression Analysis of SDED Among Undergraduate Students at the University of West Indies (n=400) |
Discussion
This study found that, the prevalence of SDED was 84.3% (95% CI, 80.8–87.5) with a standard OSDI score. This prevalence is higher than the study done university students at University of Murcia (Spain) during the COVID-19 lockdown, which was 51.8%. This discrepancy could be because of criteria difference to say SDED that, they diagnosed a participant as having SDED when she/he have OSDI score >22, in contrast to our study which uses ≥13 as a cut point.31 This prevalence is also higher than the prevalence conducted among 4393 VDU users 32.3%,20 and 3443 high-school students 21.6% in Japan.21 It was also higher than other studies among students ranging from 18.7% to 48.1%2,23 with sample size of 1139 and 700, respectively, and also two studies conducted among Turkey lecturers and Saudi Arabia population who found 52.8% and 62.4%, respectively.17,19 The higher value of this study may be because of wearing masks during the COVID-19 lockdown though we have not studied.45,46 The discrepancies may also be because of an intensified VDU use among students during the lockdown of the COVID-19 pandemic could contribute positively to the high prevalence of SDED.1 The high prevalence in this study could be also because more than half of the participants (64.8%) were females who are more likely to be vulnerable to dry eye because of hormonal changes and oral contraceptive use.47–49 This difference also may be due to difference in environmental exposures, weather parameters, and climate of study areas.50,51
This study found previous education on dry eye found to be associated with SDED. The odds that developing SDED is 2.69 times higher among students who have no previous education about dry eye as compared to participants who have previous education. It may be because of the reason that since awareness and knowledge about a disease can lead people to prevent themselves from its awareness about dry eye disease pushes people to protect themselves from the disease and its consequences.
In this study, the odds of developing SDED are 3.92 times higher for participants who do not use reading mode (blue light filter or increase the warmth of the device’s screen display when using it) than those who use it. The use of reading mode or blue light filters is highly advisable, especially for those who worked on VDU for an extended period. Therefore, blue light filters can improve comfort; the majority of symptoms of dry eye relieves with blue light filters.52
This study found that the odds of developing SDED are 3.20 times higher for ametropic participants as compared to emmetropic participants. Similar findings were obtained among studies in China and Ghana.1,2,23,25 The reason may be because of common and shared reported symptoms between refractive error and SDED as the study is based on symptoms53 or it may also be because of contact lens use of participants, which is one of the factor for SDED although our study did not assess it.21,22,33
Those participants with a history of use of systemic medication are found to be 2.80 times at risk of having SDED as compared to those not using. This finding is supported by study conducted in Saudi Arabia17 where up to 70% of the participants with systemic conditions suffer from SDED.54
Our study found that those who use VDU for 5–9, 10–14, and >14 total hours have 5.47 times, 8.70 times, and 12.39 times higher chance of developing SDED, respectively, as compared to those participants who use VDU for only 1–4 average total hours per day. This was consistent with findings from studies in China,23 Japan,20 and Turkey.19 The possible reason for the association could be due to extended exposure to VDU which is linked to increased tear evaporation and low blinking rate, which eventually leads to SDED.20
Limitations
Using a single validated questionnaire instead of a combined tool (OSDI and other objective assessments) could affect the estimates of SDED prevalence and make comparison difficult with previous studies. This study result could be inflated because visual status, the presence of uncorrected refractive error, and other ocular and near vision problems were not measured in online data collection.
Contact lens use of participants, hours of face mask use, which could be factors for SDED were not assessed.
Recall bias to social and behavioral past exposure may occur among participants.
Conclusion
In this study, symptomatic dry eye disease is a common ocular problem experienced by majority of undergraduate students at the University of West Indies. An average of >5 hours of VDU use per day, refractive error, history of systemic medication, and education about dry eye were significantly associated factors.
Disclosure
The authors declare no competing interests in this work.
References
1. Elhusseiny AM, Eleiwa TK, Yacoub MS, et al. Relationship between screen time and dry eye symptoms in pediatric population during the COVID-19 pandemic. Ocul Surf. 2021;22:117–119. doi:10.1016/j.jtos.2021.08.002
2. Asiedu K, Kyei S, Boampong F, Ocansey S. Symptomatic dry eye and its associated factors: a study of university undergraduate students in Ghana. Eye Contact Lens. 2017;43(4):262–266. doi:10.1097/ICL.0000000000000256
3. Gupta N, Prasad I, Jain R, D’Souza P. Estimating the prevalence of dry eye among Indian patients attending a tertiary ophthalmology clinic. Ann Trop Med Parasitol. 2010;104(3):247–255. doi:10.1179/136485910X12647085215859
4. Muna’AIM MA, Tey Y, Mohamad Zafarullah A, Saleh RM, Omar N. Dry eye among patients at the eye clinic of a secondary referral hospital. Malays J Med Sci. 2016;12:30–37.
5. Tong L, Waduthantri S, Wong T, et al. Impact of symptomatic dry eye on vision-related daily activities: the Singapore Malay Eye Study. Eye. 2010;24(9):1486–1491. doi:10.1038/eye.2010.67
6. Gantz L, Rosenfield M. Digital eye strain symptoms during online university learning in Israel and the USA during the COVID-19 pandemic. Invest Ophthalmol Vis Sci. 2021;62(8):1975.
7. Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders–new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;28(Suppl 1):3. doi:10.1097/01.icu.0000512373.81749.b7
8. Alshamrani AA, Almousa AS, Almulhim AA, et al. Prevalence and risk factors of dry eye symptoms in a Saudi Arabian population. Middle East Afr J Ophthalmol. 2017;24(2):67. doi:10.4103/meajo.MEAJO_281_16
9. Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–15. e2. doi:10.1016/j.ajo.2006.11.060
10. Le Q, Zhou X, Ge L, Wu L, Hong J, Xu J. Impact of dry eye syndrome on vision-related quality of life in a non-clinic-based general population. BMC Ophthalmol. 2012;12(1):22. doi:10.1186/1471-2415-12-22
11. Waduthantri S, Yong SS, Tan CH, et al. Cost of dry eye treatment in an Asian clinic setting. PLoS One. 2012;7(6):e37711. doi:10.1371/journal.pone.0037711
12. Clegg JP, Guest JF, Lehman A, Smith AF. The annual cost of dry eye syndrome in France, Germany, Italy, Spain, Sweden and the United Kingdom among patients managed by ophthalmologists. Ophthalmic Epidemiol. 2006;13(4):263–274. doi:10.1080/09286580600801044
13. Hashemi H, Khabazkhoob M, Kheirkhah A, et al. Prevalence of dry eye syndrome in an adult population. Clin Experiment Ophthalmol. 2014;42(3):242–248. doi:10.1111/ceo.12183
14. Reddy P, Grad O, Rajagopalan K. The economic burden of dry eye: a conceptual framework and preliminary assessment. Cornea. 2004;23(8):751–761. doi:10.1097/01.ico.0000134183.47687.75
15. Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30(4):379–387. doi:10.1097/ICO.0b013e3181f7f363
16. Sherry A, Aridi M, Ghach W. Prevalence and risk factors of symptomatic dry eye disease in Lebanon. Contact Lens Anterior Eye. 2019;43:355–358. doi:10.1016/j.clae.2019.08.001
17. Alharbi AJ. Prevalence of symptomatic dry eye and its risk factors among coastal population in Eastern Province of Saudi Arabia. EC Ophthalmol. 2019;10:503–509.
18. Uchino M, Nishiwaki Y, Michikawa T, et al. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology. 2011;118(12):2361–2367. doi:10.1016/j.ophtha.2011.05.029
19. Vayısoğlu SK, Öncü E, Dursun Ö, Dinç E. Investigation of dry eye symptoms in lecturers by ocular surface disease index. Turk J Ophthalmol. 2019;49(3):142. doi:10.4274/tjo.galenos.2018.67915
20. Uchino M, Schaumberg DA, Dogru M, et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology. 2008;115(11):1982–1988. doi:10.1016/j.ophtha.2008.06.022
21. Uchino M, Dogru M, Uchino Y, et al. Japan Ministry of Health study on prevalence of dry eye disease among Japanese high school students. Am J Ophthalmol. 2008;146(6):925–9. e2. doi:10.1016/j.ajo.2008.06.030
22. Zhang Y, Chen H, Wu X. Prevalence and risk factors associated with dry eye syndrome among senior high school students in a county of Shandong Province, China. Ophthalmic Epidemiol. 2012;19(4):226–230. doi:10.3109/09286586.2012.670742
23. Yu-ping H, Wen-fang Z, Peng L, Ran Z, Jin-tao X, Ying F. Prevalence of symptomatic dry eye disease among Chinese college students with associated risk factors. Int Eye Sci. 2016;16(6):1019–1025.
24. Vehof J, Kozareva D, Hysi PG, Hammond CJ. Prevalence and risk factors of dry eye disease in a British female cohort. Br J Ophthalmol. 2014;98(12):1712–1717. doi:10.1136/bjophthalmol-2014-305201
25. Ranjan R, Shukla SK, Singh CV, Mishra B, Sinha S, Sharma B. Prevalence of Dry Eye and its Association with various risk factors in rural setup of Western Uttar Pradesh in a Tertiary care hospital. Open J Prev Med. 2016;6(01):57. doi:10.4236/ojpm.2016.61005
26. Zhang S, Hong J. Risk factors for dry eye in Mainland China: a multi-center cross-sectional hospital-based study. Ophthalmic Epidemiol. 2019;26(6):393–399. doi:10.1080/09286586.2019.1632905
27. Guo B, Lu P, Chen X, Zhang W, Chen R. Prevalence of dry eye disease in Mongolians at high altitude in China: the Henan eye study. Ophthal Epidemiol. 2010;17(4):234–241. doi:10.3109/09286586.2010.498659
28. Galor A, Feuer W, Lee DJ, et al. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol. 2011;152(3):377–84. e2. doi:10.1016/j.ajo.2011.02.026
29. Ferrero A, Alassane S, Binquet C, et al. Dry eye disease in the elderly in a French population-based study (the Montrachet study: maculopathy, Optic Nerve, nuTRition, neurovAsCular and HEarT diseases): prevalence and associated factors. Ocul Surf. 2018;16(1):112–119. doi:10.1016/j.jtos.2017.09.008
30. Castellanos-González JA, Torres-Martínez V, Martínez-Ruiz A, et al. Prevalence of dry eye syndrome in residents of surgical specialties. BMC Ophthalmol. 2016;16(1):108. doi:10.1186/s12886-016-0292-3
31. García-Ayuso D, Di Pierdomenico J, Moya-Rodríguez E, Valiente-Soriano FJ, Galindo-Romero C, Sobrado-Calvo P. Assessment of dry eye symptoms among university students during the COVID-19 pandemic. Clin Exp Optomet. 2022;105(5):507–513. doi:10.1080/08164622.2021.1945411
32. Cartes C, Segovia C, Salinas-Toro D, et al. Dry eye and visual display terminal-related symptoms among university students during the coronavirus disease pandemic. Ophthalmic Epidemiol. 2021;20:1–7.
33. Tan LL, Morgan P, Cai ZQ, Straughan RA. Prevalence of and risk factors for symptomatic dry eye disease in S ingapore. Clin Exp Optomet. 2015;98(1):45–53. doi:10.1111/cxo.12210
34. Jie Y, Xu L, Wu Y, Jonas J. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye. 2009;23(3):688–693. doi:10.1038/sj.eye.6703101
35. Thomas ML, Soares J. Increasing public access to university qualifications: evolution of the University of the West Indies Open Campus. Int Rev Res Open Distributed Learn. 2009;10(1). doi:10.19173/irrodl.v10i1.537
36. Lu F, Tao A, Hu Y, Tao W, Lu P. Evaluation of reliability and validity of three common dry eye questionnaires in Chinese. J Ophthalmol. 2018;2018:125.
37. Vitale S, Goodman LA, Reed GF, Smith JA. Comparison of the NEI-VFQ and OSDI questionnaires in patients with Sjögren’s syndrome-related dry eye. Health Qual Life Outcomes. 2004;2(1):44. doi:10.1186/1477-7525-2-44
38. Group IDEWS. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:93–107. doi:10.1016/S1542-0124(12)70082-4
39. Nichols KK, Mitchell GL, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23(3):272–285. doi:10.1097/00003226-200404000-00010
40. Korb DR. Survey of preferred tests for diagnosis of the tear film and dry eye. Cornea. 2000;19(4):483–486. doi:10.1097/00003226-200007000-00016
41. Williamson JF, Huynh K, Weaver MA, Davis RM. Perceptions of dry eye disease management in current clinical practice. Eye Contact Lens. 2014;40(2):111. doi:10.1097/ICL.0000000000000020
42. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi:10.1001/archopht.118.5.615
43. Özcura F, Aydin S, Helvaci MR. Ocular surface disease index for the diagnosis of dry eye syndrome. Ocul Immunol Inflamm. 2007;15(5):389–393. doi:10.1080/09273940701486803
44. Eric D, Donnenfeld M. Chronic dry eye develops in small percentage of patients after PRK, LASIK Ocular surgery news. 2016.
45. Fan Q, Liang M, Kong W, et al. Wearing face masks and possibility for dry eye during the COVID-19 pandemic. Sci Rep. 2022;12(1):1–9. doi:10.1038/s41598-022-07724-0
46. Krolo I, Blazeka M, Merdzo I, Vrtar I, Sabol I, Petric-Vickovic I. Mask-associated dry eye during COVID-19 pandemic–how face masks contribute to dry eye disease symptoms. Med Archiv. 2021;75(2):144. doi:10.5455/medarh.2021.75.144-148
47. Chen SP, Massaro-Giordano G, Pistilli M, Schreiber CA, Bunya VY. Tear osmolarity and dry eye symptoms in women using oral contraception and contact lenses. Cornea. 2013;32(4):423–428. doi:10.1097/ICO.0b013e3182662390
48. Peck T, Olsakovsky L, Aggarwal S. Dry eye syndrome in menopause and perimenopausal age group. J Midlife Health. 2017;8(2):51–54. doi:10.4103/jmh.JMH_41_17
49. Sriprasert I, Warren DW, Mircheff AK, Stanczyk FZ. Dry eye in postmenopausal women: a hormonal disorder. Menopause. 2016;23(3):343–351. doi:10.1097/GME.0000000000000530
50. Tesón M, López-Miguel A, Neves H, Calonge M, González-García MJ, González-Méijome JM. Influence of climate on clinical diagnostic dry eye tests: pilot study. Optomet Vision Sci. 2015;92(9):e284–e9. doi:10.1097/OPX.0000000000000673
51. Berg EJ, Ying GS, Maguire MG, et al. Climatic and environmental correlates of dry eye disease severity: a report from the dry eye assessment and management (DREAM) Study. Transl Vis Sci Technol. 2020;9(5):25. doi:10.1167/tvst.9.5.25
52. Dabrowiecki A, Villalobos A, Krupinski EA. Impact of blue light filtering glasses on computer vision syndrome in radiology residents: a pilot study. J Med Imaging. 2019;7(2):022402. doi:10.1117/1.JMI.7.2.022402
53. Ilhan N, Ilhan O, Tuzcu EA, et al. Is there a relationship between pathologic myopia and dry eye syndrome? Cornea. 2014;33(2):169–171. doi:10.1097/ICO.0000000000000033
54. Wong J, Lan W, Ong LM, Tong L. Non-hormonal systemic medications and dry eye. Ocul Surf. 2011;9(4):212–226. doi:10.1016/S1542-0124(11)70034-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
