Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
High Measures of Pre-Chemoradiotherapy Platelet-to-Albumin Ratio Indicates Poor Prognosis in Locally Advanced Pancreatic Cancer Patients
Authors Kucuk A , Topkan E , Selek U , Haksoyler V , Mertsoylu H , Besen AA, Pehlivan B
Received 22 January 2022
Accepted for publication 3 April 2022
Published 14 April 2022 Volume 2022:18 Pages 421—428
DOI https://doi.org/10.2147/TCRM.S359553
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Ahmet Kucuk,1 Erkan Topkan,2 Ugur Selek,3,4 Veysel Haksoyler,5 Huseyin Mertsoylu,6 Ali Ayberk Besen,6 Berrin Pehlivan7
1Clinic of Radiation Oncology, Mersin Education and Research Hospital, Mersin, Turkey; 2Department of Radiation Oncology, Baskent University Medical Faculty, Adana, Turkey; 3Department of Radiation Oncology, Koc University School of Medicine, Istanbul, Turkey; 4Division of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; 5Clinics of Medical Oncology, Medline Hospital, Adana, Turkey; 6Department of Medical Oncology, Baskent University Medical Faculty, Adana, Turkey; 7Department of Radiation Oncology, Bahcesehir University, Istanbul, Turkey
Correspondence: Erkan Topkan, Department of Radiation Oncology, Baskent University Medical Faculty, Adana, Turkey, Tel +90-533-7381069, Fax +90-322-3444452, Email [email protected]
Purpose: In a lack of similar research, we meant to retrospectively investigate the prognostic significance of pre-chemoradiotherapy (C-CRT) platelet-to-albumin ratio (PAR) on the survival results of locally advanced unresectable pancreatic adenocarcinoma (LAPC) patients.
Patients and Methods: The present analysis included 139 LAPC patients who received C-CRT in total. The utility of pre-C-CRT cutoff(s) reshaping survival data was explored using receiver operating characteristic (ROC) curve analysis. The primary and secondary objectives were the associations between PAR levels and overall survival (OS) and progression-free survival (PFS) outcomes.
Results: At a median follow-up of 15.7 months (95% CI: 11.6– 19.8), the overall cohort’s median and 5-year OS rates were 14.4 months (95% CI: 11.8– 17) and 14.7%, respectively, while the corresponding PFS rates were 7.8 months (95% CI: 6.5– 9.1) and 11.2%. Because the ROC curve analysis found 4.9 as the optimal PAR cutoff for both OS and PFS [area under the curve (AUC): 75.4%; sensitivity: 72.4%; specificity: 70.3%], we divided the patients into two PAR cohorts: PAR< 4.9 (N=60) and PAR≥ 4.9 (N=79). Comparative analysis per PAR group exhibited significantly worse OS (11.2 vs 18.6 months, and 9.8% vs 20.9% at 5 years, P=0.003) and DFS (7 vs 14.3 months, and 7.6% vs 16.2% at 5 years, P=0.001) with PAR≥ 4.9 versus PAR< 4.9, respectively. In multivariate analysis, the N0 nodal status, CA 19– 9≤ 90 U/mL, and PAR< 4.9 were found to be independent predictors of improved OS and PFS.
Conclusion: The pre-C-CRT high PAR (≥ 4.9) robustly and independently prognosticated significantly worse OS and PFS results in inoperable LAPC patients who underwent definitive C-CRT.
Keywords: pancreas cancer, prognosis, platelet-to-albumin ratio, concurrent chemoradiotherapy, survival outcomes
Introduction
The results of the prospective randomized LAP 07 Trial comparing chemotherapy versus chemotherapy plus concurrent chemoradiotherapy (C-CRT) in patients with locally advanced unresectable pancreatic adenocarcinoma (LAPC) shifted the treatment trend in favor of systemic chemotherapy due to its high metastatic potential and the lack of universally accepted consensus on the definitive treatment of such patients.1 However, owing to its local control advantage, definitive C-CRT without induction chemotherapy is considered a feasible therapeutic option for medically fit LAPC patients.2 Owing to their poor response to existing anti-cancer treatment modalities and the inevitability of distant metastases (DM), which frequently necessitate palliative interventions, LAPC patients have a dismal median overall survival (OS) of just 9 to 13 months.3,4 Clinical researchers are searching for novel biologic markers to enable strategic prognostic stratification of unresectable LAPC patients at admittance, who may exhibit widely differing response rates and outcomes after standard therapies, despite nearly identical performance status and TNM (tumor-node-metastasis) stage.
A growing body of evidence hints that systemic inflammation plays a decisive role in carcinogenesis, tumor growth, invasiveness, and metastasis.5 Many prognostic indicators have previously been investigated in LAPC patients, including the Glasgow Prognostic Score (GPS), modified GPS (mGPS), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), prognostic nutritional index (PNI), lymphocyte-to-monocyte ratio (LMR), and C-reactive protein/albumin ratio (CAR), with provocative correlations between these indicators and therapeutic results.6–13 Preoperative platelet-to-albumin ratio (PAR) has recently been proposed as a unique independent prognostic index in predicting the survival results of patients undergoing curative pancreatic surgery.14 With the self-standing functions of platelets and albumin, the novel PAR can express the patient’s nutritional, systemic inflammatory, and immunological conditions in a simultaneous manner. In the absence of such research, we postulated that PAR, which is less susceptible to a wide variety of physiological and/or pathological circumstances, might be a reliable new biological marker for LAPC patients receiving definitive C-CRT. If the current study’s findings demonstrate a substantial correlation between pretreatment PAR values and survival outcomes, it might aid usefully in supplementing the existing TNM staging in terms of patient stratification and individualized therapies.
Patients and Methods
Study Population
We reviewed the records of our institutional database for unresectable LAPC patients who received definitive C-CRT between January 2007 and December 2019. Our study included stage III (T4N0-2M0, AJCC 8th ed.) patients who had their tumors staged by 18F-fluorodeoxyglucose- (FDG-) positron emission tomography- (PET-) CT, abdominal magnetic resonance imaging (MRI), MR angiography, and endoscopic ultrasonography (during the open abdominal exploration if necessary), lung/chest computed tomography (CT), and brain MRI. Patients who met the following criteria were considered eligible: (1) between the ages of 18 and 80; (2) Eastern Cooperative Oncology Group (ECOG) performance status 0–1; (3) histopathologically pathologically proven adenocarcinoma; (4) no history of chemotherapy or radiotherapy (RT); (5) adequate bone marrow, liver, and kidney functions; (6) receiving at least one cycle of concurrent chemotherapy during abdominal RT; and (7) available chemotherapy, RT, and follow-up examination details.
Permissions, Consent, and Ethics
Before collecting any patient data, the Institutional Review Board at Baskent University approved the current retrospective study design. All procedures followed our institutional research committee’s ethical guidelines, as well as the 1964 Helsinki Declaration and its revisions. As per our institutional norms, all patients, either themselves or legitimately authorized representatives, provided written informed consent prior to the start of therapy to collect and analyze blood samples and pathology specimens, as well as publish the results.
Treatment Protocol
As described earlier,15–17 all patients underwent definitive C-CRT consisting of a total dose of 45 Gy RT (1.8 Gy/fraction, 5-days/week, for 5-weeks) that exclusively embraced the index tumor site and involved nodes. According to our institutional standards for LAPC patients, elective nodal irradiation was not sanctioned with an end goal to lessen unavailing toxicity. All patients received continuously infused 5-fluorouracil (225 mg/m2/day) concurrent with RT and 2 to 6 courses of maintenance gemcitabine (1000 mg/m2 intravenously on days 1 and 8, every 21 days intervals).
Platelet-Albumin Ratio (PAR) Measurements
The pre-treatment PAR was calculated for each eligible patient using the complete blood count and biochemistry tests obtained on the first day of C-CRT by executing the following formula: PAR= Platelets (109/L) ÷ serum albumin (g/L).14
Treatment Response Assessment and Follow-Up
After completion of the C-CRT course, all patients had planned periodic response assessments at three-monthly (first two years), six-monthly (third to fifth years), and yearly intervals (after five years). Response assessment was accomplished incorporating PET/CT and abdominal MRI scans, as well as the complete blood count and biochemistry tests, serum CA 19–9 concentrations in accordance with EORTC 1999 guidelines.18 For patients with a confirmed complete metabolic response on PET-CT scans, MRI was the chosen follow-up imaging tool. Additional re-staging tools were used only when absolutely necessary.
Statistical Analysis
The primary endpoint was OS (the time from the first day of C-CRT to death or last follow-up), whereas the secondary endpoint was progression-free survival (the time from the first day of C-CRT to the date of emergence of local, regional, or distant relapses or death/last follow-up). Medians and intervals were used to characterize continuous variables, whereas frequency distributions were utilized to express categorical variables. The Chi-square test, Student’s t-test, Fisher’s exact test, and Spearman correlations, as fitting, were executed to investigate intergroup correlations. Using receiver operating feature (ROC) curve analysis, we sought a PAR cutoff that could divide the research population into two fundamentally distinct OS and PFS outcomes. We utilized Kaplan-Meier estimates and Log rank tests to evaluate the potential influence of various risk variables on OS and PFS findings. The multivariate Cox proportional hazard model was used to assess the possible interactions between different variables and survival outcomes. All comparisons were two-tailed, with a statistical significance level of P < 0.05.
Results
Our retrospective search yielded a total of 139 patients who fulfilled the current research’s eligibility criteria (Table 1). The median age for the entire cohort was 55 years (range: 26–79 yearsThe most prevalent tumor location was the pancreatic head (n = 103, 74%). Nodal metastasis was present in 71 patients (51%). According to the landmark study Charité Onkologie 001 (CONKO-001), 87 patients (62.5%) had critical CA19-9 measurements (> 90 U/mL).
 |
Table 1 Baseline Patient and Disease Characteristics for the Entire Study Group and per Low and High Platelet-to-Albumin Ratio Subgroups |
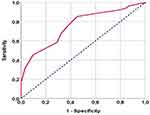
After a median follow-up time of 15.7 months (95% CI: 11.6–19.8), the median and 5-year OS rates for the entire cohort were 14.4 months (95% CI: 11.8–17) and 14.7%, respectively, while corresponding PFS rates were 7.8 months (95% CI: 6.5–9.1) and 11.2%. The ROC curve analysis found a rounded 4.9 [area under the curve (AUC): 75.4%; sensitivity: 72.4%; specificity: 70.3%] as the optimal PAR cutoff for both OS and PFS endpoints (Figure 1). On the basis of this cutoff value, we split the patients into two PAR groups for subsequent comparative analysis: PAR<4.9 (N=60) and PAR≥4.9 (N=79) groups. In comparison to the PAR<4.9 patient group, the PAR≥4.9 patients exhibited significantly higher rates of nodal metastases (N1-2: 35% vs 63%; P=0.001) and higher CA 19–9>90 U/mL (47% vs 75%; P<0.001) (Table 1).
In univariate analysis, CA 19–9> 90 (vs ≤90 U/mL), N1-2 node stage (vs N0), and PAR≥4.9 (vs PAR<4.9) were found to be linked to significantly inferior OS and PFS results, with their independent significance maintained in multivariate analyses (Table 2). Comparative Kaplan-Meier analyses exhibited that the PAR≥4.9 group had significantly shorter median PFS (7.0 vs 14.3 months; P=0.001) and OS (11.2 vs 18.6 months; P=0.003) durations than the PAR<4.9 group (Figure 2 and Table 3). As shown in Table 3, the 5-year PFS and OS rates were likewise inferior in the PAR≥4.9, CA 19–9 > 90 U/mL, and node-positive patients groups (Table 3).
 |
Table 2 Outcomes of Uni- and Multi-Variate Analyses |
 |
Table 3 Survival Outcomes per Factors Demonstrating Independent Multivariate Prognostic Significance |
Discussion
The current retrospective study was conducted in the absence of comparable antecedents to assess the prognostic quality of the pretreatment PAR in unresectable LAPC patients treated with definitive C-CRT. Our findings revealed that the novel pre-C-CRT PAR stratifies unresectable LAPC patients into two fundamentally different prognostic groups in terms of OS (18.6 months vs 11.2 months; P=0.003) and PFS (7.0 months vs 14.3 months; P=0.001) outcomes, with the PAR< 4.9 group outperforming its PAR≥4.9 counterpart, which may serve usefully in tailoring treatment for such patients.
Cancer-related inflammation, as the seventh characteristic of cancer, is present at all stages of carcinogenesis, including initiation, development, and metastasis.18 Cancer cells produce inflammatory cytokines and growth factors such as interleukin (IL1 and IL6) and tumor necrosis factor-α,19,20 which promote thrombocytosis.21 Platelets, which are a component of PAR, have been shown to play a significant role in the interwoven mechanisms of tumor invasion and metastasis by stimulating cell-to-cell communication, provoking the progression of aggressive tumor behaviors, forming aggregation and thrombus around cancer cells, and adhesion to the vascular endothelium to protect cancer cells from immune termination. Platelets were indeed exposed to facilitate the development of a more malignant phenotype by commencing the epithelial-mesenchymal transition and boosting invasive, migratory, and early metastatic capabilities.22–28 Platelets have also been shown to recruit granulocytes to the tumor site, invigorating an early pro-metastatic milieu by increasing inflammation and suppressing the immune response.29 Thrombocytosis has been recommended by several researchers as a significant predictor of poor prognosis, as platelets might increase cancer-cell aggressiveness and shield them from the immune system.30–34 Some platelet receptors, such as P-selectin and glycoprotein, are involved in tumor cell-induced platelet aggregation and adherence to tumor cells. These receptors play a direct role in tumor growth and metastasis via negotiating between platelets and circulating tumor cells (CTCs) and facilitating the binding of the tumor cell-platelet complex to the microvascular environment at metastatic sites.35–37 In addition to its crucial roles in host immunity and systemic inflammation, albumin, the other component of the PAR, has been acknowledged as a legitimate nutritional biomarker of cancer-related malnutrition and cachexia in the Washington Consensus definition.38,39 Malnutrition and inflammation are strongly related to tumor progression and poor prognosis.4,40 Albumin synthesis is inhibited in the context of an exaggerated systemic inflammatory response and related cytokines, such as IL-6 secreted by cancer-induced Kupffer cells.41,42 The resulting hypoalbuminemia is not only a robust biomarker of malnutrition and cancer-related cachexia, but it also indicates that the immune system is malfunctioning, which can lead to tumor development and metastatic disease progression.43
Because thrombocytosis and hypoalbuminemia are related with cancer-induced inflammation and malnutrition, combining these factors as a prognostic indicator seems reasonable. Shirai et al examined the prognostic role of preoperative PAR in 107 patients with pancreatic cancer who underwent curative-intent resection.12 Aside from nodal involvement, the authors discovered PAR as a significant prognostic marker in multivariate analysis for OS (P=0.014) and PFS (P=0.017). The same group examined the prognostic significance of preoperative PAR in 59 cholangiocarcinoma patients and confirmed its prognostic relevance in this patient group as well.44 In their study of hepatocellular carcinoma (HCC) patients after liver resection, Li et al discovered that a high preoperative PAR recall was associated with an increased risk of recurrence and a lower survival rate, implying that PAR could be used to predict the prognosis of HCC patients after liver resection.45 Goo et al investigated the potential utility of PAR in NSCLC patients having curative-intent resection and found comparable predictive value to that reported for other tumor sites.46
We pursued the utility of PAR in the setting of pre-C-CRT of LAPC, which had not been addressed earlier, as we had previously explored the advanced lung cancer inflammation index (ALI), another biomarker of immunity and inflammation, in the same context and established its prognostic utility in stratifying patients into two distinct survival groups.13 The new PAR proved to be as successful as the ALI in stratifying these patients into significantly different OS (18.6 vs 11.2 months for PAR≥4.9; P=0.003) and DFS (14.3 vs 7.0 months for PAR≥4.9; P=0.001) in our present investigation, utilizing a cutoff of 4.9. As PAR4.9, which had higher platelets and lower albumin levels, successfully distinguished unresectable LAPC patients with a poor prognosis, PAR status may be taken into account when deciding whether C-CRT should be used for patients with low progression-free and overall survival prospects. Such data may further highlight the importance of discovering and integrating more effective systemic medications into the treatment algorithms of these patients. In this sense, our unique findings may prompt the usage of PAR as a reliable biomarker of underlying systemic inflammation and malnutrition in the prognostic categorization of unresectable LAPC patients, alongside other well-established independent prognostic factors like N-stage and pre-treatment CA 19–9 levels, as shown here.
The current research has some weaknesses. First, we believe that our findings should be valued as hypothesis-generating rather than firm recommendations, given the inherent biases of any single institutional retrospective small cohort study. Second, although PAR is a dynamic indicator of nutritional and inflammatory conditions that fluctuate dramatically during and after C-CRT periods, the current study focused only on the pre-C-CRT PAR measures. As a result, future research should concentrate on the dynamics of PAR to define potentially more robust cutoffs (s). Third, because varied neoadjuvant, adjuvant, and/or rescue therapy preferences may affect the results by favoring one PAR group, the current findings are unlikely to be generalized to all unresectable LAPC patients. Fourth, we may have forfeited the opportunity to delineate the subtle linkages between the PAR measures and patient prognosis given the absence of evaluations integrating the current PAR with other biomarkers such as IL-6, tumor necrosis factor-alpha, and phagocytosis indicators. As a result, well-designed future study findings might be crucial in solving these essential concerns. Despite these obstacles, these results and a fast-growing number of others for other tumor locations suggest that PAR is a simple-to-achieve, simple-to-calculate, and low-cost biomarker.
Conclusions
Our retrospective analysis insinuated that, surpassing traditional TNM staging alone, pre-treatment L-PAR<4.9 was a potent independent predictor of better prognosis for LAPC patients undergoing definitive C-CRT, and thus calls for additional research to substantiate this prognostic biomarker in large-scale cohorts.
Data Sharing Statement
The data cannot be shared publicly because the data is owned and saved by Baskent University Medical Faculty. Data are available from the Baskent University Radiation Oncology Institutional Data Access/Ethics Committee (contact via Baskent University Ethics Committee) for researchers who meet the criteria for access to confidential data: contact address, [email protected].
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors have declared no conflicts of interest for this work.
References
1. Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. doi:10.1001/jama.2016.4324
2. Ioka T, Furuse J, Fukutomi A, et al. Randomized Phase II study of chemoradiotherapy with versus without induction chemotherapy for locally advanced pancreatic cancer: Japan Clinical Oncology Group trial, JCOG1106. Jpn J Clin Oncol. 2021;51:235–243. doi:10.1093/jjco/hyaa198
3. Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi:10.1056/NEJMra0901557
4. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi:10.1016/j.cell.2010.01.025
5. La Torre M, Nigri G, Cavallini M, et al. The Glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19:2917–2923. doi:10.1245/s10434-012-2348-9
6. Jamieson NB, Mohamed M, Oien KA, et al. The relationship between tumor inflammatory cell infiltrate and outcome in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2012;19:3581–3590. doi:10.1245/s10434-012-2370-y
7. Stotz M, Gerger A. Eisner A, et al.. Increased Neutrophil-Lymphocyte Ratio is a Poor Prognostic Factor in Patients with Primary Operable and Inoperable Pancreatic Cancer. Br J Cancer. 2013;109:416–421. doi:10.1038/bjc.2013.332
8. Shirai Y, Shiba H, Sakamoto T, et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery. 2015;158(2):360–365. doi:10.1016/j.surg.2015.03.043
9. Kanda M, Fujii T, Kodera Y, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–274. doi:10.1002/bjs.7305
10. Haruki K, Shiba H, Shirai Y, et al. The C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J Surg. 2016;40:2254–2260. doi:10.1007/s00268-016-3491-4
11. Ahmad J, Grimes N, Farid S, Morris-Stiff G. Inflammatory response related scoring systems in assessing the prognosis of patients with pancreatic ductal adenocarcinoma: a systematic review. Hepatobiliary Pancreat Dis Int. 2014;13:474–481. doi:10.1016/s1499-3872(14)60284-8
12. Shirai Y, Shiba H, Haruki K, et al. Preoperative platelet-to-albumin ratio predicts prognosis of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Anticancer Res. 2017;37:787–793. doi:10.21873/anticanres.11378
13. Topkan E, Mertsoylu H. Ozdemir Y, et al.. Prognostic Usefulness of Advanced Lung Cancer Inflammation Index in Locally-Advanced Pancreatic Carcinoma Patients Treated with Radical Chemoradiotherapy. Cancer Manag Res. 2019;11:8807–8815. doi:10.2147/CMAR.S222297
14. Topkan E, Mertsoylu H, Kucuk A, et al. Low systemic inflammation response index predicts good prognosis in locally advanced pancreatic carcinoma patients treated with concurrent chemoradiotherapy. Gastroenterol Res Pract. 2020;2020:5701949. doi:10.1155/2020/5701949
15. Topkan E, Yavuz AA, Aydin M, et al. Comparison of CT and PET-CT based planning of radiation therapy in locally advanced pancreatic carcinoma. J Exp Clin Cancer Res. 2008;27:41. doi:10.1186/1756-9966-27-41
16. Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. Eur J Cancer. 1999;35:1773–1782. doi:10.1016/s0959-8049(99)00229-4
17. Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi:10.1001/jama.297.3.267
18. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi:10.1038/nature07205
19. Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi:10.1002/ijc.23173
20. Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–2736. doi:10.1002/cncr.20672
21. Alexandrakis MG, Passam FH, Moschandrea IA, et al. Levels of serum cytokines and acute phase proteins in patients with essential and cancer-related thrombocytosis. Am J Clin Oncol. 2003;26:135–140. doi:10.1097/00000421-200304000-00007
22. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi:10.1038/nrc3004
23. Dovizio M, Alberti S, Guillem-Llobat P, Patrignani P. Role of platelets in inflammation and cancer: novel therapeutic strategies. Basic Clin Pharmacol Toxicol. 2014;114:118–127. doi:10.1111/bcpt.12156
24. Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24:130–137. doi:10.1016/j.ccr.2013.05.008
25. Ariad S, Seymour L, Bezwoda WR. Platelet-derived growth factor (PDGF) in plasma of breast cancer patients: correlation with stage and rate of progression. Breast Cancer Res Treat. 1991;20:11–17. doi:10.1007/BF01833352
26. Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur J Intern Med. 2013;24:393–400. doi:10.1016/j.ejim.2013.01.017
27. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi:10.1016/j.ccr.2011.09.009
28. Dovizio M, Maier TJ, Alberti S, et al. Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Mol Pharmacol. 2013;84:25–40. doi:10.1124/mol.113.084988
29. Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci USA. 2014;111:E3053–3061. doi:10.1073/pnas.1411082111
30. Kawai K, Kitayama J, Tsuno NH, Sunami E, Watanabe T. Thrombocytosis before pre-operative chemoradiotherapy predicts poor response and shorter local recurrence-free survival in rectal cancer. Int J Colorectal Dis. 2013;28:527–535. doi:10.1007/s00384-012-1594-4
31. Cakar B, Karaoglanoglu M, Sayici Y, Gonullu Demirag G, Yucel I. The prognostic value of thrombocytosis in newly diagnosed lung cancer patients: a retrospective analysis. J BUON. 2011;16:677–681. PMID: 22331721.
32. Hwang SG, Kim KM, Cheong JH, et al. Impact of pretreatment thrombocytosis on blood-borne metastasis and prognosis of gastric cancer. Eur J Surg Oncol. 2012;38:562–567. doi:10.1016/j.ejso.2012.04.009
33. Chadha AS, Kocak-Uzel E, Das P, et al. Paraneoplastic thrombocytosis independently predicts poor prognosis in patients with locally advanced pancreatic cancer. Acta Oncol. 2015;54:971–978. doi:10.3109/0284186X.2014.1000466
34. Baranyai Z, Josa V, Toth A, et al. Paraneoplastic thrombocytosis in gastrointestinal cancer. Platelets. 2016;27:269–275. doi:10.3109/09537104.2016.1170112
35. Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelets effects on tumor growth. Semin Oncol. 2014;41:359–369. doi:10.1053/j.seminoncol.2014.04.006
36. Qi C, Li B, Guo S, et al. P-selectin-mediated adhesion between platelets and tumor cells promotes intestinal tumorigenesis in Apc(Min/+) mice. Int J Biol Sci. 2015;11:679–687. doi:10.7150/ijbs.11589
37. Qi C, Wei B, Zhou W, et al. P-selectin-mediated platelet adhesion promotes tumor growth. Oncotarget. 2015;6:6584–6596. doi:10.18632/oncotarget.3164
38. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi:10.1093/carcin/bgp127
39. Lambert JW, Ingham M, Gibbs BB, et al. Using preoperative albumin levels as a surrogate marker for outcomes after radical cystectomy for bladder cancer. Urology. 2013;81:587–592. doi:10.1016/j.urology.2012.10.055
40. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi:10.1038/nature01322
41. Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32:S118–125. doi:10.1016/s0272-6386(98)70174-x
42. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi:10.1186/1475-2891-9-69
43. Lin MY, Liu WY, Tolan AM, et al. Preoperative serum albumin but not prealbumin is an excellent predictor of postoperative complications and mortality in patients with gastrointestinal cancer. Am Surg. 2011;77:1286–1289. PMID: 22127071. doi:10.1177/000313481107701002
44. Saito N, Shirai Y, Horiuchi T, et al. Preoperative platelet to albumin ratio predicts outcome of patients with cholangiocarcinoma. Anticancer Res. 2018;38:987–992. doi:10.21873/anticanres.12313
45. Li C, Peng W, Zhang XY, Wen TF, Chen LP. The preoperative platelet to albumin ratio predicts the prognosis of hepatocellular carcinoma patients without portal hypertension after liver resection. Medicine. 2019;98:e17920. doi:10.1097/MD.0000000000017920
46. Guo M, Sun T, Zhao Z, Ming L. preoperative platelet to albumin ratio predicts outcome of patients with non-small-cell lung cancer. Ann Thorac Cardiovasc Surg. 2021;27:84–90. doi:10.5761/atcs.oa.20-00090
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.