Back to Journals » Journal of Inflammation Research » Volume 13
High-Intensity Interval Training Reversed High-Fat Diet–Induced M1-Macrophage Polarization in Rat Adipose Tissue via Inhibition of NOTCH Signaling
Authors Shanaki M, Khosravi M , Khoshdooni-Farahani A, Dadashi A, Heydari MF, Delfan M , Jafary H, Gorgani-Firuzjaee S
Received 2 November 2019
Accepted for publication 22 January 2020
Published 17 March 2020 Volume 2020:13 Pages 165—174
DOI https://doi.org/10.2147/JIR.S237049
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Mehrnoosh Shanaki, 1 Maryam Khosravi, 2 Arezoo Khoshdooni-Farahani, 3 Alireza Dadashi, 4 Mohammad Foad Heydari, 5 Maryam Delfan, 6 Hanieh Jafary, 3 Sattar Gorgani-Firuzjaee 5
1Department of Medical Laboratory Sciences, School of Allied Health Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 2Department of Immunology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; 3Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran; 4Department of Infectious Disease, School of Medicine, AJA University of Medical Sciences, Tehran, Iran; 5Department of Medical Laboratory Sciences, School of Allied Health Medicine, AJA University of Medical Sciences, Tehran, Iran; 6Department of Exercise Physiology, Faculty of Physical Education and Sport Sciences, Alzahra University, Tehran, Iran
Correspondence: Sattar Gorgani-Firuzjaee
Email [email protected]
Introduction: There is accumulating evidence on the beneficial effect of exercise intervention in the management of metabolic disorders; however, the molecular mechanism is still unclear. Here, the current study aimed to compare the effect of high-intensity interval training (HIIT) and continuous endurance training (CET) on serum and adipose-tissue markers of M1/M2 macrophage polarization.
Methods: A total of 45 healthy male Wistar rats were divided into groups of normal chow (n=10) and high-fat diet (HFD) (n=35). Then, rats receiving the HFD were randomly divided into four groups. Training programs were performed for 5 days/week over 10 weeks. The CET protocol included 30 minutes running at 50%– 60% of VO 2max. The HIIT protocol consisted of five repeated intervals of 2-minute sprints on the treadmill at 80%– 90% VO 2max workload with 1 minute’s 30%– 35% VO 2max interval for each rat. Then, biochemical parameters were assessed. Macrophage-polarization markers were assessed at mRNA and protein levels by real-time PCR and Western blotting, respectively.
Results: Both exercise-training programs, especially HIIT, reversed increased serum biochemical parameters (glucose, triglycerides, cholesterol, Homeostatic Model Assessment of Insulin Resistance, and hsCRP), M1-polarization markers (circulating IL6, TNFα, and adipose-tissue mRNA expression of IL6, TNFα and iNOS), M2 markers (CD206, CD163, and IL10 expression), as well as pIκKB, pNFκB, and NICD expression in HFD-induced diabetes.
Conclusion: Our findings suggest that despite devoting less time, the HIIT workout is a more effective intervention for diabetes management. Moreover, HIIT reverses HFD-induced macrophage polarization by targeting the NFκB and NOTCH signaling pathways.
Keywords: obesity, diabetes, macrophage polarization, high-intensity interval training, continuous endurance training
Introduction
Type 2 diabetes mellitus (T2DM), the most common metabolic disorder, is characterized by peripheral insulin resistance in different tissue.1 Metabolic syndrome is a constellation of abnormalities, such as obesity, T2DM, dyslipidemia, and hypertension.2 It is evident that chronic low-grade inflammation resulting from activation of the immune system is involved in the pathogenesis of obesity-related metabolic disorders, such as insulin resistance and T2DM.3 Low-grade inflammation affects many tissue types, such as muscle, hepatocyte, and adipose. Aside from its principal role in lipid storage, adipose tissue secretes a wide range of molecules, such as resistin, adiponectin, TNFα, and interleukins.4
Adipose tissue is composed of heterogeneous cells: adipocytes, preadipocytes, and fibroblasts — vascular endothelial, and immune. There is evidence that the number and phenotype of such cells change in obesity-related metabolic disorders.3 Specifically, the activation of M1 and M2 macrophages is associated with inducing and suppressing inflammation, respectively.5 M1 polarization induces proinflammatory mediators, such as TNFα, CD11C, IL 2, and iNOS, while M2-phenotype markers are arginase 1, IL12, and CD206.5
Adipose-tissue macrophage polarization is crucial in mediating local and systemic inflammation of adipose tissue and the whole body.6,7 It has been shown that M1 polarization and local adipose-tissue inflammation lead to diabetogenic adipokine oversecretion, such as TNFα and IL6, which trigger insulin resistance and probably DM.4 Recently, NOTCH signaling has been introduced as a possible underlying mechanism of metabolic abnormalities.8 A Notch signaling pathway is required for maintaining cellular homeostasis, cell–cell communication, and development, and is initiated by activation of different Notch receptors with Notch ligands.9 Notch-receptor activation triggers proteolytic cleavage of Notch, which in turn leads to the release of NICD.8,9 Consequently, NICD translocates to the nucleus, binds to RBPJ in the nucleus, promotes M1-macrophage polarization via the synthesis of IFR8 and NFκB, and finally inhibits M2-macrophage polarization by downregulating JMJD3.8 Also, induction of the Notch pathway in adipocytes promotes the production of proinflammatory cytokines (TNFa, IL1β) in a mechanism dependent on induction of NFκB signaling that causes infiltration of macrophages, induction of low-grade systemic inflammation, and insulin resistance. In obesity, infiltrated macrophages activate NFκB signaling.10 Notch-signaling suppression in high-fat diet (HFD)-induced obesity induces the browning of WAT by enhancement of UCP1 expression and ameliorated hepatic insulin resistance.11 Additionally, inhibition of the Notch-signaling pathway ameliorates obesity in HFD-induced obese mice and reduces blood-glucose levels.11 In vitro studies have shown that defects in Notch signaling, such as upstream regulators of gluconeogenesis and lipogenesis, cause hyperglycemia and fatty-liver disease. These findings demonstrated that Notch regulates hepatic gluconeogenesis in a mechanism mediated by NICD and FoxO1.10 Also, it has been shown that expression of M1-phenotypic markers, iNOS, TNFα, and IL1β was induced in livers of Notch-activated mice.12 Moreover, LPS-induced M1 markers were significantly reduced in Notch1–/– hepatic macrophages.12 Despite the aforementioned evidence, the biological role of Notch signaling in adipose-tissue macrophages is still unclear.8
It is well established that sport intervention and lifestyle changes can prevent obesity-related metabolic diseases, such as DM, hypertension, and cardiovascular disease. Today, new training protocols, such as high-intensity interval training (HIIT), have been developed for the management of metabolic diseases.13 Ample evidence has revealed that an HIIT program has beneficial effects on the management of metabolic diseases, such as polycystic ovary syndrome, obesity, fatty liver, and DM.14 Our previous studies focused on the underlying mechanism of the beneficial effect of continuous endurance training (CET) and HIIT on glycemic control and hypolipidemic impacts in HFD-induced diabetic rats by targeting microRNAs. The results demonstrated that exercise intervention, especially HIIT, efficiently alleviated antidiabetic and hypolipidemic markers.13,15 Recent evidence indicates that HIIT can improve insulin sensitivity and metabolic syndrome; however, the molecular mechanism of HIIT effects has not yet been well-defined.16 Adipose-tissue inflammation is essential among several molecular mechanisms of the beneficial effects of training. In this study, we investigated the effect of exercise intervention (CET and HIIT) on adipose-tissue macrophage M1/M2 polarization and possible upstream molecular mechanisms. The results showed that both exercise interventions significantly reduced M1 markers and enhanced M2 markers, probably via the NOTCH-signaling pathway.
Methods
The HFD (Razi Institute, Iran), glucose-detection kit (Pars Azmon, Iran), miScript II RT kit, miScript SYBR Green PCR kit, DNase Treatment (Fermentas), first-strand cDNA-synthesis kit (Roche), PCR master mix (amplicon), β-actin, Histone H3, pNFκB antibody (Abcam), iNOS, NICD, IKB, pIKB antibody (Cell Signaling), TNFα, IL6, and IL10 (Raybitech), as well as other reagents and solvents used in this study, were of analytical grade.
Male Wistar rats (n=45) with mean initial body weight of 200±10 g were purchased from the Razi Institute of Iran. Animals were housed individually in cages, with controlled temperature (22ºC) room, humidity, and light (12-hour light–dark cycle), and provided with laboratory chow and tap water ad libitum. All animal treatments were considered humanely, and procedures were approved by the ethics committee for animal experiments at AJA University of Medical Sciences in compliance with the recommendation of the principles of laboratory animal care (NIH publication 85–23, revised 1985).
After 1 week's adaptation, rats were divided into two groups:normal chow and HFD (16 weeks). After 3 months, four rats from group 2 and two from group 1 were selected randomly and killed for DM confirmation. In order to induce obesity and type 2 DM, the rats received an HFD (35% fat, 25% fructose, and 40% standard chow diet) for 16 weeks. To confirm DM after 12 weeks, blood-glucose levels were determined by a glucose meter. To be categorized in the diabetic group, blood sugar had to be >250 mg/dL (13.5 mM/L). Any animal with a blood-sugar range pf 250–320 mg/dL was assigned to the study. No diabetic rats received any treatment with insulin during the study.
After DM induction, animals were randomly divided into three groups (n=8): diabetic control (these did not participate in any exercise training), CET (continuous exercise training for 10 weeks), HIIT (HIIT for 10 weeks), and ndiabetic control/normal chow (these rats did not participate in any exercise training. For equivalent situations, they were placed on an immobilized treadmill five times a week for 10–15 minutes every session. Training protocols were conducted for 10 weeks. During the first week, animals were adapted to the treadmill, and at the end of the week maximal oxygen uptake (VO2max) was measured with a slowly modified ramp test protocol, as previously described in detail.17,18 Training protocols were performed 5 days per week, and on the sixth day of every week, VO2max was determined. HIIT in every session compriseded 5 minutes' running at 30%–40% VO2max to warm up, the main practice cycle of 3 minutes' running at 85%–90% VO2max, and 1 minute's recovery. This cycle was repeated four times in each session, and finished with 5 minutes' cooling down by running at 30%–40%VO2max. The CET program comprised 40 minutes' running. Every session contained 5 minutes' running at 30%–40% VO2max to warm up, 30 minutes' running at 60%–65% VO2max, and terminated by 5 minutes' cooling down by running at 30%–40% VO2max. The control group did not practice any exercise program; however, to create the same environmental conditions, they were placed on an immobilized treadmill five times a week for 10–15 minutes each session. At 48 hours after the last training, the animals were killed following ketamine–xylazine anesthesia. After collection of whole-body blood, fat tissue was dissected and frozen in liquid nitrogen. The tissue was stored at −80°C for further analysis.
At the end of the training intervention, fasting blood samples were collected from overnight-fasted rats. Immediately after complete anesthesia with ketamine–xylazine, blood was collected from the animal hearts into a centrifuge tube. Blood was centrifuged (4,000 rpm, 5minutes), and sera stored in aliquots at −20°C for further analyses. Additionally, at the beginning of every week, 4-hour fasted tail-vein blood glucose was determined with a FreeStyle blood-glucose meter (Johnson & Johnson). Fasting plasma insulin was determined with a rat insulin–determination kit (90010; Crystal Chem) and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) values calculated.19 Then, total cholesterol and triglyceride were determined using an automated Hitachi analyzer and Pars Azmoon kit (Tehran, Iran). Serum levels of TNFα, IL6, and IL10 were determined with a commercial ELISA kit (RayBiotech) according to the manufacturer’s instructions.
To analyze mRNA expression, 50–70 mg visceral adipose tissue was homogenized in Trizol, total RNA extracted, and quality and quantities confirmed. Total RNA was reverse-transcribed using the M-Mulv reverse transcriptase and random hexamer primer (miScript II RT Kit, Qiagen). Gene expression was quantified using specific primers for iNOS with SYBR Green PCR Master Mix (miScript SYBR Green PCR kit, Qiagen). Levels of target-gene transcripts were normalized relative to GAPDH. The amplification protocol for 40 cycles was 10 seconds at 95ºC for initial activation, 5 seconds at 95ºC for denaturation, and 20 seconds at 60ºC for annealing/extension.
To assay gene expression at the protein level, cellular protein extracted from visceral adipose tissue by homogenization of 70–100 mg tissue in modified RIPA buffer (50 mm Tris–HCl, pH 7.4, 1% Triton X-100, 0.2% sodium deoxycholate, 0.2% SDS, 1 mm Na-EDTA, and 1 mm PMSF) was supplemented with a protease-inhibitor cocktail and PMSF (Roche). Then, protein concentrations were determined and equal amounts of protein subjected to SDS-PAGE, followed by transfer onto PVDF membrane. The blocking process continued with 2 hours' incubation at room temperature with 5% nonfat dry milk or BSA for unbinding proteins sitting in TBS with 0.5% Tween.20 Blots were subjected to overnight incubation with primary antibodies against iNOS (Cell Signaling Technology, Beverly, MA, USA) and β-actin (Abcam, Cambridge, MA, USA) at 4ºC. To stabilize protein bands, an enhanced chemiluminescent substrate was used after incubation with secondary HRP-conjugated antibodies. Band density was analyzed by densitometry withImage J software.
Descriptive values are shown as means ± SD. The Kolmogorov–Smirnov test was used to assess the normality of the data. We used one-way ANOVA to determine statistical significance in the studied group. The significance level for all statistical tests was p<0.05. Statistical analyses were conducted using SPSS.19
Results
Effects of Training Intervention on Biochemical Indices in HFD-Induced Obese and Diabetic Rats
General characteristics of the animals used in this study are shown in Table 1. The HFD increased FBS, cholesterol, and triglycerides, while concentrations of FBS, cholesterol, and triglycerides decreased in both the CET and HIIT groups. Improvement in metabolic profile was more evident in the HIIT group than the CET group. Furthermore, the HOMA-IR, an index of insulin resistance, was reduced: both interventions improved insulin sensitivity. HIIT intervention decreased insulin resistance more effectively than CET (1.9 versus 2.78, p<0.05). Our results also showed that HFD treatment enhanced serum levels of hsCRP as an index of whole-body inflammation, and training programs reversed these. Additionally, exercise intervention caused weight loss in the HFD-induced obesity model.
 |
Table 1 Biochemical and Clinical Analyses of the Rats’ Sera |
Reduction in M1-Polarization Markers in Adipose Tissue byExercise Training
To find the molecular mechanism of the beneficial effect of exercise on inflammation and macrophage polarization, serum levels of TNFα and IL6 were determined. The results showed that both intervention programs, particularly HIIT, reduced HFD-induced inflammation through decreasing TNFα and IL6 (Figure 1, A and C). In order to confirm the system-level origin of these cytokines, adipose-tissue mRNA expression of TNFα and IL6 was analyzed, and the results demonstrated that both interventions reduced mRNA expression of TNFα and IL6 (Figure 1, B and D). In addition, iNOS expression (M1-macrophage marker) was analyzed. The results showed that HFD induced iNOS expression in an HFD and exercise training significantly reduced both mRNA and protein expression (P<0.05, Figure 1, E and F).
More Potently Induced Markers of M2 Polarization in Adipose Tissue of Rats Undergoing HIIT
To prove the importance of CET and HIIT interventions in M2-macrophage polarization, expression of M2 markers (IL10, CD163, and CD206) was analyzed. The results demonstrated that HFD significantly reduced all M2 markers. CET intervention induced mRNA expression of IL10, CD163, and CD206, with fold changes of 1.59, 1.67, and 1.48, respectively (Figure 2, A–C). Moreover, the HIIT program enhanced mRNA expression of IL10, CD163, and CD206 by 2.01-, 2.22-, and 2.16-fold respectively. Our results also showed that HFD reduced IL10 serum levels and that exercise intervention reversed this (Figure 2D).
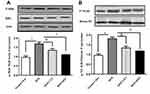
Effects of Exercise Intervention on the IκBα–NFκB Signaling Pathway in Rat Adipose Tissue
Protein-expression analysis of the NFκB signaling pathway, an upstream component of adipose -issue inflammation, was determined by Western blotting. In comparison with the normal-chow group, the HFD group had significantly increased ratios of pIκBα to IκBα in adipose tissue, and exercise intervention reversed this effect (Figure 3A). Importantly, adipose tissue pNFκB increased in the HFD group and decreased after CET and HIIT exercise interventions. As shown in Figure 4B, HIIT intervention more effectively reduced NFκB expression.
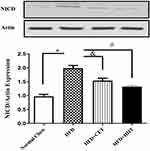
Effects of Exercise Intervention on the NOTCH-Signaling Pathway in Adipose Tissue of HFD-Fed Rats
The NOTCH-signaling pathway was evaluated via mRNA-expression analysis of NICD to show the upstream molecular mechanism by which exercise intervention in HFD induced adipose-tissue macrophage polarization. The results showed that HFD treatment significantly induced NICD expression and exercise intervention reversed it. HIIT reduced HFD-induced NICD induction in comparison with normal chow (Figure 4).
Discussion
In agreement with other studies, we found that the HFD caused an increase in fasting blood sugar, total cholesterol, triglycerides, and HOMA-IR, while training programs markedly reversed them.20–22 More importantly, our results strengthened the concept that HIIT can control blood glucose in diabetic patients.23 In parallel, HIIT reduced hyperglycemia in patients with T2DM.24 Another study showed that HIIT caused a decrease in fasting blood sugar more than CET.25 Acute HIIT reduced the postprandial glucose response and hyperglycemia prevalence in patients with T2DM.23 Moreover, the HIIT program (three times a week) for 12 weeks improved fasting blood glucose considerably in T2DM patients.26 Also, another study indicated that low-volume HIIT can rapidly improve glucose control and induce adaptations in skeletal muscle, which in turn could enhance metabolic status in patients with T2DM.27 Kriska et al noted that higher physical activity was associated with lower insulin concentration in more than 5,000 Pimas and Mauritians with various body compositions, pointing to influences of activity independent of body composition.28 Based on a cohort on overweight and obese men, HIIT for 2 weeks is sufficient to induce beneficial alterations in resting inflammatory profile and adipose-tissue proteome.29
Chronic low-grade inflammation and immune-system activation are involved in the pathogenesis of obesity-related insulin resistance and T2DM.30 The infiltration of macrophages and other immune cells is associated with a cell-population shift from an anti-inflammatory to a proinflammatory profile.30 These cells are crucial for the production of proinflammatory cytokines, which act in an autocrine and paracrine manner to interfere with insulin signaling in peripheral tissues or induce β-cell dysfunction and subsequent insulin deficiency.30 Few studies have investigated the role of exercise training on macrophage-phenotype switching in adipose tissue.
Oliveira et al reported that two single bouts of swim exercise induced an M1–M2 phenotype switch in WAT and stromal vascular fraction in rats receiving an HFD. This finding was along with an increase in protein expression of macrophage galactose–type C-type lectin 1 as an M2-macrophage marker.31 Moderate acute exercise induced macrophage polarization toward the M2 phenotype and improved inflammatory status and insulin signaling in adipocytes and stromal vascular fraction.31 Moreover, our previous studies showed that aerobic endurance training improved hepatocyte fat accumulation via inducing autophagy induction.32 Further, HIIT training reduced NAFLD-related features by targeting miR122 induction in the liver of high-fat, high-fructose diet–induced diabetic rats.15 Also, Khakdan et al demonstrated that HIIT intervention effectively improved heart function in an miR195 dependent manner and alleviated high-fat, high-fructose diet–induced cardiomyopathy in diabetic rats.13
The main finding of this study was that the HFD considerably increased M1 markers and exercise interventions reversed them. More importantly, HIIT intervention was more effective. Furthermore, M2-macrophage markers (CD206, CD163, and IL10) were reduced by HFD treatment and reversed by exercise intervention, specially the HIIT program. In macrophages,increased iNOS expression is associated with an increase in arginase expression, which competes with iNOS for arginine in rabbits.33 A few studies have shown that iNOS is overexpressed in metabolic tissue of both dietary and genetic models of obesity and plays a pivotal role in the pathogenesis of IR and glucose intolerance in mice.33 For the first time, Lee et aldemonstrated the presence of Akt-independent iNOS expression in a Goto–Kakizakinonobese insulin-resistant diabetic rat model. Moreover, defective insulin-induced vasodilation in the diabetic vasculature can be restored by the overexpression of active Akt, which advocates a novel therapeutic strategy for treating T2DM.34 Exercise training decreases the expression of adhesion molecules plus iNOS and ameliorates the severe vascular dysfunction induced by high-cholesterol feeding.35 Also, there is accumulating evidence that exercise training can improve overall immunofunction via reversing M1 macrophages to M2 polarization and reduce obesity-induced inflammation.36 In parallel, Ruffino et al showed that an 8-week walking-intervention program induced M2-biomarker expression in humans.37 In another study, Kawanishi et al showed that exercise training reduced macrophage clusters in adipose tissue and increased the number of CD8+ T cells in obese mice.38 It was shown that exercise training in HFD-fed mice also inhibited adipose-tissue inflammation by inhibiting TNFα, TLR4, and the number of F4/80 macrophages.39 In agreement with our data, exercise training improved local and systemic inflammation, possibly through inducing a phenotypic switch of M1 macrophages to M2 macrophages in adipose tissue;40 however, there was little evidence comparing different training programs. Also, the results demonstrated anti-inflammatory effects of exercise intervention, possibly achieveded via the NOTCH-signaling pathway. This signaling pathway promotes M1-macrophage polarization by the induction of NFκB and suppresses M2-macrophage polarization via reduction of JMJD3.41 In addition, Notch signaling triggers the reduction of proinflammatory cytokines (TNFα, IL1β) via NFκB signaling in adipose tissue, due to infiltration of macrophages, low-grade systemic inflammation, and insulin resistance. In imbalanced metabolic situations, such as obesity and DM, infiltrated macrophages stimulate the NFκB pathway by DLL4 ligand 10. Our results showed that exercise training reduced the HFD-induced NFκB pathway and its upstream molecule in the Notch-signaling pathway (NICD). To the best of our knowledge, there are no data on the direct role of training in regulating Notch- and Wnt-signaling pathways; however, Fujimaki et al revealed that treadmill running promoted satellite cells, possibly through activating Notch- and Wnt-signaling pathways.42
The present study shows that despite devoting less time, an HIIT workout is a more effective intervention than CET via switching macrophage polarization to the M2 phenotype. Furthermore, our results reveal that exercise intervention reduces M1-polarization markers in a mechanism dependent on inhibiting Notch signaling and reducing the NFκB pathway. However, more studies are needed to demonstrate the direct role of exercise intervention in the macrophage-polarization process and Notch signaling–pathway regulation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yu X-Y, Geng Y-J, Lei H-P, et al. IGF-1 prevents high glucose-induced cell cycle arrest in cardiomyocytes via β-catenin pathway. Int J Cardiol. 2013;168(3):2869–2870. doi:10.1016/j.ijcard.2013.03.145
2. Alberti K, Eckel RH, Grundy SM. International diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; international association for the study of obesity: harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi:10.1161/CIRCULATIONAHA.109.192644
3. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98. doi:10.1038/nri2925
4. Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, et al. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res. 2016;167(1):228–256. doi:10.1016/j.trsl.2015.08.011
5. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889. doi:10.1038/ni.1937
6. Kraakman MJ, Murphy AJ, Jandeleit-Dahm K, Kammoun HL, et al. Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front Immunol. 2014;5:470.
7. Türk Y, Theel W, Kasteleyn MJ, et al. High intensity training in obesity: a meta‐analysis. Obes Sci Pract. 2017;3(3):258–271. doi:10.1002/osp4.109
8. Appari M, Channon KM, McNeill E, et al. Metabolic regulation of adipose tissue macrophage function in obesity and diabetes. Antioxid Redox Signal. 2018;29(3):297–312. doi:10.1089/ars.2017.7060
9. Kopan R, Ilagan MXG. The canonical notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–233. doi:10.1016/j.cell.2009.03.045
10. Bi P, Kuang S. Notch signaling as a novel regulator of metabolism. Trends Endocrinol Metab. 2015;26(5):248–255. doi:10.1016/j.tem.2015.02.006
11. Bi P, Shan T, Liu W, et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nature Med. 2014;20(8):911.
12. Xu J, Chi F, Guo T, et al. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J Clin Invest. 2015;125(4):1579–1590. doi:10.1172/JCI76468
13. Khakdan S, Delfan M, Heydarpour Meymeh M, et al. High-intensity interval training (HIIT) effectively enhances heart function via miR-195 dependent cardiomyopathy reduction in high-fat high-fructose diet-induced diabetic rats. Archiv Physiol Biochem. 2018;1–8.
14. Jelleyman C, Yates T, O’Donovan G, et al. The effects of high‐intensity interval training on glucose regulation and insulin resistance: a meta‐analysis. Obes Rev. 2015;16(11):942–961. doi:10.1111/obr.12317
15. Kalaki-Jouybari F, Shanaki M, Delfan M, Gorgani-Firouzjae S, Khakdan S. High-intensity interval training (HIIT) alleviated NAFLD feature via miR-122 induction in liver of high-fat high-fructose diet induced diabetic rats. Archiv Physiol Biochem. 2018;3:1–8.
16. Marcinko K, Sikkema SR, Samaan MC, et al. High intensity interval training improves liver and adipose tissue insulin sensitivity. Mol Metab. 2015;4(12):903–915. doi:10.1016/j.molmet.2015.09.006
17. Høydal MA, Wisløff U, Kemi OJ, et al. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14(6):753–760. doi:10.1097/HJR.0b013e3281eacef1
18. Leandro CG. A program of moderate physical training for Wistar rats based on maximal oxygen consumption. J Strength Cond Res. 2007;21(3):751.
19. Cacho J, Sevillano J, de Castro J, et al. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol –Endocrinol Metab. 2008;295(5):E1269–E1276. doi:10.1152/ajpendo.90207.2008
20. Stanford KI, Middelbeek RJW, Townsend KL, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64(6):2002–2014. doi:10.2337/db14-0704
21. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16(1):37. doi:10.1186/s12933-017-0518-6
22. Bakker EA, Lee DC, Sui X, et al. Association of Resistance Exercise, Independent of and Combined with Aerobic Exercise, with the Incidence of Metabolic Syndrome. In Mayo Clinic Proceedings. Elsevier; 2017.
23. Gillen J, Little JP, Punthakee Z, et al. Acute high‐intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes Metab. 2012;14(6):575–577. doi:10.1111/dom.2012.14.issue-6
24. Little JP, Gillen JB, Percival ME, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111(6):1554–1560. doi:10.1152/japplphysiol.00921.2011
25. Little JP, Jung ME, Wright AE, et al. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Appl Physiol Nutr Metab. 2014;39(7):835–841. doi:10.1139/apnm-2013-0512
26. Fex A, Leduc-Gaudet J-P, Filion M-E, et al. Effect of elliptical high intensity interval training on metabolic risk factor in Pre- and type 2 diabetes patients: a pilot study. J Phys Act Health. 2015;12(7):942–946. doi:10.1123/jpah.2014-0123
27. Dunstan DW, Daly RM, Owen N, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729–1736. doi:10.2337/diacare.25.10.1729
28. Kriska AM, Pereira MA, Hanson RL, et al. Association of physical activity and serum insulin concentrations in two populations at high risk for type 2 diabetes but differing by BMI. Diabetes Care. 2001;24(7):1175–1180. doi:10.2337/diacare.24.7.1175
29. Leggate M, Carter WG, Evans MJC, et al. Determination of inflammatory and prominent proteomic changes in plasma and adipose tissue after high-intensity intermittent training in overweight and obese males. J Appl Physiol. 2012;112(8):1353–1360. doi:10.1152/japplphysiol.01080.2011
30. Esser N, Legrand-Poels S, Piette J, et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–150. doi:10.1016/j.diabres.2014.04.006
31. Oliveira AG, Araujo TG, Carvalho BM, et al. Acute exercise induces a phenotypic switch in adipose tissue macrophage polarization in diet‐induced obese rats. Obesity. 2013;21(12):2545–2556. doi:10.1002/oby.20402
32. Ghareghani P, Shanaki M, Ahmadi S, et al. Aerobic endurance training improves nonalcoholic fatty liver disease (NAFLD) features via miR-33 dependent autophagy induction in high fat diet fed mice. Obes Res Clin Pract. 2018;12(1):80–89. doi:10.1016/j.orcp.2017.01.004
33. Heusch P, Aker S, Boengler K, et al. Increased inducible nitric oxide synthase and arginase II expression in heart failure: no net nitrite/nitrate production and protein S-nitrosylation. Am J Physiol -Heart Circul Physiol. 2010;299(2):H446–H453. doi:10.1152/ajpheart.01034.2009
34. Lee JH, Palaia T, Ragolia L. Impaired insulin-mediated vasorelaxation in diabetic Goto-Kakizaki rats is caused by impaired Akt phosphorylation. Am J Physiol Cell Physiol. 2009;296(2):C327–C338. doi:10.1152/ajpcell.00254.2008
35. Yang A-L, Chen H-I. Chronic exercise reduces adhesion molecules/iNOS expression and partially reverses vascular responsiveness in hypercholesterolemic rabbit aortae. Atherosclerosis. 2003;169(1):11–17. doi:10.1016/S0021-9150(03)00013-3
36. Bishop N, Walsh N, Gleeson M. Exercise Immunology. Routledge; 2013.
37. Ruffino J, Davies NA, Morris K, et al. Moderate-intensity exercise alters markers of alternative activation in circulating monocytes in females: a putative role for PPARγ. Eur J Appl Physiol. 2016;116(9):1671–1682. doi:10.1007/s00421-016-3414-y
38. Kawanishi N, Mizokami T, Yano H, et al. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med Sci Sports Exercise. 2013;45(9):1684–1693. doi:10.1249/MSS.0b013e31828ff9c6
39. Macpherson REK, Huber JS, Frendo-Cumbo S, et al. Adipose tissue insulin action and IL-6 signaling after exercise in obese mice. Med Sci Sports Exercise. 2015;47(10):2034–2042. doi:10.1249/MSS.0000000000000660
40. Jeong JH, Lee YR, Park HG, Lee WL, et al. The effects of either resveratrol or exercise on macrophage infiltration and switching from M1 to M2 in high fat diet mice. J Exercise Nutr Biochem. 2015;19(2):65.
41. Xu H, Zhu J, Smith S, et al. Notch–RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol. 2012;13(7):642.
42. Fujimaki S, Wakabayashi T, Asashima M, et al. Treadmill running induces satellite cell activation in diabetic mice. Biochem Biophys Rep. 2016;8:6–13. doi:10.1016/j.bbrep.2016.07.004
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.