Back to Journals » Infection and Drug Resistance » Volume 13
High-Dose Tigecycline in Elderly Patients with Pneumonia Due to Multidrug-Resistant Acinetobacter baumannii in Intensive Care Unit
Authors Bai XR, Jiang DC, Yan SY
Received 11 February 2020
Accepted for publication 21 April 2020
Published 18 May 2020 Volume 2020:13 Pages 1447—1454
DOI https://doi.org/10.2147/IDR.S249352
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Xiang-Rong Bai,1,2 De-Chun Jiang,1,2 Su-Ying Yan1,2
1Department of Pharmacy, Xuanwu Hospital Capital Medical University, Beijing, People’s Republic of China; 2National Clinical Research Center for Geriatric Disorder, Beijing, People’s Republic of China
Correspondence: De-Chun Jiang Email [email protected]
Purpose: The association between clinical and microbiological outcomes and high-dose tigecycline (TGC) was assessed in elderly (≥ 60 years old) patients with hospital-acquired and ventilator-associated pneumonia due to multidrug-resistant Acinetobacterbaumannii(A. baumannii). This study also assessed tigecycline combination with different antibiotics and its influence on the outcome.
Patients and Methods: An observational retrospective cohort study was conducted. Patients over 60 years old were treated with standard-dose (SD) TGC (100-mg intravenous TGC initially, followed by 50-mg doses administered intravenously twice daily) and high-dose (HD) TGC (200-mg intravenous TGC initially, followed by 100-mg doses administered intravenously twice daily) for a microbially confirmed infection. The outcome was 30-day crude mortality, co-administered antimicrobial agent and the microbial eradication percentage in both groups.
Results: A total of 48 multidrug-resistant A. baumannii respiratory patients were identified. Tigecycline was administered to 85% of ventilation-associated pneumonia (VAP) patients (28/33) in the SD group and 80% of VAP patients (12/15) in the HD group. Combined therapy was the major treatment option in both groups, accounting for 85% and 87%, respectively. Median treatment duration in both groups was 7.36 vs 8.6 days, respectively. Survival days were 13.61 vs 12.4 days (P=0.357), respectively. The 30-day crude mortality was 39.4% (13/33) for the SD group and 14% (2/15) for the HD group (P=0.098). The microbial eradication rate of respiratory specimens in the SD group was higher than that in the HD group (P=0.02). The variables associated with 30-day crude mortality were chronic obstructive pulmonary disease (hazard ratio [HR] 11.63, 95% CI 1.094– 123.058; P=0.042), tigecycline treatment duration (HR 0.690, 95% CI 0.515– 0.926; P=0.013), and surgery before infection (HR 79.276, 95% CI 6.983– 899.979; P=0.000). High-dose tigecycline was not associated with 30-day crude mortality (adjusted HR 0.329, 95% CI 0.074– 1.460; P=0.145). Combined antibiotics was also not different between the two groups.
Conclusions: High-dose tigecycline was not associated with 30-day crude mortality in elderly patients with pneumonia due to multidrug-resistant A. baumannii, although the microbial eradication rate was high.
Keywords: pneumonia, critical ill patients, high-dose tigecycline, drug-resistant, Acinetobacter baumannii
Introduction
Nosocomial pneumonia is a leading cause of death in critically ill patients.1 One of the most common pathogens of nosocomial pneumonia is Acinetobacter baumannii (A. baumannii). The prevalence of multidrug-resistant (MDR) or extensively drug-resistant (XDR) A. baumannii has increased to at least 80% in the past decades.2 A. baumannii is the most commonly isolated from endotracheal aspirates.3 Patients hospitalized in the intensive care unit (ICU) mostly received wide spectrum antibiotic treatment leads to isolation of A.baumannii strains frequently.4 Empirical coverage of A. baumannii is recommended for severe infections (severe sepsis or septic shock) in the ICU.5 Considering the efficacy of various antimicrobial treatments for MDR/XDR A. baumannii pneumonia, guided selection of an optimal antimicrobial treatment for this infection is urgently needed.
Tigecycline is a bacteriostatic activity against multidrug-resistant (MDR) A. baumannii group.6 A retrospective study shows that the relatively low clinical success rate and high microbial eradication rate were observed for multidrug-resistant A. baumannii infection.7 International guidelines considered tigecycline as second-line antibiotic therapy.8 Infectious Diseases Society of America guideline recommended against the use of tigecycline for hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) because current label dose worsened clinical outcomes.9 However, tigecycline was low level of resistance for A. baumannii in China.10 Publish data showed that high-dose tigecycline was associated with clinical outcomes and survival time.11 A meta-analysis indicated high-dose tigecycline was more effective than standard-dose tigecycline or the comparators for the treatment of HAP.12 It also may be used higher-than-licensed dosing such as 100mg twice daily for infections due to MDR in critical care.13 A recent meta-analysis indicated that high-dose tigecycline reduced all-cause mortality in nosocomial-acquired pneumonia.14 But, the main pathogens were Klebsiella pneumoniae. In fact, the clinical efficacy of high-dose tigecycline is unknown for elderly patients. So we conducted a retrospective cohort study to determine clinical and microbial outcomes in elderly patients treated with high-dose vs standard-dose tigecycline for severe infections caused by MDR Gram-negative A. baumannii.
Patients and Methods
Study Design and Patients
The retrospective cohort study was conducted at the 6 ICU of Xuan Wu Hospital Capital Medical University, a 1100-bed, academic, tertiary care medical centre in China. Data were collected between 1 January 2016 and 31 December 2017 in medical records. The inclusion criterion was critical patients ≥60 years of age. Respiratory specimens for Gram stain and culture were obtained via endotracheal aspirate (ventilated patients), expectorated or induced sputum (non-ventilated patients) before using of TGC.15 The pathogens were A. baumannii and simultaneous empirical tigecycline had been given. Results of 2 times ≥3+colony forming units were considered as positive bacterial culture.16 Patients diagnosed with hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) which was defined as pneumonia with an onset 48 h or longer after admission. Ventilator-associated pneumonia was defined as parenchymal lung infection with an onset 48 h or longer after endotracheal intubation and mechanical ventilation according to the guidelines.17 Patients were stratified to two treatment groups: (1) standard-dose (SD) of patients was treated with loading dose (LD) TGC 100mg, followed by maintenance dose (MD) 50 mg administered intravenously twice daily, for at least 5 days) and (2) high-dose of (HD) patients was treated with loading dose (LD) TGC200 mg, followed by maintenance dose (MD) 100mg administered intravenously twice daily, for at least 5 days). Exclusion criteria were as follows: <60 years of age; pregnant women; severe liver dysfunction (Child-Pugh class C); treated with vancomycin and antifungal medications. The study was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University (NO2018003). This dataset exempted from patient consent owing to retrospective medical data, in which patients cannot be contacted directly. Clinical diagnosis and treatment were collected in the medical records, except patients' names. We have confidential measures for the privacy of patients. This work was conducted according to the provisions of the Declaration of Helsinki.
Study Variables
Data were collected upon ICU admission and at the start and end of tigecycline treatment. Only patients who received tigecycline therapy for at least 5 days were included. Patients who began tigecycline therapy before culture were considered empirical therapy. Survival time was calculated from the time tigecycline therapy was initiated. Clinical data were collected, including demographics, the Charlson index,18 the Mean Acute Physiology and Chronic Health Evaluation (APACHE) II score,19 the nosocomial pneumonia source,20 surgery before infection and septic shock before antibiotic therapy.21 Data on microbiological and clinical outcomes and concomitant antimicrobial agents (carbapenem, aminoglycosides, Beta-lactamase) were also collected.
Microbiological Data
Antibiotic susceptibility profiling of the isolates was performed using the Vitek 2 system (bioMérieux, Marcy-l’Etoile, France). Tigecycline susceptibility breakpoints were determined according to the FDA standard (MIC <2mg/L, sensitive; MIC ≥8mg/L, resistant) by using the E-test method (BIO-KONT, Wenzhou, China).22,23 Multidrug-resistance (MDR) was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories; extensive drug resistance (XDR) was defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories.24–26 The MDR/XDR infection was determined according to the results of the bacterial culture and clinical symptoms.27
Outcomes
The primary outcome measure in this study was 30-day crude mortality after TGC treatment, and the secondary outcome measure was assessed pathogen eradication. Thirty-day crude mortality, defined as the incidence of deaths from any cause in the ICU within the approximately 30-day follow-up duration, was chosen as the primary outcome variable for comparing antimicrobial effectiveness.28 Microbial eradication was defined as no growth of A. baumannii or susceptibility change from MDR strains to susceptible strains in A. baumannii in follow-up respiratory tract cultures before and 7 days after treatment cessation.29 Two physicians who are blinded to the treatment to evaluate the outcome, and when judgments are discordant (about 5% of patients), the results reassessed by other physicians to reach a consensus decision.
Statistical Analysis
Continuous data are presented as the median and interquartile range (IQR), and categorical data are presented counts and percentages. The Kolmogorov–Smirnov test was used to evaluate the distribution of the variables. Non-normally distributed data were assessed using the Mann–Whitney test, and the median and selected centile (25th to 75th) values are provided. Normally distributed data were assessed using Student’s t-test. Categorical variables are presented as proportions and were analysed using the chi-square test or Fisher’s exact test, as appropriate. A P-value <0.05 was considered significant. The crude odds ratio (OR) and 95% CI were calculated for each variable. We used Kaplan–Meier survival curves to show the patients’ cumulative survival rates, and the Log-rank test was used to compare patient survival rates between the two groups. Cox regression analyses were performed to evaluate factors independently influencing clinical outcomes. All statistical analyses were performed using SPSS, version 23, for Windows (SPSS, Chicago, IL, USA).
Results
Patient Characteristics
During the study period, forty-eight elderly patients were included: 33 patients in the standard-dose (SD) group and 15 in the high-dose (HD) group (Table 1). The mean ages were 77.64±9.26 years and 70.33±9.01 years without significant differences between study groups (P=0.093). The pneumonia sources were hospital-acquired or ventilator-acquired. The sources did not differ between the SD TGC and HD TGC groups. No significant differences were noted between the Charlson index and APACHE II scores in both groups, which suggested that the disease severity baselines were similar. Patients in the two groups with chronic obstructive pulmonary disease (COPD) did not differ (P=0.103). Previous carbapenem use and hospital stay before treatment did not differ between groups.
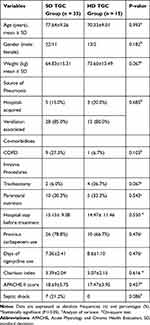 |
Table 1 Demographic Characteristics of the 48 Elderly Patients with the Standard-Dose (SD) and High-Dose (HD) Tigecycline |
Primary and Secondary Outcome Outcomes
The number of 30-day deaths did not significantly differ between the two groups (P= 0.098). Survival days and number of hospital days did not differ. Microbiological eradication percentages were higher when TGC was used at higher doses (61.5% versus 66.7%; P =0.02) (Table 2)
 |
Table 2 The Primary and Secondary Outcomes in Both Groups |
Combined Various Antimicrobial Medication
We also analysed the combined antimicrobial medication in both groups (Table 3). Combined carbapenems were more frequent in the high-dose TGC group (P =0.017). Combined antibiotics did not differ between the two groups, but no patients had combined antibiotics in the HD TGC group. No specific antibiotic combination was associated with a better outcome.
 |
Table 3 Combined Various Antimicrobials in Both Groups |
Predictors of 30-Day Crude Mortality in Patients
Univariate regression analysis (Table 4) of the 48 patients showed that individuals with clinical failure were older, had COPD, longer lengths of hospital stay and TGC treatment and surgery duration before infection. Cox regression analysis indicated that COPD independently predicted 30-day mortality (HR 11.63, 95% CI 1.094–123.058) and surgery before infection (HR 79.276, 95% CI 6.983–899.979). The predictor was days of tigecycline use (HR 0.690, 95% CI 0.515–0.926).
 |
Table 4 Cox Regression Analysis of Factors Associated with 30-Day Mortality in 48 Elderly Patients |
Our study also analysed 30-day follow-up patient survival in both groups (Figure 1). A Log-rank test comparison confirmed no significant difference in 30-day survival (Log-rank test 2.414, P= 0.12).
 |
Figure 1 Kaplan–Meier curves for mortality as a function of SD TGC or HD TGC by survival days. |
Discussion
All patients in this study were elderly and diagnosed with nosocomial pneumonia. In this retrospective study, we collected the patient comorbidities, disease severity (Charlson index, APACHE II score),30 invasive procedures (tracheotomy, parenteral nutrition), status (sepsis), and co-administered antibiotics, which has an association with mortality in ICU.31 No statistically significant differences were seen in these variables for both groups.
In this study, we found that there was no significant difference between the two groups in the 30-day deaths. The high dose of TG did not improve the survival days and number of hospital days for the elderly. A meta-analysis study indicated that all-cause mortality was higher in the tigecycline group than in the other groups, but the difference was not significant (odds ratio 1.28, 95% CI 0.97–1.69).32 Our previous meta-analysis results indicated that there was no significant association between high dose of TGC and mortality.33 A review study showed that mortality with high-dose tigecycline in the cohort studies ranged from 8.3% to 26%, while mortality in the low-dose groups (50 mg q12 h) ranged from 8% to 61% and depended on the underlying infection severity. Available data are limited regarding the effectiveness and safety of high-dose tigecycline.34 A recent network meta-analysis of A. baumannii studies compared the efficacy of fifteen antimicrobial treatments for drug-resistant A. baumannii pneumonia in critically ill patients. For survival benefit, sulbactam monotherapy appeared to be the best treatment, high-dose tigecycline was not preferred.35
Our study indicated that the eradication rate in the HD TGC group was significantly higher than in the SD HGC group. Synergistic effect against MDR A. baumannii with the combination of sulbactam and carbapenem had been reported.36 A combination with carbapenem is more frequent in the HD TGC group than SD TGC group. So, it may play a role in giving a high airway eradication rate. However, tigecycline group had a lower microbiological eradiation rate comparison with other control groups.37 Some scholars have suggested that combination therapy may be an option.38 However, combined therapy with two active drugs was superior to therapy with a single active drug.39 The study showed TGC combination therapy did not improve microbiologic eradication or all-cause mortality. It can only be recommended when other optimized therapeutics, such as colistin, are unavailable.40 An observational cohort study included colistin monotherapy (9 million UI/day) or combined therapy with colistin and tigecycline (100 mg/day) and found the combined targeted therapy with high-dose colistin and standard-dose tigecycline was unassociated with a lower 30-day crude mortality of bacteremia due to carbapenem-resistant A. baumannii in critically ill patients.41 A study showed 42 patients with XDR A. baumannii infection randomized into two groups: the tigecycline group and the tigecycline and cefoperazone-sulbactam (1:1) group. The total combined efficacy rate (including all patients who demonstrated improved conditions) was significantly higher in the tigecycline and cefoperazone-sulbactam.42 In vitro synergistic activities of tigecycline combined with cefoperazone-sulbactam against MDR A. baumannii were demonstrated.43 This combination may be an option for critically ill patients; however, due to the small number of current research samples, a large clinical trial is needed to verify the best combination.
In our study, COPD and surgery before infection were associated with 30-day mortality. Survival analysis found high-dose tigecycline was unassociated with lower crude mortality. A study indicated thatThe microbial success was 21.0 times higher for every 1g/dl increase in albumin (P<0.001) and 8.59 times higher for patients without VAP compared to those with VAP (P<0.003).44 Although total protein generally is unaffected by aging, the plasma albumin portion has been shown to decrease from 4 g/dL in young adults to approximately 3.5 g/dL in patients over 80.45 This may also explain tigecycline’s poor efficacy in elderly patients.
Several potential limitations should be considered when interpreting these results. First, the number of subjects included was small. Second, we did not assess the impact of high-dose tigecycline on adverse drug reactions such as the rate of abnormal laboratory measures or patients requiring TGC discontinuation. One study showed high-dose tigecycline-associated alterations in coagulation parameters, such as the plasma-fibrinogen concentration, international normalised ratio (INR) and activated partial thromboplastin time (aPTT), especially in elderly patients.46,47 Third, we also did not analyze the clinical response. Fourth, the different number of patients and number of carbapenem combinations in both groups were also heterogeneity in this study.
In conclusion, high-dose tigecycline was unassociated with lower crude mortality in elderly patients with pneumonia due to multidrug-resistant A. baumannii, and it benefitted eradicating A. baumannii in the ICU. Combination therapy may be an option. Prospective cohort studies or random clinical trials are needed to confirm these preliminary results.
Abbreviations
APACHE, Acute Physiology and Chronic Health Evaluation; CI, confidence interval; ICU, intensive care unit; HR, hazard ratio; SD; VAP, ventilator-associated pneumonia; HAP, hospital-acquired pneumonia; MDR, multidrug-resistant; XDR, extensively drug-resistant.
Acknowledgments
The authors thank Dr Guojie Teng and Yan Li for their dedicated contributions in clinical outcome evaluation.
Funding
This research was funded by Beijing Municipal Administration of Hospitals Incubating Program (PX2020038).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Kalanuria AA, Ziai W, Zai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18(2):208. doi:10.1186/cc13775
2. Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. Multidrug resistance, inappropriate empiric therapy, and hospital mortality in Acinetobacter baumannii pneumonia and sepsis. Crit Care. 2016;20(1):221. doi:10.1186/s13054-016-1392-4
3. Goel V, Hogade SA, Karadesai S. Ventilator associated pneumonia in a medical intensive care unit: microbial aetiology, susceptibility patterns of isolated microorganisms and outcome. Indian J Anaesth. 2012;56(6):558–562. doi:10.4103/0019-5049.104575
4. Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46(8):1254–1263. doi:10.1086/529198
5. Garnacho-Montero J, Dimopoulos G, Poulakou G, et al. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med. 2015;41(12):2057–2075. doi:10.1007/s00134-015-4079-4
6. Stein GE, Babinchak T. Tigecycline: an update. Diagn Microbiol Infect Dis. 2013;75(4):331–336. doi:10.1016/j.diagmicrobio.2012.12.004
7. Shin JA, Chang YS, Kim HJ, et al. Clinical outcomes of tigecycline in the treatment of multidrug-resistant Acinetobacter baumannii infection. Yonsei Med J. 2012;53(5):974–984. doi:10.3349/ymj.2012.53.5.974
8. Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J. 2017;50(3). doi:10.1183/13993003.00582-2017
9. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–61e111. doi:10.1093/cid/ciw353
10. Hu F, Guo Y, Yang Y, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. 2019;38(12):2275–2281. doi:10.1007/s10096-019-03673-1
11. Geng TT, Xu X, Huang M. High-dose tigecycline for the treatment of nosocomial carbapenem-resistant Klebsiella pneumoniae bloodstream infections: a retrospective cohort study. Medicine (Baltimore). 2018;97(8):e9961. doi:10.1097/MD.0000000000009961
12. Xu L, Wang YL, Du S, Chen L, Long LH, Wu Y. Efficacy and safety of tigecycline for patients with hospital-acquired pneumonia. Chemotherapy. 2016;61(6):323–330. doi:10.1159/000445425
13. Hawkey PM, Warren RE, Livermore DM, et al. Treatment of infections caused by multidrug-resistant gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother. 2018;73(suppl_3):iii2–2iii78. doi:10.1093/jac/dky027
14. Zha L, Oho AUID, Pan L, et al. Effectiveness and safety of high dose tigecycline for the treatment of severe infections: a systematic review and meta-analysis. Adv Ther. 2020;37(3):1049–1064. doi:10.1007/s12325-020-01235-y
15. Giuliano C, Patel CR, Kale-Pradhan PB. A guide to bacterial culture identification and results interpretation. P T. 2019;44(4):192–200.
16. Xia G, Jiang R. Clinical study on the safety and efficacy of high-dose tigecycline in the elderly patients with multidrug-resistant bacterial infections: a retrospective analysis. Medicine (Baltimore). 2020;99(10):e19466. doi:10.1097/MD.0000000000019466
17. American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi:10.1164/rccm.200405-644ST
18. Roffman CE, Buchanan J, Allison GT. Charlson comorbidities index. J Physiother. 2016;62(3):171. doi:10.1016/j.jphys.2016.05.008
19. Man SY, Chan KM, Wong FY, et al. Evaluation of the performance of a modified Acute Physiology and Chronic Health Evaluation (APACHE II) scoring system for critically ill patients in emergency departments in Hong Kong. Resuscitation. 2007;74(2):259–265. doi:10.1016/j.resuscitation.2006.12.015
20. Hammond JM, Potgieter PD. Source of infection in nosocomial pneumonia. Lancet. 1993;341(8856):1358.
21. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi:10.1007/s00134-017-4683-6
22. Sader HS, Farrell DJ, Jones RN. Tigecycline activity tested against multidrug-resistant Enterobacteriaceae and Acinetobacter spp. isolated in US medical centers (2005–2009). Diagn Microbiol Infect Dis. 2011;69(2):223–227. doi:10.1016/j.diagmicrobio.2010.10.020
23. Wang H, Song Y, Wang M, Ni Y, Ma Y, Jiankang R. Expert consensus on operating procedures for tigecycline in vitro susceptibility testing. Chin J Lab Med. 2013;7(36):584–587. doi:10.3760/cma.j.issn.1009-9158.2009.11.002
24. Falagas ME, Karageorgopoulos DE. Pandrug resistance (PDR), extensive drug resistance (XDR), and multidrug resistance (MDR) among Gram-negative bacilli: need for international harmonization in terminology. Clin Infect Dis. 2008;46(7):1121–1122. doi:10.1086/528867
25. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
26. Paterson DL, Doi Y. A step closer to extreme drug resistance (XDR) in gram-negative bacilli. Clin Infect Dis. 2007;45(9):1179–1181. doi:10.1086/522287
27. Ren Y, Ma G, Peng L, Ren Y, Zhang F. Active screening of multi-drug resistant bacteria effectively prevent and control the potential infections. Cell Biochem Biophys. 2015;71(2):1235–1238. doi:10.1007/s12013-014-0333-6
28. Torres A, Zhong N, Pachl J, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, Phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18(3):285–295. doi:10.1016/S1473-3099(17)30747-8
29. Ye JJ, Lin HS, Yeh CF, et al. Tigecycline-based versus sulbactam-based treatment for pneumonia involving multidrug-resistant Acinetobacter calcoaceticus-Acinetobacter baumannii complex. BMC Infect Dis. 2016;16(1):374. doi:10.1186/s12879-016-1717-6
30. Sun JW, Rogers JR, Her Q, et al. Validation of the combined comorbidity index of charlson and elixhauser to predict 30-day mortality across ICD-9 and ICD-10. Med Care. 2018;56(9):812. doi:10.1097/MLR.0000000000000954
31. Despotovic A, Milosevic B, Milosevic I, et al. Hospital-acquired infections in the adult intensive care unit-Epidemiology, antimicrobial resistance patterns, and risk factors for acquisition and mortality. Am J Infect Control. 2020:1–5. doi:10.1016/j.ajic.2020.01.009
32. Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis. 2011;11(11):834–844. doi:10.1016/S1473-3099(11)70177-3
33. Bai XR, Liu JM, Jiang DC, Yan SY. Efficacy and safety of tigecycline monotherapy versus combination therapy for the treatment of hospital-acquired pneumonia (HAP): a meta-analysis of cohort studies. J Chemother. 2018;1–7. doi:10.1080/1120009X.2018.1425279
34. Falagas ME, Vardakas KZ, Tsiveriotis KP, Triarides NA, Tansarli GS. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents. 2014;44(1):1–7. doi:10.1016/j.ijantimicag.2014.01.006
35. Jung SY, Lee SH, Lee SY, et al. Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: a systemic review and Bayesian network meta-analysis. Crit Care. 2017;21(1):319. doi:10.1186/s13054-017-1916-6
36. Lee NY, Wang CL, Chuang YC, et al. Combination carbapenem-sulbactam therapy for critically ill patients with multidrug-resistant Acinetobacter baumannii bacteremia: four case reports and an in vitro combination synergy study. Pharmacotherapy. 2007;27(11):1506–1511. doi:10.1592/phco.27.11.1506
37. Mei H, Yang T, Wang J, Wang R, Cai Y. Efficacy and safety of tigecycline in treatment of pneumonia caused by MDR Acinetobacter baumannii: a systematic review and meta-analysis. J Antimicrob Chemother. 2019;74(12):3423–3431. doi:10.1093/jac/dkz337
38. Li J, Fu Y, Zhang J, et al. Efficacy of tigecycline monotherapy versus combination therapy with other antimicrobials against carbapenem-resistant Acinetobacter baumannii sequence type 2 in Heilongjiang Province. Ann Palliat Med. 2019;8(5):651–659. doi:10.21037/apm.2019.11.06
39. Tuon FF, Graf ME, Merlini A, et al. Risk factors for mortality in patients with ventilator-associated pneumonia caused by carbapenem-resistant Enterobacteriaceae. Braz J Infect Dis. 2017;21(1):1–6. doi:10.1016/j.bjid.2016.09.008
40. He H, Zheng Y, Sun B, Tang X, Wang R, Tong Z. Tigecycline combination for ventilator-associated pneumonia caused by extensive drug-resistant Acinetobacter baumannii. J Thorac Dis. 2016;8(10):2784–2792. doi:10.21037/jtd.2016.10.29
41. Amat T, Gutierrez-Pizarraya A, Machuca I, et al. The combined use of tigecycline with high-dose colistin might not be associated with higher survival in critically ill patients with bacteraemia due to carbapenem-resistant Acinetobacter baumannii. Clin Microbiol Infect. 2017:1–5. doi:10.1016/j.cmi.2017.09.016
42. Qin Y, Zhang J, Wu L, Zhang D, Fu L, Xue X. Comparison of the treatment efficacy between tigecycline plus high-dose cefoperazone-sulbactam and tigecycline monotherapy against ventilator-associated pneumonia caused by extensively drug-resistant Acinetobacter baumannii. Int J Clin Pharmacol Ther. 2018;56:120. doi:10.5414/CP203102
43. Liu B, Bai Y, Liu Y, et al. In vitro activity of tigecycline in combination with cefoperazone-sulbactam against multidrug-resistant Acinetobacter baumannii. J Chemother. 2015;27(5):271–276. doi:10.1179/1973947814Y.0000000203
44. Bhavnani SM, Rubino CM, Hammel JP, et al. Pharmacological and patient-specific response determinants in patients with hospital-acquired pneumonia treated with tigecycline. Antimicrob Agents Chemother. 2012;56(2):1065–1072. doi:10.1128/AAC.01615-10
45. Greenblatt DJ. Reduced serum albumin concentration in the elderly: a report from the boston collaborative drug surveillance program. J Am Geriatr Soc. 1979;27(1):20–22. doi:10.1111/j.1532-5415.1979.tb01715.x
46. Routsi C, Kokkoris S, Douka E, Ekonomidou F, Karaiskos I, Giamarellou H. High-dose tigecycline-associated alterations in coagulation parameters in critically ill patients with severe infections. Int J Antimicrob Agents. 2015;45(1):90–93. doi:10.1016/j.ijantimicag.2014.07.014
47. Zhang Q, Zhou S, Zhou J. Tigecycline treatment causes a decrease in fibrinogen levels. Antimicrob Agents Chemother. 2015;59(3):1650–1655. doi:10.1128/AAC.04305-14
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
