Back to Journals » Infection and Drug Resistance » Volume 12
High dose of vancomycin plus gentamicin incorporated acrylic bone cement decreased the elution of vancomycin
Authors Li T, Fu L, Wang J, Shi Z
Received 31 January 2019
Accepted for publication 3 July 2019
Published 18 July 2019 Volume 2019:12 Pages 2191—2199
DOI https://doi.org/10.2147/IDR.S203740
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Tao Li,1,* Lilan Fu,2,* Jian Wang,1 Zhanjun Shi1
1Department of Orthopedics, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 2Nanfang PET Center, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Purpose: Low doses of vancomycin and gentamicin were commonly incorporated into acrylic bone cement (antibiotic-impregnated bone cement, AIBC) during revision arthroplasty. Previous studies showed that only a very small amount of antibiotics could be eluted from AIBC. Given the fact that a high dose of antibiotic would elute high concentration of antibiotic, this study investigated the influence of a high dose of dual-antibiotic loading on the properties of cement.
Methods: A total of 8 groups of AIBC containing either gentamicin or vancomycin or both with different amounts of antibiotics (1 g, 2 g and 4 g) were tested on material properties, elution profiles, antibacterial activity and cytological toxicity.
Results: A high dose of gentamicin and vancomycin AIBC (with 2 g gentamicin and 2 g vancomycin loaded) regiment showed acceptable compressive strength of 74.25±0.72 MPa. No cytotoxicity or antibacterial activity reduction was observed in any group tested in this study. The elution profiles indicated that incorporating 2 g vancomycin resulted in 4.77% (1049.57±3.74 μg) released after 28 days. However, after 2 g gentamicin was added, the vancomycin released was significantly reduced to 2.42% (532.24±1.77 μg) (p<0.001), approximately 50% reduction. No significant influence of vancomycin on gentamicin was observed.
Conclusion: These findings suggest that the addition of 2 g vancomycin and 2 g gentamicin into acrylic bone cement was preferred while considering this dual-antibiotic AIBC regiment with acceptably material properties and effective antibacterial activity. However, special attention should be drawn to the reduction of vancomycin elution when incorporated with gentamicin.
Keywords: antibiotic impregnated bone cement, elution, mechanical characteristic, antibacterial activity, toxicity
Introduction
Periprosthetic joint infection (PJI) is a disastrous failure of total joint replacement with the current occurrence of 1–2%.1 Large numbers of studies have demonstrated effective strategies for PJI prevention and treatment, including perioperative usage of antibiotics, adjustment of comorbidities and one-stage or two-stage revision.1 Antibiotic-impregnated bone cement (AIBC) is another effective material for the prevention and treatment of PJI. In this procedure, an appropriate antibiotic is impregnated into bone cement during primary or revision total joint replacement.2–4
Prior studies have investigated the properties of a few kinds of antibiotics after being impregnated into bone cement. Several antibiotics showed excellent eluting and material properties, including vancomycin, gentamicin, daptomycin and levofloxacin.2,5–9 However, the regiment of AIBC should be changed due to the change of bacterial spectrum and drug resistance isolated from PJI. Most of the time, one single antibiotic is not enough for the treatment of complicated PJI.10,11 Currently, it is generally accepted that a combination of a glycopeptide (eg, vancomycin) and aminoglycoside (eg, tobramycin or gentamicin) is the optimal antibiotic for inclusion into acrylic bone cement for PJI eradication.12 After impregnated with two antibiotics, the bone cement showed a phenomenon known as passive opportunism, referring to a synergistic elution of one or both of the impregnated antibiotics resulting from increased polymethylmethacrylate (PMMA) porosity.13,14 For example, in the study of Slane et al, after addition of 1 g vancomycin to tobramycin-incorporated bone cement, it resulted in approximately 38% increase in the elution of tobramycin.15
Different ratios (ranging from low to high) of antibiotics impregnated with bone cement have been investigated.2,5–8 Nevertheless, the ideal ratio of antibiotics added is still controversial. It is generally thought that a high dose of antibiotic would elute high concentration of antibiotic. So high antibiotic loading should be considered because only a small ratio of antibiotic could finally be eluted from AIBC. But this is not always true. The studies of Paz et al and Bishop et al had demonstrated that low antibiotic loading might provide the same antibacterial activity (ie concentration eluted from AIBC was above the minimum inhibitory concentration).16,17 Restriction of mechanical properties is another obstacle for high-dose antibiotic because high antibiotic loading has a detrimental impact on the mechanical properties such as compressive strength and elastic modulus. Previous studies showed that upper ratios of vancomycin and gentamicin were restricted by mechanical properties.17,18 Unfortunately, prior studies ignored the local cytological toxicity of AIBC due to a large amount of antibiotic eluted at the local site and the potential risk of polymerization of cement affected by antibiotic addition.
The purpose of this study was to investigate the influence of high-dose dual-antibiotic loading on the mechanical and antibacterial properties of acrylic bone cement. To this end, two antibiotics commonly used to treat PJI, gentamicin and vancomycin, were incorporated at various ratios into a commercially available bone cement. The elution properties and cytological toxicity were subsequently characterized.
Materials and methods
Cement preparation
A commercial bone cement (Palacos R, Heraeus) was used in this study as control and basic material for different AIBC formulations. Multiple lots of powder and monomer were mixed together to diminish batch-to-batch differences. Different amounts (Table 1) of gentamicin powder (gentamicin sulfate, Amresco CO) and vancomycin powder (Vancocin, Eli Lily) were added homogenized to the cement powder. Liquid monomer was added next by a standard 2:1 polymer powder to liquid monomer ratio. The mixture was then stirred by hand at atmospheric conditions according to the product instructions. During the early period of dough phase, the cement was transferred into stainless molds (φ6 mm×12 mm) with 30 MPa pressure at both ends. The resulting cylindrical cement was then sterilized by 20 kGy γ-ray irradiation and stored at 4°C until the time of analysis.
 |
Table 1 Composition of cement groups tested in this study |
Material characteristics
Compressive strength and compressive modulus were determined according to ISO 583319 by a materials testing machine (Allround, Zwick/Roell, Germany). The cylindrical cement was stored in 50 mL of saline at 37°C for 28 days prior to testing. Six cylindrical samples per group were tested at a crosshead speed of 20 mm/min till the sample ruptured or the yield point appeared. The stress–strain curves were obtained after the testing stopped. And then compressive strength and compressive modulus were shown or calculated as described in ISO 5833.19
The porosity of AIBCs was calculated using Archimedes’ principle. Six cylindrical samples of each group were submerged in 50 mL of normal saline for 28 days at 37°C. Then, the apparent density was measured using a density determination kit (A&D Weighing, Adelaide, Australia). The maximum density of Palacos R bone cement is 1.2967 g.cm−3 and is defined as the true density of the cement, which means it is completely free of pores and voids. The percentages of porosity of AIBCs were then calculated.20
Antibiotic elution
Six cylindrical cement samples per group were immersed in 10 mL of saline in an incubator at 37°C with constant shaking at 60 rpm before testing. At specific time points (1 hr, 6 hrs, 12 hrs, 24 hrs, 7 days, 14 days, 21 days and 28 days), samples were removed from the liquid, gently washed with 10 mL of saline and then immersed in another tube of 10 mL of fresh saline. The washed liquid was gathered together with prior immersed liquid and then transferred into a cryotube at −80°C until the time of analysis. All these manipulations were conducted under sterile conditions.
The concentrations of vancomycin and gentamicin were detected by chemiluminescent microparticle immunoassay with ARCHITECT i2000SR detection system (Abbott, USA). Vancomycin test kit ivancomycin (Biokit S.A, Spain) and gentamicin test kit iGentamicin (Max-Planck-Ring2, Germany) were used to detect these two antibiotics, respectively, according to the manufacturer’s instructions.
Antibacterial activity and cytological toxicity
Three different microbes, which were most commonly found in PJI,10 were tested against for the detection of antibacterial activity. In this procedure, Staphylococcus aureus ATCC 43300, Enterococcus faecalis ATCC 29212 and Escherichia coli ATCC 35218 were diluted to a concentration of 1.5×108 CFU/mL and then 10 mL bacteria medium was incubated with a cylindrical sample, respectively, under 37°C. At specific time points (24 hrs, 48 hrs, 7 days, 14 days and 21 days), 10 μL medium was inoculated in Mueller–Hinton agar plate. After 24 hrs of inoculation, the bacterial colonies were counted and bacterial concentration was calculated.
Cytotoxicity and proliferation were detected with CCK8 procedure.21 Prior to testing, cylindrical samples were immersed in 10 mL of saline in an incubator at 37°C with constant shaking at 60 rpm for 24 hrs. And then the medium was collected for testing. One hundred microliters of MEM-EBSS with 5×103 cells/mL of L929 connective tissue cells (ATCC CCL-1) were added to each of 96-well culture plates except for the reference group. Ten-microliter medium was added next and 0.1% sterilized phenol was designed as positive control. The cells were then cultured for 7 days under 37°C with 5% CO2. At a specific time (1 days, 3 days, 5 days and 7 days), 10 μL CcK8 reagent (Dojindo, Japan) was added in each well. The absorbance at 450 nm (OD450) was measured by the microplate reader (Thermo Multiskan Spectrum, Thermo Scientific, USA) after 2 hrs of incubation. The cell relative growth rate was calculated and the cytotoxicity scale was evaluated according to ISO 10993.22
Statistical analysis
Statistical analyses were conducted in SPSS Statistics 22. The level of significance was set at α=0.05 (P<0.05) and all the tests were 2-tailed. Results of continuous variables were expressed as means ± SD, and frequency and ratio were used for ordinal variables. Shapiro–Wilk tests were used to investigate a normal distribution. Homogeneity of variance tests was conducted with Levene test. Comparisons between two groups were performed using one-way ANOVA. In case of homogeneity of variance, LSD t-test was used for post-hoc analysis, while Tamhane T2 was used in case of heterogeneity of variance. Chi-square test or Fisher exact test was used for discontinuous variables. Mann–Whitney u tests were used for non-normal distributed variables.
Results
Antibiotic elution
The total amount of gentamicin and vancomycin eluted from cement is shown in Table 2. The percentages of antibiotics eluted were between 2.33% and 6.16% (Table 2). For each group of single antibiotic AIBC, a higher ratio of antibiotic added seemed to elute more antibiotic in 28 days (p<0.01). Gentamicin always eluted higher percentage than vancomycin (p<0.01). In these dual-antibiotic AIBCs, G2V2 was found to elute a higher percentage of both antibiotics than V1G1 but a reduction of vancomycin when compared with G0V2 (532.24±1.77 vs 1049.57±3.74, p<0.001). No significant difference was found in terms of gentamicin when compared with V0G2 (815.47±5.62 Vs 818.53±4.81, p=0.33). The same phenomenon was also observed in the group of G1V1 compared with G0V1 and G1V0.
 |
Table 2 Accumulated antibiotics elution (Mean ± SD) for each group and the release ratios (%) after 28 days |
The elution profile of vancomycin and gentamicin is exhibited in Figure 1. All cement groups showed a burst effect during the first 24 hrs. Almost 80% of the eluted antibiotics happened in this stage. After 24 hrs following submersion, G2V2 eluted almost the same gentamicin at all time points with G2V0 (p>0.05) and both were lower than G4V0 (p<0.001). In regard to vancomycin, G2V2 released much less vancomycin than G0V2 (p<0.001) but a little bit more than G0V1 and much more than G1V1 (p<0.001).
 |
Figure 1 The elution profiles (mean ± SD) of gentamicin (A) and vancomycin (B) over a 28-day period. |
The phenomenon of synergistic effect former described was not observed in these formulas of dual AIBCs. For example, in the group of G0V2, incorporating 2 g (5.0%, wt/wt) of vancomycin resulted in 4.77% (1049.57±3.74 μg) released after 28 days. However, after the addition of 2 g gentamicin (group G2V2), the vancomycin released was significantly reduced to 2.42% (532.24±1.77 μg) (p<0.001), approximately 50% reduction. Similar results were found in the group G1V1. For gentamicin, the group G2V0 eluted a total of 818.53±4.81 μg (3.72%) gentamicin of 28 days. Although incorporation of 2 g vancomycin (G2V2) resulted in a little less of gentamicin released (815.47±5.62 μg, 3.71%). However, no statistically significant difference was observed between these two groups.
Material characterization
Significant decreases in compressive strength and modulus were found with the addition of antibiotics (Figure 2). Generally, incubation with saline was not required for determination of characteristics according to ISO 5833.19 In this study, we detected the material characteristics of AIBC after submerging into saline at 37°C for 28 days. This was done to simulate in vivo conditions. The results showed that all the groups with antibiotics showed a lower compressive strength and modulus than the control. The largest reduction of compressive strength was seen in the group of G0V4, which was just above the requirement established by ISO 5833 (ie 70 MPa). For this dual-antibiotic AIBC, G2V2 (74.25±0.72 MPa) showed better compressive strength and modulus than G0V4 (70.52±1.43 MPa, p<0.05), but worse than G4V0 (76.46±1.42 MPa, p<0.05).
 |
Figure 2 Compressive strength (A) and compressive modulus (B) of tested cement. The dashed line on the left represents the lower limit of 70 MPa established by ISO 5833. |
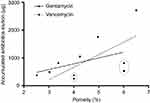
With regard to the porosity, the statistical analysis showed significantly increased cement porosity in the group of antibiotics added than control (Table 3). The highest level was observed in the group of G0V4 (6.51±0.14%). The porosity of vancomycin-impregnated cement seemed to be larger than that of gentamicin (p<0.05). A significant correlation was observed between porosity and gentamicin elution and vancomycin elution (Figure 3).
 |
Table 3 The porosity (%) of each group after submerged in saline at 37°C for 28 days |
Antibacterial activity and toxicity
Cement without vancomycin did not show any inhibitory effect on Staphylococcus aureus growth. Cement without gentamicin showed no inhibitory effect on Escherichia coli. High dose of antibiotic loaded showed better antibacterial activities. The most powerful effect was seen in the group of G2V2 because no bacterial growth was observed during the first 48 hrs (Figure 4).
The results of cytotoxicity and proliferation detected by CCK 8 procedures are mentioned in Table 4. No significant difference was detected between any group of AIBC and the control. The results indicated no toxicity of these formula of AIBC and no extra effect on proliferation.
 |
Table 4 The cell relative growth rate ([RGR] %) of L929 cell at different time periods detected with CCK8 procedure and the cytotoxicity scale (CTS) evaluated according to ISO10993. |
Discussion
It is universally accepted that AIBC is one of the most effective methods in the management of PJI.1 However, it is a fact that one single antibiotic was usually not effective enough to treat complicated PJI, which was mostly related to multiple bacteria and drug resistance.10 Then, investigating the properties of dual-antibiotic-impregnated bone cement is of great value to optimize the regiments of cement spacer while used in PJI.11 In this study, gentamicin and vancomycin were used to cover the current bacterial spectrum isolated from PJI. Previous studies demonstrated the increasing tendency of PJI pathogen pattern of methicillin-resistant Staphylococcus aureus (MRSA), enterococcus and extended-spectrum beta-lactamase bacteria (ESBL) (eg. ESBL+ Escherichia coli).10 Then, a combination of gentamicin and vancomycin might be effective for the current bacterial spectrum. The results of this study showed effective antibacterial activity and no cytological toxicity, which demonstrated our hypothesis.
Generally, PMMA was not an optimal drug delivery system because only a small amount of antibiotic could finally be eluted from AIBC.12 In this study, 3.39–3.99% of gentamicin and 2.33–6.16% of vancomycin were finally released. In the study by Neut et al, only 4% of gentamicin was finally eluted from gentamicin-loaded bone cement.18 Another study of van de Belt et al showed 8.4% of gentamicin eluted.23 The same was seen in vancomycin-loaded bone cement.17,24 Fortunately, with special techniques, the elution ratio might be increased. In the study of Pithankuakul et al, 24% of vancomycin was finally eluted from a special modified vancomycin-loaded bone cement.25 Previous studies and results of this study indicated that the total amount of antibiotic delivered would increase by increased loading percentage.8 However, mechanical properties decreased significantly with high dose antibiotic loaded. We had observed a drop from 93.86±2.13 Mpa to 70.52±1.43 MPa when the loading ratio of vancomycin increased from 2.5% to 10%. In the study by Lee et al, the compressive strength of AIBC decreased to 38% when the loaded vancomycin increased from 1.25% to 10%.24 Although a high dose of antibiotic-loaded AIBC was necessary for the clinician, mechanical properties were the main restriction. To the best of our acknowledgment, the upper limit of gentamicin and vancomycin adding to acrylic bone cement were not defined yet. In the study by Lilikakis et al, the results indicated that 5% to 10% incorporating ratio of vancomycin into acrylic cement would be safe in concern to material properties.26 However, another study by Bishop et al demonstrated that not more than 1.25% of vancomycin should be loaded.17 The different results might be related to different brands of cement, different procedures in preparing of AIBC and different types of antibiotics.25,27,28 In this study, we investigated the relatively high dose of gentamicin- and vancomycin-loaded AIBC, and the results showed compression strength of 74.25±0.72 MPa and elastic modulus of 1619.83±48.38 MPa with 2 g (5%, wt/wt) of each antibiotic incorporated. These properties met the lower limit of ISO standard19 for acrylic bone cement, indicating that the loading percentage of this study might be close to the upper limit under the condition of a normal procedure for the preparation of antibiotic-loaded Palacos R bone cement.
The result of this study showed a large amount of gentamicin and vancomycin released during the initial stage. The elution rate gradually reduced after 24 hrs. This phenomenon was in accordance with previous studies.15,17 An interesting phenomenon was that after combination of gentamicin, the vancomycin released was largely reduced compared with single vancomycin loaded cement. Meanwhile, little effect occurred on that of gentamicin. Previous studies showed ambiguous results about this dual-antibiotic-loaded cement. To the best of our acknowledgement, four different studies concerned about this topic currently. The study by Boelch et al compared the elution of gentamicin in several different premixed gentamicin-loaded bone cement with manually bending vancomycin. The results showed no significant difference in gentamicin elution.29 However, this study ignored the influence of gentamicin on the elution of vancomycin. And no conclusion could be made based on the design of this study. Another study by Frew et al compared commercially prepared gentamicin plus vancomycin bone cement by manually adding vancomycin to commercially available gentamicin-only bone cement (hand-made preparation). The results showed that hand-made preparation increased the elution of both antibiotics.27 Nevertheless, this study could only demonstrate different ways of vancomycin added might influent antibiotic elution. It failed to explain the influence of each antibiotic added to each other. The study by Hsieh et al investigated liquid gentamicin- and powder vancomycin-loaded bone cement. Although the results indicated enhanced elution of both antibiotics. There was no doubt that the material characteristics of liquid antibiotic-loaded cement were far below the clinical requirement.30 And the enhancement might have resulted from water instead of gentamicin or vancomycin. The study by Bertazzoni Minelli et al investigated 2.5% gentamicin- and 5% vancomycin-loaded Cemex bone cement. The results observed little effect of vancomycin on gentamicin elution. But a reduction of vancomycin was shown with gentamicin loaded.31 The results were in accordance with our findings. However, the study by Bertazzoni Minelli et al observed 34.1% loss of microbiological activity of vancomycin.31 This might have resulted from different acrylic cement regiment. Another shortage of the study was the ignorance of material properties, which was a limitation for clinical usage.
The release of antibiotic from cement was thought to be a combination of surface phenomenon and matrix diffusion.32 Under scan electron microscopy, the cross-section of acrylic cement showed a porous structure with pore size around 30–100 μm. After the antibiotic was added, the pore size was enlarged to 40–250 μm.33,34 The bulk porosity was the foundation of drug delivery and pore size could affect drug elution. Actually we do observed the relationship between porosity percentages with elution, however we didn’t investigate the pore size and distribution generated by different antibiotics. The formation of the porous structure might be complex. Based on this study, given the fact that gentamicin generated less porosity than vancomycin under the same incorporating ratio, it was reasonable that the pore size generated by gentamicin might be much smaller than vancomycin. This might be the reason for reduced elution of vancomycin from bone cement after being combined with gentamicin. Unfortunately, this was only one theoretical hypothesis.
Conclusion
The results of this study indicated that gentamicin and vancomycin complicated acrylic cement was an effective regiment for concurrent PJI bacterial spectrum. With a high dose of 5% of each antibiotics, its material properties complied with standards and released effective and large enough amount of antibiotics without local cytotoxicity. The results of this study indicated that this dual-antibiotic AIBC would be useful in the management of PJI.
Acknowledgments
This work was supported by the Natural Science Foundation of Guangdong Province (grant number 2016A030310398) and the Special Foundation of President of the Nanfang Hospital, Southern Medical University (grant number 2015C014).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–e25. doi:10.1093/cid/cis803
2. Wouthuyzen-Bakker M, Lowik CAM, Knobben BAS, et al. Use of gentamicin-impregnated beads or sponges in the treatment of early acute periprosthetic joint infection: a propensity score analysis. J Antimicrob Chemother. 2018;73(12):3454–3459. doi:10.1093/jac/dky354
3. Kunutsor SK, Beswick AD, Whitehouse MR, et al. Debridement, antibiotics and implant retention for periprosthetic joint infections: A systematic review and meta-analysis of treatment outcomes. J Infect. 2018;77(6):479–488. doi:10.1016/j.jinf.2018.08.017
4. Oh EJ, Oh SH, Lee IS, et al. Antibiotic-eluting hydrophilized PMMA bone cement with prolonged bactericidal effect for the treatment of osteomyelitis. J Biomater Appl. 2016;30:1534–1544. doi:10.1177/0885328216629823
5. Neut D, de Groot EP, Kowalski RS, et al. Gentamicin-loaded bone cement with clindamycin or fusidic acid added: biofilm formation and antibiotic release. J Biomed Mater Res A. 2005;73:165–170.
6. Penalba Arias P, Furustrand Tafin U, Betrisey B, et al. Activity of bone cement loaded with daptomycin alone or in combination with gentamicin or PEG600 against Staphylococcus epidermidis biofilms. Injury. 2015;46:249–253.
7. Matos AC, Ribeiro IA, Guedes RC, et al. Key-properties outlook of a levofloxacin-loaded acrylic bone cement with improved antibiotic delivery. Int J Pharm. 2015;485:317–328.
8. Galvez-Lopez R, Pena-Monje A, Antelo-Lorenzo R, et al. Elution kinetics, antimicrobial activity, and mechanical properties of 11 different antibiotic loaded acrylic bone cement. Diagn Microbiol Infect Dis. 2014;78:70–74.
9. Chang Y, Tai CL, Hsieh PH, et al. Gentamicin in bone cement: A potentially more effective prophylactic measure of infectionin joint arthroplasty. Bone Joint Res. 2013;2:220–226.
10. Rosteius T, Jansen O, Fehmer T, et al. Evaluating the microbial pattern of periprosthetic joint infections of the hip and knee. J Med Microbiol. 2018;67(11):1608–1613. doi:10.1099/jmm.0.000835
11. Hsu YH, Hu CC, Hsieh PH, et al. Vancomycin and ceftazidime in bone cement as a potentially effective treatment for knee periprosthetic joint infection. J Bone Joint Surg Am. 2017;99:223–231. doi:10.2106/JBJS.16.00290
12. Anagnostakos K. What do we (not) know about antibiotic-loaded hip spacers? Orthopedics. 2014;37:297–298. doi:10.3928/01477447-20140430-03
13. Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939–944.
14. Saleh KJ, El Othmani MM, Tzeng TH, et al. Acrylic bone cement in total joint arthroplasty: A review. J Orthop Res. 2016;34:737–744. doi:10.1002/jor.23184
15. Slane J, Gietman B, Squire M. Antibiotic elution from acrylic bone cement loaded with high doses of tobramycin and vancomycin. J Orthop Res. 2018;36:1078–1085. doi:10.1002/jor.23722
16. Paz E, Sanz-Ruiz P, Abenojar J, et al. Evaluation of elution and mechanical properties of high-dose antibiotic-loaded bone cement: comparative “in vitro” study of the influence of vancomycin and cefazolin. J Arthroplasty. 2015;30:1423–1429. doi:10.1016/j.arth.2015.02.040
17. Bishop AR, Kim S, Squire MW, et al. Vancomycin elution, activity and impact on mechanical properties when added to orthopedic bone cement. J Mech Behav Biomed Mater. 2018;87:80–86. doi:10.1016/j.jmbbm.2018.06.033
18. Neut D, Dijkstra RJ, Thompson JI, et al. Antibacterial efficacy of a new gentamicin-coating for cementless prostheses compared to gentamicin-loaded bone cement. J Orthop Res. 2011;29:1654–1661. doi:10.1002/jor.21433
19. International Organization for Standardization. Implants for Surgery- Acrylic Resin Cements. Geneva, Switzerland. 2002:5833
20. Dunne NJ, Orr JF. Influence of mixing techniques on the physical properties of acrylic bone cement. Biomaterials. 2001;22:1819–1826.
21. Cell Counting Kit-8 Assay. Cell Proliferation/Cytotoxicity Assay Kit. Dojindo molecular technologies, Inc; 2019. Available from: https://www.dojindo.com/store/p/456-Cell-Counting-Kit-8.html.
22. International Organization for Standardization. Biological Evaluation of Medical Devices. Geneva, Switzerland. 2018.10993-1.
23. van de Belt H, Neut D, Uges DR, et al. Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials. 2000;21:1981–1987.
24. Lee SH, Tai CL, Chen SY, et al. Elution and mechanical strength of vancomycin-loaded bone cement: in vitro study of the influence of brand combination. PLoS One. 2016;11:e0166545. doi:10.1371/journal.pone.0166545
25. Pithankuakul K, Samranvedhya W, Visutipol B, et al. The effects of different mixing speeds on the elution and strength of high-dose antibiotic-loaded bone cement created with the hand-mixed technique. J Arthroplasty. 2015;30:858–863. doi:10.1016/j.arth.2014.12.003
26. Lilikakis A, Sutcliffe MP. The effect of vancomycin addition to the compression strength of antibiotic-loaded bone cements. Int Orthop. 2009;33:815–819. doi:10.1007/s00264-008-0521-3
27. Frew NM, Cannon T, Nichol T, et al. Comparison of the elution properties of commercially available gentamicin and bone cement containing vancomycin with ‘home-made’ preparations. Bone Joint J. 2017;99-b:73–77. doi:10.1302/0301-620X.99B1.BJJ-2016-0566.R1
28. Chang YH, Tai CL, Hsu HY, et al. Liquid antibiotics in bone cement: an effective way to improve the efficiency of antibiotic release in antibiotic loaded bone cement. Bone Joint Res. 2014;3:246–251. doi:10.1302/2046-3758.38.2000305
29. Boelch SP, Jordan MC, Arnholdt J, et al. Loading with vancomycin does not decrease gentamicin elution in gentamicin premixed bone cement. J Mater Sci Mater Med. 2017;28:104. doi:10.1007/s10856-017-5915-6
30. Hsieh PH, Tai CL, Lee PC, et al. Liquid gentamicin and vancomycin in bone cement: a potentially more cost-effective regimen. J Arthroplasty. 2009;24:125–130. doi:10.1016/j.arth.2008.01.131
31. Bertazzoni Minelli E, Caveiari C, Benini A. Release of antibiotics from polymethylmethacrylate cement. J Chemother. 2002;14:492–500. doi:10.1179/joc.2002.14.5.492
32. Giers MB, McLaren AC, Schmidt KJ, et al. Distribution of molecules locally delivered from bone cement. J Biomed Mater Res B Appl Biomater. 2014;102:806–814. doi:10.1002/jbm.b.33062
33. Kuehn KD, Ege W, Gopp U. Acrylic bone cements: composition and properties. Orthop Clin North Am. 2005;36:17–28. doi:10.1016/j.ocl.2004.06.010
34. Waertel G. The role of antibiotic-loaded cement in the treatment of an infection after a hip replacement. J Bone Joint Surg Am. 1996;78:472–473.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.