Back to Journals » International Journal of General Medicine » Volume 15
High-Mobility Group AT-Hook 1 Served as a Prognosis Biomarker and Associated with Immune Infiltrate in Hepatocellular Carcinoma
Authors Wei YG , Yang CK, Wei ZL, Liao XW , He YF, Zhou X, Huang HS, Lan CL, Han CY, Peng T
Received 17 October 2021
Accepted for publication 23 December 2021
Published 14 January 2022 Volume 2022:15 Pages 609—621
DOI https://doi.org/10.2147/IJGM.S344858
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yong-Guang Wei, Cheng-Kun Yang, Zhong-Liu Wei, Xi-Wen Liao, Yong-Fei He, Xin Zhou, Hua-Sheng Huang, Chen-Lu Lan, Chuang-Ye Han, Tao Peng
Department of Hepatobiliary Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, 530021, Guangxi Zhuang Autonomous Region, People’s Republic of China
Correspondence: Tao Peng
Department of Hepatobiliary Surgery, The First Affiliated Hospital of Guangxi Medical University, Shuang Yong Road 6, Nanning, Guangxi Zhuang Autonomous Region, People’s Republic of China
Tel +86-771-5356528
Fax +86-771-5350031
Email [email protected]
Background: The protein high-mobility group AT-hook 1 (HMGA1) has been demonstrated that modulated cellular proliferation, invasion, and apoptosis with a poor prognosis in miscellaneous carcinomas. However, the mechanism of circumstantial carcinogenesis and association with the immune microenvironment of HMGA1 in hepatocellular carcinoma (HCC) had not been extensively explored.
Methods: The gene expression, clinicopathological correlation, and prognosis analysis were performed in the data obtained from TCGA. The results were further validated by ICGC and GEO database and external validation cohort from Guangxi. The HMGA1 protein expression was further examined in the HPA database. Biological function analyses were conducted by GSEA, STRING database, and Coexpedia online tool. Using TIMER and CIBERSORT method, the relationship between immune infiltrate and HMGA1 was investigated.
Results: In HCC, HMGA1 had much higher transcriptional and proteomic expression than in corresponding paraneoplastic tissue. Patients with high HMGA1 expression had a poor prognosis and unpromising clinicopathological features. High HMGA1 expression was closely related to the cell cycle, tumorigenesis, substance metabolism, and immune processes by regulating complex signaling pathways. Notably, HMGA1 may be associated with TP53 mutational carcinogenesis. Moreover, increased HMGA1 expression may lead to an increase in immune infiltration and a decrease in tumor purity in HCC. CIBERSORT analysis elucidated that the amount of B cell naive, B cell memory, T cells gamma delta, macrophages M2, and mast cell resting decreased when HMGA1 expression was high, whereas T cells follicular helper, macrophages M0, and Dendritic cells resting increased.
Conclusion: In conclusions, HMGA1 is a potent prognostic biomarker and a sign of immune infiltration in HCC, which may be a potential immunotherapy target for HCC.
Keywords: hepatocellular carcinoma, HMGA1, prognostic signature, bioinformatics, immune filtration
Introduction
Hepatocellular carcinoma (HCC), which accounts for 75–85% of all cases, is the predominant type of liver cancer with a highly malignant nature due to its insidious onset, rapid progression, and intrahepatic and distant metastasis.1,2 Liver cancer is one of the most common malignancies and the second most frequent cause of cancer-associated mortality worldwide. More than 80% of HCC cases are estimated to occur in the countries with low-medium resources, particularly in Eastern Asia and sub-Saharan Africa.1 An investigation showed that liver cancer was one of the five leading causes of years of life lost (YLLs) in 2017 in China, suggesting it had brought an grave burden to society.3
Over the past few decades, there has been considerable progress in comprehending the epidemiology, risk factors, and molecular profiles of HCC. On the etiology, HCC arises from infection of chronic hepatitis (B and C) virus and cirrhosis, excessive drinking, metabolic liver diseases, particularly nonalcoholic fatty liver disease (NAFLD), prolonged dietary toxins exposure such as aflatoxin and aristolochic acid, type 2 diabetes mellitus, obesity, and smoking.4,5 These ongoing risk factors mediate the liver cells damage, chronic inflammation, and further genomic changes. Indeed, HCC can actually be clinically cured with an early diagnosis with a satisfactory prognosis when potentially curative approaches are applicable.1,6 In recent years, the researchers have made headway on the operative treatment and medication of HCC.7–11 Immune checkpoint inhibitor (ICI) therapy has become an incrementally utilized treatment modality across the various stages of HCC. This provides a new therapeutic prospect for patients with HCC and underscores the crucial role of the tumor microenvironment (TME) in the development of HCC.12–15 Unfortunately, due to the complexity of immunotherapy and the genetic heterogeneity, this method took effect only for partial patients.16 Emerging evidence demonstrated that the change of tumor-induced immune infiltrate cells was a momentous mechanism that carcinomas develop to evade immune surveillance. Meanwhile, tumor immune infiltration carries a clinicopathological significance in predicting prognosis and therapeutic efficacy.17–19
HMGA1 is a non-histone nuclear protein as well as a chromatin architectural protein. In contrast to the high expression levels in various carcinomas, HMGA1 expression levels in normal tissue were low or undetectable. Several studies demonstrated that a high level of HMGA1 expression correlated with adverse clinical outcomes and more advanced disease in cancers.20–23 Previous researches have shown that HMGA1 connected with hepatitis B virus- mediated changes and liver fibrosis.24,25 Further, it acted as an oncogenic driver of progression and migration with a poor prognosis in HCC.26,27 Higher HMGA1 mRNA and protein levels were discovered in HCCs with intrahepatic metastases compared to those without intrahepatic metastases, indicating the aggressive nature of HMGA1 in HCC development.28,29 The reviews concluded that the ability to modulate gene expression and chromatin remodeling led to complex biological activities of HMGA1, resulting in the transformation of different molecular pathways.23,30–32 Despite this, the exact mechanisms of HMGA1 on hepatocarcinogenesis and its immune relevance remain to be further elucidated to lay the foundation for the next analysis at a deeper level. Therefore, this study comprehensively evaluated the prognosis significance, latent biological functions, and the association with immune infiltrating cells of HMGA1 in HCC.
Materials and Methods
Hepatocellular Carcinoma (HCC) Datasets and Data Processing
The Cancer Genome Atlas (TCGA) database portal (https://portal.gdc.cancer.gov/cart; up to May 15, 2019) was employed to retained data of HCC samples on gene expression (Workflow Type: HT Seq-FPKM), clinical features (Data Type: Clinical Supplement), and genetic mutation. Samples lacking information on survival time, pathological grade, lymphatic, and distant metastasis were excluded. Processed RNA-Seq FPKM data of 374 pathologically verified HCC and 50 adjacent normal tissues were included. In addition, 232 HCC patients were included in The combined International Cancer Genomics Consortium (ICGC) cohort, and the Gene Expression Omnibus (GEO) cohort (GSE14520 datasets) included 212 HCC patients. Their expression and the clinical information was respectively obtained from the ICGC (http://dcc.icgc.org) and GEO database (https://www.ncbi.nlm.nih.gov/geo/). For the Kaplan-Meier survival analysis, the patients were divided into high- and low-HMGA1 expression groups according to the optimal cutoff value figured out by a user-friendly and web-based integrative tool (ESurv, https://www.giantonline.org/).33
Differential mRNA Expression, Prognosis Value, and Clinicopathological Correlation Analyses
The differentially expressed HMGA1 in HCC and normal tissues were detected using the Wilcoxon test method. To investigate the prognostic value of HMGA1, the Kaplan–Meier analysis was performed to compare overall survival (OS) time between high- and low-HMGA1 expression groups. Log rank test was used for statistical comparison. The log-rank p-values and the hazard ratios were figured. Univariate Cox proportional hazards regression and multivariate Cox regression analyses were utilized to assess the relationship between HMGA1 and OS time. The receiver operating characteristic (ROC) curve was also implemented. Logistic regression analysis was utilized to figure out whether there are significant variances in HMGA1 expression under different clinicopathological statuses. The associations between HMGA1 expression and certain clinicopathological parameters were analyzed using the Student’s t-test.
Biological Function Analysis
To gain insight into the biological processes associated with the HMGA1 regulatory network that could underlie HCC development, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed based on normalized RNA-Seq data obtained from the TCGA dataset using Gene Set Enrichment Analysis (GSEA). The count of permutations was set to 1000. Functional categories with a false discovery rate (FDR) < 0.050 and a nominal p-value < 0.050 were regarded as statistically significant pathways. Furthermore, we explored the STRING (search tool for recurring instances of neighboring genes) website (https://string-db.org/) to construct a protein-protein interaction (PPI) network among HMGA1 and the neighboring 10 genes significantly associated with it. An interaction score of 0.4 was set as a cut-off value.34 The HMGA1 co-expression network was assessed utilizing Coexpedia online tools (http://www.coexpedia.org/) and was reconstructed by Cytoscape.35 To examine the possible mechanism by which HMGA1 acts on the p53 signaling pathway, Pearson correlation analyses were performed on HMGA1 and some TP53-related genes respectively.
Immune Infiltrates Analysis
TIMER (Tumor Immune Estimation Resource) is a comprehensive resource, based on statistical computation known as deconvolution,36 which is used to perform interactive analysis of the associations between the abundance of immune infiltrates and gene expression level (https://cistrome.shinyapps.io/timer/).37 Using TIMER, the abundance of six tumor-infiltrating immune cells (TIICs), including B cells, CD4 T cells, CD8 T cells, macrophages, neutrophils, and dendritic cells as well as infiltrate purity was approximated. Besides, CIBERSORT is an analytical tool that estimates the abundance of member cell types in a mixed cell population using gene expression data (http://cibersort.stanford.edu/).38 Through the CIBERSORT (Cell type Identification through the Estimating Relative Subsets of RNA Transcripts) algorithm, we evaluated the immune response of 22 TIICs in HCC based on the TCGA dataset and then evaluated their abundance between the high and low HMGA1 expression groups. A p-value < 0.05 makes known the statistical significance of deconvolution outcomes.
Collection and Reverse Transcription‑Quantitative PCR (RT‑qPCR) of HCC Samples in the Guangxi Cohort
From 2017 to 2018, surgical biopsies were collected from 29 patients who underwent curative hepatic resections for HCC at The First Affiliated Hospital of Guangxi Medical University (Guangxi, China) without the preoperative therapies. OS information was retrieved through electronic medical records or telephone follow-up. All patients enrolled signed informed consent forms to donate their tissue samples for biomedical research, which was authorized by the ethics committee of The First Affiliated Hospital of Guangxi Medical University [Approval Number: 2021 (KY-E-032)]. In our previous study, the way of specimen preservation and the specific procedures of RNA extraction and reverse transcription-quantitative PCR had been mentioned.39 The sequence of primers was as following: GAPDH, forward GTCAGCCGCATCTTCTTT, reverse CGCCCAATACGACCAAAT. HMGA1, forward AAACCAAGGGGCAGACCCAA, reverse CTGTGTAGTGTGGTGGTGAGG. Using the 2−ΔΔCt method, the expression levels of HMGA1 were estimated to conduct validation analysis.
Validation of Differential Expression, Prognosis Value of HMGA1
The differential expression in HCC vs para-carcinoma tissues and prognosis value of HMGA1 were further validated by investigating the data from ICGC, GSE14520, and Guangxi cohorts. Furthermore, the HPA database (https://www.proteinatlas.org) was used to exam the protein expression of HMGA1 and to accurately assess protein localization.
Statistical Analysis
Unless otherwise noted, all analyses were performed using R software (version 3.6.3) and p-value < 0.05 was considered to be significant.
Result
Discrepant mRNA and Protein Expression of HMGA1
The HMGA1 expression levels were significantly up-regulated in HCC tissues in comparison with the non-tumor controls (Figure 1A, P < 0.001). Such result was further verified by mining Guangxi (Figure 1B, P < 0.001), ICGC (Figure 1C, P < 0.001), and GSE14520 (Figure 1D, P < 0.001) cohorts. Consistent with the mRNA expression analysis, the proteins encoded by HMGA1 were strongly positive in HCCs (Figure 1E and F), while were low-expression in normal liver tissues in the human Protein Profiles (Figure 1G and H). Besides, HMGA1 protein was mainly localized to nuclei.
Prognosis Value and Clinicopathological Correlation of HMGA1
In the TCGA cohort, the augmented expression of HMGA1 corresponded with worse OS at the optimal cut-off point of 54.73 (Figure 2A, P < 0.001). Consistently, the enhanced mRNA expression of HMGA1 linked to the poor OS in the ICGC (Figure 2B, P < 0.05) and GSE14520 cohorts (Figure 2C, P < 0.05). Nevertheless, survival analysis was not statistically significant in Guangxi cohort, which may be due an insufficient sample size and adequate follow-up time (Figure 2D). The connections between HMGA1 expression and clinical information in patients with HCC were analyzed (Table 1). As for cancer status, HMGA1 upregulation expressions were prone to be found in the HCCs with poorer clinicopathological features, including histological grade, T stage, and pathological stage (Figure 3A–C, all P < 0.001). Interestingly, HMGA1 expression showed a monotone increasing from histological grade I to IV, pathological stage I to III, and T1 stage to T3 stage. Nevertheless, HMGA1 expression in pathological stage IV or T4 stage is not the highest. This may be attributed to the cause that operation should not be considered as a major treatment measure for advanced-stage patients. Logistic regression analysis demonstrated that T stage (II and III vs I), stage (II and III vs I) and grade (II and III vs I) were significantly associated with increased HMGA1 expression level in HCC (Table 2). Furthermore, a notable discovery indicated that HCCs with TP53 mutations had higher HMGA1 expression levels in the TCGA (Figure 3D, P < 0.001). Univariate analysis indicated that pathological stage, T and M stage were markedly correlated with OS (Table 3, P < 0.05). Following that, multivariate analysis indicated that HMGA1 overexpression remained as an independent predictive marker of OS (Table 2, Figure 3E, P < 0.001). Meanwhile, HMGA1 expression showed a promising prognostic power as the ROC curve illustrated that the area under the curve (AUC) of HMGA1 expression for predicting OS was 0.622 (Figure 3F).
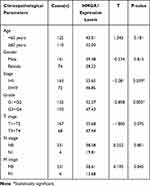 |
Table 1 Association Between Clinicopathological Features of Patients and HMGA1 Expression in the Cancer Genome Atlas |
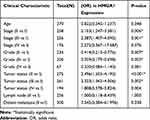 |
Table 2 Association Between HMGA1 Expression and Clinicopathological Features (Logistic Regression) |
 |
Table 3 Univariate Cox Proportional Hazards Regression and Multivariate Cox Regression Analyses of HMGA1 and Clinical Features in HCC |
Biological Function of HMGA1
According to the results of GO terms enrichment analysis, upregulated HMGA1 was significantly concentrated in “RNA catabolic process, mRNA binding, cell cycle G2/M phase transition, and mRNA processing” and downregulated HMGA1 was enriched in “cellular amino acid catabolic process, alpha-amino acid catabolic process, organic acid catabolic process, protein activation cascade, and complement activation alternative pathway” (Figure 4A). KEGG pathway analysis elucidated that the upregulated HMGA1 was mainly involved in “DNA replication, pathway in cancer, cell cycle, MAPK signaling pathway, NOTCH signaling pathway, VEGF signaling pathway, p53 signaling pathway, and WNT signaling pathway”, while “complement and coagulation cascades, tryptophan metabolism, PPAR signaling pathway, fatty acid metabolism, and primary bile acid biosynthesis” were enriched in downregulated HMGA1 (Figure 4B). Moreover, a PPI network constructed from the STRING database showed that TP53, LMNB1, RB1, RPS6KB1, EP400, HMGCR, INSIG1, HMGA2, CEBPB, and C6orf1 proteins were significantly related to HMGA1 protein (Figure 4C). The HMGA1 co-expression network was analyzed and created by Coexpedia, in which heterogeneous nuclear ribonucleoprotein U-like 1 (hnRPUL1) carried the greatest correlation with the highest edges’ LLS (log-likelihood score) of 9.276 (Figure 4D). Furthermore, Pearson correlation analyses uncovered that HMGA1 was significant positive correlation to TP53 (R=0.4), RB1 (R=0.17), CCNB1 (R=0.54), PAK1 (R=0.42), CDK1 (R=0.37), CDK2 (R=0.34), CDK4 (R=0.55), E2F1 (R=0.22), E2F2 (R=0.39), HNRNPUL1 (R=0.45), while was negatively correlated to GADD45A (R = - 0.23) (Figure 5A–L, all P < 0.001).
Immune Cells Infiltration and HMGA1
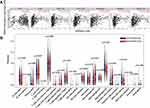
In TIMER, increased HMGA1 expression resulted in a rise in immune infiltration level, including infiltrated B cells, CD4+, CD8+ T cells, neutrophils, macrophages, and dendritic cells (DCs) in HCC, as well as a reduction in infiltrating purity (Figure 6A, all P < 0.001). Notably, macrophages had the highest correlation coefficient among these immune infiltrate cells. Moreover, CIBERSORT analysis elucidated that the amount of B cell naive, B cell memory, T cells gamma delta, Macrophages M2, and mast cell resting decreased when HMGA1 expression was high, whereas T cells follicular helper, Macrophages M0, and Dendritic cells resting increased (Figure 6B, P < 0.05). The resulting heat map manifested that there was a weak to moderate correlation among diverse TIICs subpopulations (Figure 7).
 |
Figure 7 Resulting heat map visualizing the correlation matrix of diverse tumor-infiltrating immune cells subpopulations. Each tile indicates coefficients calculated by Pearson’s correlation test. |
Discussion
Several studies have elucidated that high mobility group A1 (HMGA1), an important member of the HMGA family, played a central role in the pathogenesis and progression of diverse malignant tumors.23,40 HMGA1 is overexpressed during embryogenesis but at relatively low levels in normal adult tissues, implying that HMGA1 may act as a critical molecular switch to form transcriptional networks that remain primitive, poorly differentiated, and stem-like state in both cancer and normal development.41–43 Amounting studies proved that the suppression of oncogenic HMGA1 significantly inhibited some cancer cell proliferation and migration, providing a novel clue to probe into anti-cancer approaches.21,29,32,44,45 Furthermore, HMGA1 is up-regulated in a significant proportion of HCC, and its overexpression is associated with a poor prognosis. A research in vitro models ulteriorly elucidated that HMGA1 overexpression may act a pivotal role in cell viability and migration and promote anchorage-independent growth to induce transformation in liver cancer cell lines.26,27 Consistently, our study demonstrated that HMGA1 expression was amplified in HCC and high-expression HMGA1 was closely associated with advanced pathological stage, unpromising T stage, and poor pathological differentiation. Accordingly, oncogenic properties and aggressive malignancy of HMGA1 were uncovered. Microtubule Interacting and Trafficking Domain containing 1 (MITD1) can serve as a novel predictor for human HCC prognosis, which had been demonstrated. By contrast, HMGA1 had a better prognostic evaluation effectiveness with a higher AUC.46
Recent studies had identified many frequently mutated genes in HCC genomic alterations, including TERT promoter, TP53 (tumor protein P53), and CTNNB1 (b-catenin) and so on, wherein TP53, a crucial cancer-driver mutation gene, accounted for about 30% of cases.1 As a tumor suppressor gene, TP53 activates its protein expression to regulate its downstream genes and subsequently inhibit malignant transformation by inducing cell cycle arrest, apoptosis, DNA repair, and senescence.47,48 Its mutation is significantly associated with the advanced clinical stage, poor prognosis, and occurrence of liver cancer.49,50 HMGA1 regulated the p53 signaling pathway at the transcriptional level by regulating the balance between symmetric and asymmetric divisions in cancer stem cells and suppressing apoptosis, according to the new studies.31,42,51 Besides, hnRPUL1 was demonstrated to interact directly with p53, inhibit p53 transcriptional activity in response to UV radiation, and play a role in DNA damage response, especially in the process of alcohol-related cirrhosis.52,53 Additionally, substantial epidemiological and experimental evidence had revealed that HMGA1 may regulate carcinogenesis and development of cancer by activating the Wnt/β-catenin pathway.20,22,30,54 Xian et al uncovered that such activating pattern conformed to a feed-forward amplification loop, whereby Wnt induces HMGA1, which in turn, enhances signaling.54 Moreover, β-catenin activation promotes immune evasion and resistance to anti-PD-1 therapy in HCC.55 Our study demonstrated that high expression level HMGA1 phenotype closely linked with several cancer-related pathways (e.g. cell cycle, MAPK signaling pathway, NOTCH signaling pathway, VEGF signaling pathway, p53 signaling pathway, and WNT signaling pathway). Furthermore, HCC patients with TP53 dysregulation had higher HMGA1 expression than non-mutations, and HMGA1 was weakly - moderately correlated with TP53 downstream genes and hnRPUL1. Accordingly, it can be inferred that HMGA1 may exert an important effect on TP53 mutational carcinogenesis. These outcomes may explain why HMGA1 functions as an oncogene and its overexpression portends an aggressive malignancy of cancers.
As a crucial part of TME, the cellular composition of immune infiltration is tightly regulated by the various chemokines, which modulate tumor immunity and the biological phenotype of the tumors and further impact tumor progression, therapy, and prognosis.1,12–14,36 These days, emerging insights into tumor biology suggested that successful therapeutics in the future depended on rescuing T cells from the homing and dysfunction state. Tumor-associated macrophages (TAM) function as a vital mediator of tissue homeostasis. Macrophages M1 activity inhibits cell proliferation and causes tissue damage, while M2 activity promotes cell proliferation and tissue repair. Adjuvant immunotherapy targeting macrophages was considered beneficial for HCC patients.56–59 Most cancers, including HCC, overexpress inhibitory ligands to evade the immune response by dampening T cell function, thus contributing to cancer progression. A study proved that by activating HMGA1-dependent signaling pathways including the PI3K/Akt and MEK/ERK pathways, PD-L1 maintained colorectal cancer stem cells and promoted the expansion of cancer.60 The results of this study demonstrated that HMGA1 immune infiltration in HCC. Remarkably, the amount of M0 macrophages displayed a significant rise in the high HMGA1 expression phenotype, while Macrophages M2 decreased. Accordingly, it was speculated that HMGA1 may exert dual influences on tumor-infiltrating immune system, namely recruitment and management of tumor-infiltrating cells and helping the immune evade by regulating TAM via activating of the relevant signal pathways.
Conclusions
In conclusion, HCC with a high HMGA1 expression level may be the one with malignant nature and dismal prognosis. Moreover, excess levels of HMGA1 have a crucial role in the regulation of immune infiltrating cells and may be an accomplice to help the immune escape in HCC. However, precise approaches by which HMGA1 with multifaceted functions promotes hepatocarcinogenesis and links with liver cirrhosis or viral hepatitis need further investigation.
Data Sharing Statement
All raw online RNA sequencing dataset and clinical information of HCC patients, which were included in the current study, can be downloaded from the TCGA (https://portal.gdc.cancer.gov/), ICGC (http://dcc.icgc.org), and GEO database (https://www.ncbi.nlm.nih.gov/geo/). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
The present study was approved by the Ethics Committee of The First Affiliated Hospital of Guangxi Medical University [Approval Number: 2021 (KY-E-032)] and all patients provided written informed consent. This trial was conducted in accordance with the ethical principles in the Declaration of Helsinki.
Acknowledgments
The authors thank the contributors of The Cancer Genome Atlas (https://cancergenome.nih.gov/), ICGC (http://dcc.icgc.org), and GEO database (https://www.ncbi.nlm.nih.gov/geo/) for sharing the HCC dataset on open access. In addition, we would like to acknowledge the helpful comments on this article received from our reviewers.
Disclosure
The authors declare that they have no competing interests.
References
1. Villanueva A, Longo DL. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi:10.1016/S0140-6736(19)30427-1
4. Fujiwara N, Friedman SL, Goossens N, et al. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68(3):526–549. doi:10.1016/j.jhep.2017.09.016
5. Marengo A, Rosso C, Bugianesi E. Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu Rev Med. 2016;67:103–117. doi:10.1146/annurev-med-090514-013832
6. Dimitroulis D, Damaskos C, Valsami S, et al. From diagnosis to treatment of hepatocellular carcinoma: an epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23(29):5282–5294. doi:10.3748/wjg.v23.i29.5282
7. Baig B, Halim SA, Farrukh A, et al. Current status of nanomaterial-based treatment for hepatocellular carcinoma. Biomed Pharmacother. 2019;116:108852. doi:10.1016/j.biopha.2019.108852
8. Benassi E, Fan H, Sun Q, et al. Generation of particle assemblies mimicking enzymatic activity by processing of herbal food: the case of rhizoma polygonati and other natural ingredients in traditional Chinese medicine. Nanoscale Adv. 2021;3(8):2222–2235. doi:10.1039/D0NA00958J
9. Hamza AA, Heeba GH, Hamza S, et al. Standardized extract of ginger ameliorates liver cancer by reducing proliferation and inducing apoptosis through inhibition oxidative stress/ inflammation pathway. Biomed Pharmacother. 2021;134:111102. doi:10.1016/j.biopha.2020.111102
10. Xie Y, Mu C, Kazybay B, et al. Network pharmacology and experimental investigation of Rhizoma polygonati extract targeted kinase with herbzyme activity for potent drug delivery. Drug Deliv. 2021;28(1):2187–2197. doi:10.1080/10717544.2021.1977422
11. Nazarbek G, Kutzhanova A, Nurtay L, et al. Nano-evolution and protein-based enzymatic evolution predicts novel types of natural product nanozymes of traditional Chinese medicine: cases of herbzymes of Taishan-Huangjing (Rhizoma polygonati) and Goji (Lycium chinense). Nanoscale Adv. 2021;3(23):6728–6738. doi:10.1039/D1NA00475A
12. Sim HW, Knox J. Hepatocellular carcinoma in the era of immunotherapy. Curr Probl Cancer. 2018;42(1):40–48. doi:10.1016/j.currproblcancer.2017.10.007
13. Pinato DJ, Fessas P, Sapisochin G, et al. Perspectives on the neoadjuvant use of immunotherapy in hepatocellular carcinoma. Hepatology. 2020. doi: 10.1002/hep.31697
14. Wu X, Gu Z, Chen Y, et al. Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J. 2019;17:661–674. doi:10.1016/j.csbj.2019.03.006
15. Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019;12(1):92. doi:10.1186/s13045-019-0779-5
16. Barcena-Varela M, Lujambio A. The endless sources of hepatocellular carcinoma heterogeneity. Cancers. 2021;13(11):2621. doi:10.3390/cancers13112621
17. Stephen B, Hajjar J. Overview of basic immunology and clinical application. Adv Exp Med Biol. 2020;1244:1–36. doi:10.1007/978-3-030-41008-7_1
18. Dong LQ, Peng L-H, Ma L-J, et al. Heterogeneous immunogenomic features and distinct escape mechanisms in multifocal hepatocellular carcinoma. J Hepatol. 2020;72(5):896–908. doi:10.1016/j.jhep.2019.12.014
19. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi:10.1038/ni.2703
20. Han X, Cao Y, Wang K, et al. HMGA1 facilitates tumor progression through regulating Wnt/β-catenin pathway in endometrial cancer. Biomed Pharmacother. 2016;82:312–318. doi:10.1016/j.biopha.2016.05.004
21. Qi C, Cao J, Li M, et al. HMGA1 overexpression is associated with the malignant status and progression of breast cancer. Anat Rec. 2018;301(6):1061–1067. doi:10.1002/ar.23777
22. Xing J, Cao G, Fu C. HMGA1 interacts with β-catenin to positively regulate Wnt/β-catenin signaling in colorectal cancer cells. Pathol Oncol Res. 2014;20(4):847–851. doi:10.1007/s12253-014-9763-0
23. Sumter TF, Xian L, Huso T, et al. The high mobility group A1 (HMGA1) transcriptome in cancer and development. Curr Mol Med. 2016;16(4):353–393. doi:10.2174/1566524016666160316152147
24. Nakanishi F, Ohkawa K, Ishida H, et al. Alteration in gene expression profile by full-length hepatitis B virus genome. Intervirology. 2005;48(2–3):77–83. doi:10.1159/000081732
25. Zhang Z, Yao Z, Zhao S, et al. Interaction between autophagy and senescence is required for dihydroartemisinin to alleviate liver fibrosis. Cell Death Dis. 2017;8(6):e2886. doi:10.1038/cddis.2017.255
26. Chang ZG, Yang L-Y, Wang W, et al. Determination of high mobility group A1 (HMGA1) expression in hepatocellular carcinoma: a potential prognostic marker. Dig Dis Sci. 2005;50(10):1764–1770. doi:10.1007/s10620-005-2934-9
27. Andreozzi M, Quintavalle C, Benz D, et al. HMGA1 expression in human hepatocellular carcinoma correlates with poor prognosis and promotes tumor growth and migration in in vitro models. Neoplasia. 2016;18(12):724–731. doi:10.1016/j.neo.2016.10.002
28. Chuma M, Saeki N, Yamamoto Y, et al. Expression profiling in hepatocellular carcinoma with intrahepatic metastasis: identification of high-mobility group I(Y) protein as a molecular marker of hepatocellular carcinoma metastasis. Keio J Med. 2004;53(2):90–97. doi:10.2302/kjm.53.90
29. Di Cello F, Shin J, Harbom K, et al. Knockdown of HMGA1 inhibits human breast cancer cell growth and metastasis in immunodeficient mice. Biochem Biophys Res Commun. 2013;434(1):70–74. doi:10.1016/j.bbrc.2013.03.064
30. Resar L, Chia L, Xian L. Lessons from the crypt: HMGA1-amping up Wnt for stem cells and tumor progression. Cancer Res. 2018;78(8):1890–1897. doi:10.1158/0008-5472.CAN-17-3045
31. Frasca F, Rustighi A, Malaguarnera R, et al. HMGA1 inhibits the function of p53 family members in thyroid cancer cells. Cancer Res. 2006;66(6):2980–2989. doi:10.1158/0008-5472.CAN-05-2637
32. Schuldenfrei A, Belton A, Kowalski J, et al. HMGA1 drives stem cell, inflammatory pathway, and cell cycle progression genes during lymphoid tumorigenesis. BMC Genomics. 2011;12:549. doi:10.1186/1471-2164-12-549
33. Pak K, Oh SO, Goh TS, et al. A user-friendly, web-based integrative tool (ESurv) for survival analysis: development and validation study. J Med Internet Res. 2020;22(5):e16084. doi:10.2196/16084
34. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi:10.1093/nar/gky1131
35. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi:10.1101/gr.1239303
36. Li B, Severson E, Pignon J-C, et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17(1):174. doi:10.1186/s13059-016-1028-7
37. Li T, Fan J, Wang B, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi:10.1158/0008-5472.CAN-17-0307
38. Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi:10.1038/nmeth.3337
39. Wang X, Liao X, Yu T, et al. Analysis of clinical significance and prospective molecular mechanism of main elements of the JAK/STAT pathway in hepatocellular carcinoma. Int J Oncol. 2019;55(4):805–822. doi:10.3892/ijo.2019.4862
40. Pallante P, Sepe R, Puca F, et al. High mobility group a proteins as tumor markers. Front Med. 2015;2:15. doi:10.3389/fmed.2015.00015
41. Shah SN, Kerr C, Cope L, et al. HMGA1 reprograms somatic cells into pluripotent stem cells by inducing stem cell transcriptional networks. PLoS One. 2012;7(11):e48533. doi:10.1371/journal.pone.0048533
42. Puca F, Colamaio M, Federico A, et al. HMGA1 silencing restores normal stem cell characteristics in colon cancer stem cells by increasing p53 levels. Oncotarget. 2014;5(10):3234–3245. doi:10.18632/oncotarget.1914
43. Parisi S, Piscitelli S, Passaro F, et al. HMga proteins in stemness and differentiation of embryonic and adult stem cells. Int J Mol Sci. 2020;21(1).
44. Shah SN, Cope L, Poh W, et al. HMGA1: a master regulator of tumor progression in triple-negative breast cancer cells. PLoS One. 2013;8(5):e63419.
45. Hassan F, Lossie SL, Kasik EP, et al. A mouse model study of toxicity and biodistribution of a replication defective adenovirus serotype 5 virus with its genome engineered to contain a decoy hyper binding site to sequester and suppress oncogenic HMGA1 as a new cancer treatment therapy. PLoS One. 2018;13(2):e0192882. doi:10.1371/journal.pone.0192882
46. Shen H, Wang Z, Ren S, et al. Prognostic biomarker MITD1 and its correlation with immune infiltrates in hepatocellular carcinoma (HCC). Int Immunopharmacol. 2020;81:106222. doi:10.1016/j.intimp.2020.106222
47. Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15(1):2–8. doi:10.1038/ncb2641
48. Murali C, Mudgil P, Gan C-Y, et al. Camel whey protein hydrolysates induced G2/M cellcycle arrest in human colorectal carcinoma. Sci Rep. 2021;11(1):7062. doi:10.1038/s41598-021-86391-z
49. Xue R, Li R, Guo H, et al. Variable intra-tumor genomic heterogeneity of multiple lesions in patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):998–1008. doi:10.1053/j.gastro.2015.12.033
50. Takai A, Dang HT, Wang XW. Identification of drivers from cancer genome diversity in hepatocellular carcinoma. Int J Mol Sci. 2014;15(6):11142–11160. doi:10.3390/ijms150611142
51. Pierantoni GM, Rinaldo C, Esposito F, et al. High mobility group A1 (HMGA1) proteins interact with p53 and inhibit its apoptotic activity. Cell Death Differ. 2006;13(9):1554–1563. doi:10.1038/sj.cdd.4401839
52. Barral PM, Rusch A, Turnell AS, et al. The interaction of the hnRNP family member E1B-AP5 with p53. FEBS Lett. 2005;579(13):2752–2758. doi:10.1016/j.febslet.2005.03.095
53. Innes H, Buch S, Hutchinson S, et al. Genome-wide association study for alcohol-related cirrhosis identifies risk loci in MARC1 and HNRNPUL1. Gastroenterology. 2020;159(4):1276–1289.e7. doi:10.1053/j.gastro.2020.06.014
54. Xian L, Georgess D, Huso T, et al. HMGA1 amplifies Wnt signalling and expands the intestinal stem cell compartment and Paneth cell niche. Nat Commun. 2017;8:15008. doi:10.1038/ncomms15008
55. Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, et al. β-catenin activation promotes immune escape and resistance to Anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9(8):1124–1141. doi:10.1158/2159-8290.CD-19-0074
56. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–382. doi:10.1038/s41577-019-0127-6
57. Xia Y, Rao L, Yao H, et al. Engineering macrophages for cancer immunotherapy and drug delivery. Adv Mater. 2020;32(40):e2002054. doi:10.1002/adma.202002054
58. Degroote H, Van Dierendonck A, Geerts A, et al. Preclinical and clinical therapeutic strategies affecting tumor-associated macrophages in hepatocellular carcinoma. J Immunol Res. 2018;2018:7819520. doi:10.1155/2018/7819520
59. Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi:10.1038/nrclinonc.2016.217
60. Wei F, Zhang T, Deng S-C, et al. PD-L1 promotes colorectal cancer stem cell expansion by activating HMGA1-dependent signaling pathways. Cancer Lett. 2019;450:1–13. doi:10.1016/j.canlet.2019.02.022
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.