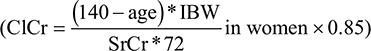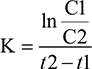Back to Journals » Infection and Drug Resistance » Volume 11
High-dose amikacin for achieving serum target levels in critically ill elderly patients
Authors Sadeghi K, Hamishehkar H, Najmeddin F, Ahmadi A, Hazrati E, Honarmand H, Mojtahedzadeh M
Received 5 September 2017
Accepted for publication 30 November 2017
Published 13 February 2018 Volume 2018:11 Pages 223—228
DOI https://doi.org/10.2147/IDR.S150839
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Kourosh Sadeghi,1 Hadi Hamishehkar,2 Farhad Najmeddin,1 Arezoo Ahmadi,3 Ebrahim Hazrati,4 Hooshyar Honarmand,1 Mojtaba Mojtahedzadeh1,5
1Department of Clinical Pharmacy, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran; 2Department of Clinical Pharmacy, Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran; 3Department of Anesthesia and Intensive Care, Sina Hospital, Tehran University of Medical Science, Tehran, Iran; 4Department of Anesthesia and Intensive Care, Imam Reza Hospital, Army University of Medical Sciences, Tehran, Iran; 5Pharmaceutical Sciences Research Center, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Introduction: To achieve target concentrations, the application of higher-than-standard doses of amikacin is proposed for the treatment of sepsis due to an increase in volume of distribution and clearance, but little data are available on aminoglycoside administration in critically ill elderly patients.
Patients and methods: Forty critically ill elderly patients (aged over 65 years) who required amikacin therapy due to severe documented, or suspected gram-negative infections, were randomly assigned to two treatment groups. Group A (20 patients) received 15 mg/kg amikacin and Group B (20 patients) received 25 mg/kg amikacin per day as a single daily dose. All the patients were monitored for renal damage by the daily monitoring of serum creatinine. The amikacin peak (Cmax) and trough (Cmin) serum concentrations were measured on Days 3 and 7 postadministration.
Results: Data from 18 patients in Group A and 15 patients in Group B were finally analyzed. On Day 3, the amikacin mean Cmax levels in the standard and high-dose treatment groups were 30.4±11 and 52.3±16.1 µg/mL (P<0.001), and the Cmin levels were 3.2±2.1 and 5.2±2.8 µg/mL, respectively (P=0.035). On Day 7, the Cmax levels in the standard and high-dose groups were 33±7.3 and 60.0±17.6 µg/mL (P=0.001), and the Cmin levels were 3.2±2.9 and 9.3±5.6 µg/mL, respectively (P=0.002). In only six (40%) of the patients in the high-dose groups and none of the patients in the standard-dose group, amikacin Cmax reached the target levels (>64 µg/mL), whereas the amikacin mean Cmin levels in the high-dose group were above the threshold of toxicity (5 µg/mL).
Conclusion: Our results suggest that the optimum dose of amikacin should be determined for elderly critically ill patients. It seems that higher-than-standard doses of amikacin with more extended intervals might be more appropriate than standard once-daily dosing in the elderly critically ill patients.
Keywords: amikacin, elderly, high-dose, critical illness, pharmacokinetics, therapeutic drug monitoring
Introduction
Aminoglycosides are polar and low-protein binding antibiotics. They demonstrate interesting pharmacokinetic and pharmacodynamic characteristics, making them a valuable class of antibiotics in the treatment of invasive microorganisms. The emerging bacterial resistance and decline in microbiological susceptibility for aminoglycosides has not been developed extensively during the last decades, in comparison to other classes of antibiotics.1
In critically ill patients, the pharmacokinetic properties of aminoglycosides may differ. The most important change is the increase in the volume of distribution (VD) of polar drugs, which can lead to suboptimal dosing.2 The amount of increase in VD is related to the severity of illness, and the VD of aminoglycosides may be a predictor of capillary leakage that is derived from sepsis.3 The antibacterial activity of aminoglycosides is best related to plasma peak concentration (Cmax) and the ratio of Cmax to minimum inhibitory concentration (MIC) for specific bacteria. It is suspected that a Cmax/MIC ratio greater than eight is required for optimum bactericidal effects and can improve the patients’ outcome, particularly if highly resistant bacteria are responsible for the infection.4 For serious infections and those caused by multidrug-resistant pathogens in an intensive care unit (ICU) setting, such as Pseudomonas aeruginosa and Enterobacteriaceae, the MIC clinical breakpoint is 8 µL/mL for amikacin.5 Thus, to achieve the target Cmax/MIC, reaching a Cmax ≥64 µL/mL would be necessary.
An increase in VD can diminish the achievement of desirable Cmax with standard recommended doses in critically ill patients. It has been demonstrated that the administration of higher-than-standard doses of amikacin (ie, 25–30 mg/kg) in critically ill patients might result in more patients achieving appropriate target peak levels without additional toxicity.6
Aging is associated with various physiological changes in the human body. These changes can alter the pharmacokinetic properties of prescribed drugs in the elderly. The most important changes that result in the alteration of pharmacokinetic properties are: reduction in renal capacity to eliminate drugs and changes in body water and fat content that can alter the drug’s volume of distribution.7 A decrease in the VD of water-soluble drugs such as aminoglycosides in geriatric patients acts contrary to the increase in VD by sepsis and edema. This can cause the dosing of aminoglycosides to be more complex in this population.
The aim of this study was to evaluate the suitability of high-dose amikacin for critically ill elderly patients and to compare this to the standard 15 mg/kg dose in achieving desired peak levels and monitoring related trough levels for toxicity concerns.
Patients and methods
This was a prospective, randomized, multicenter study performed in three ICUs. Forty elderly (age ≥65 years) patients with a documented or suspected severe gram-negative infection in whom amikacin treatment was indicated, and who have a normal serum creatinine (SrCr) level (≤1.2 mg/dL), were consecutively enrolled in the study and randomly assigned to two treatment groups. Group A patients received 15 mg/kg amikacin once daily and Group B patients received 25 mg/kg amikacin once daily. Amikacin was infused over one hour in all cases. All the patients received other standard treatments and care based on staff decisions. Amikacin was administered for 7 days during the study period, and the physician made a continuation decision after 7 days, usually based on the culture results. All the patients were monitored for at least 10 days for changes in the renal function tests. The ethical committee of Tehran University of Medical Sciences and Health Services approved the study protocol. In all cases, a written informed consent form was obtained from the patient’s closest relatives or guardian, which was accepted by the ethical committee. Amikacin was given in combination with a broad-spectrum β-lactam or carbapenem based on the local protocols. Patients were excluded from the study if they had any of the following conditions: dissatisfaction of patients or family, receiving an aminoglycoside in the last 2 weeks prior to the study, baseline creatinine clearance (ClCr) <40 mL/min, increase in SrCr by 0.3 mg/dL or more, need for amikacin dose adjustment for any reason, viral hepatitis, rise in hepatic aminotransferases to more than three times the normal upper limit, BMI ≥35 kg/m2, severe heart failure (ejection fraction <30%), neoplastic disorders with a history of chemotherapy, neuromuscular disease, and a history of allergy to the aminoglycosides. Ideal body weight (IBW) was used to calculate the amikacin dose for every patient using the following formula: IBW =50 kg (45 kg in women) + 2.3 kg for each inch over five feet. In patients whose body weight was over 30% of their IBW, the adjusted body weight (ABW) was used to calculate the amikacin doses:8
ABW = IBW +0.4*(TBW – IBW)
The blood samples of the patients were collected on baseline and on Days 3 and 7, after 1-hour infusion for measuring serum amikacin peak (Cmax) levels, and 30 min before the next dose for measuring the serum amikacin trough (Cmin) levels. The samples were collected in 5-mL plain tubes (without anticoagulant). After clots were completely formed (15–30 min), all blood samples were centrifuged for 10 min in 3,000 rpm and then serum was separated. All samples were stored at −70°C until analysis.
Demographic data, comorbidities, and admission diagnoses were recorded for all patients. Disease severity was characterized by the Acute Physiology and Chronic Health Evaluation II score.9 Positive microbiological cultures were recorded. Biological data, including coagulation parameters, complete blood count, electrolytes, urea, and creatinine were recorded at inclusion and daily thereafter. ClCr was estimated with the Cockcroft–Gault equation:
|
Renal dysfunction was diagnosed when SrCr was >1.2 mg/dL. Acute renal failure was defined by a rise in SrCr by 0.3 mg/dL or higher and/or urine output <0.5 mL/kg per hour for more than 6 hours, based on AKIN criteria.10
The amikacin serum concentrations were quantified by fluorescence polarization immunoassay with the COBAS INTEGRA analyzer (Roche GmbH, D-68298 Mannheim).
Statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA) for Windows. Descriptive statistics were computed for all study variables. A Kolmogorov–Smirnov test was used, and histograms and normal quantile plots were examined to verify the normality of distribution of continuous variables. Discrete variables were expressed as counts (percentage), and continuous variables as mean ± SD. The demographic and clinical differences between study groups were assessed using χ2, Fisher’s exact test, Student’s t-test, or the Mann–Whitney U-test, as appropriate.
For every individual patient, the elimination constant was calculated using the following equation:
|
Amikacin half-life (t½), VD, and clearance (Cl) for every patient was calculated using the following equations:
|
Results
Forty patients (≥65 years) were enrolled in the study over a 14-month period. Seven patients were excluded from the study due to different reasons: Four patients died during the study period, two patients were excluded due to early discharge from ICU, and in one patient amikacin was discontinued on the second day because of the culture results. Finally, data from a total of 33 patients were analyzed: 18 patients in Group A received 15 mg/kg amikacin per day and 15 patients in Group B received 25 mg/kg amikacin per day. The mean age of the patients was 72.90 years, SD: ±7.7. The results of the independent sample t-test revealed that there were no significant differences in age, sex, baseline ClCr, initial Acute Physiology and Chronic Health Evaluation II score, and IBW between two treatment groups. Table 1 shows the baseline parameters as well as the SD of the mean amounts and the P-value of differences in Groups A and B.
Seven patients (21% of the total) had positive blood cultures: one for Escherichia coli, one for P. aeruginosa, one for Acinetobacter baumannii, three for Klebsiella spp., and one with mixed Klebsiella and A. baumannii.
The mean amikacin dose in Group A and B patients was 927 mg, SD: ±180 (range: 600–1,250 mg), and 1,423 mg, SD: ±277 (range: 1,000–1,750 mg) respectively. The average Cmax on Day 3 in Group A patients with 15 mg/kg/d amikacin was 30.41 µg/mL, SD: ±11.09 (range: 16.72–56.64) and average Cmin was 3.26 µg/mL, SD: ±2.13 (range: <0.3–8.7). In Group B, the average Cmax on Day 3 was 52.28 µg/mL, SD: ±16.11 (range: 23.86–70.28) and the average Cmin was 5.22 µg/mL, SD: ±2.87 (range: <0.3–11.4). On Day 7 of therapy, the average Cmax in Group A was 33.05 µg/mL, SD: ±7.31 (range: 22.18–50.02) and the average Cmin was 3.20 µg/mL, SD: ±2.92 (range: <0.3–9.9). In Group B, the average Cmax on Day 7 was 60.01 µg/mL, SD: ±17.64 (range: 37.79–81.53) and the average Cmin was 9.28 µg/mL, SD: ±5.62 (range 2–20.4). There was no significant difference between Cmax and Cmin of Day 3 and Day 7 in the patients in low-dose group (Group A), but in the high-dose group (Group B), the Cmin of amikacin on Day 7 was significantly higher than on Day 3 (P=0.012) although the peak levels were not different.
The average amikacin VD on Day 3 in Group A was 0.54 L/kg, SD: ±0.19 (range: 0.25–1.06) and on Day 7 was 0.45 L/kg, SD: ±0.09 (range: 0.33–0.63), (P=0.279). In Group B, the average VD on Day 3 was 0.53 L/kg, SD: ±0.25 (range: 0.31–1.21) and on Day 7 was 0.49 L/kg, SD: ±0.18 (range: 0.31–0.85). The mean pharmacokinetic parameters of amikacin in both groups are shown in Table 2.
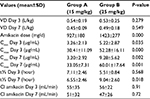  | Table 2 Mean pharmacokinetic parameters of amikacin in Groups A and B Abbreviations: Cl, clearance; t½, half-life; VD, volume of distribution. |
When considering all the patients in both groups, there were no significant differences between VD of Day 3 and Day 7 (P=0.14). Changes in VD during Days 3–7 was also compared between each group, and again there were no significant differences (for Group A: P=0.125, for Group B: P=0.731).
As a primary outcome, no patient in Group A and only 40% of the patients in Group B attained target peak levels (Cmax >64 µg/mL) for killing the resistant pathogens. On the other hand, five patients in Group A (27.8%) and 13 patients (86.7%) in Group B had at least one trough level above 5 µg/mL. No patient in Group A and six patients (40%) in Group B had at least one trough level above 10 µg/mL.
The ClCr of the patients in Groups A and B was calculated using the Cockcroft–Gault formula. The mean values of ClCr for each group on Days 0, 3, 5, 7, and 10 of the study are shown in Table 3. The average ClCr of patients in each group was compared point-by-point using a t-test. Analysis showed that there were no significant differences between the ClCr of the baseline and the ClCr of Days 3, 5, and 7 in both groups and the ClCr of Day 10 in Group A. However, in Group B, ClCr was significantly reduced in comparison with the baseline on Day 10 (P=0.016).
  | Table 3 Mean values for CrCl in Groups A and B Abbreviation: ClCr, clearance of creatinine. |
Although there was no statistically significant difference in the baseline ClCr between the two groups, when comparing the mean differences between the groups, ClCr was significantly lower in the high-dose group on Day 3 (P=0.037), Day 7 (P=0.049), and Day 10 (P=0.004).
A repeated-measure analysis of ClCr also revealed that there was a significant difference in trend of ClCr between two groups and in Group B, and that the trend was decreasing (P=0.038).
Amikacin clearance was calculated for every patient using individual elimination constant and VD on Days 3 and 7. ClCr and amikacin clearance did not significantly change in Group A during Days 3–7, but in Group B, although ClCr did not change significantly, amikacin clearance reduced significantly from Days 3 to 7 (P=0.048). As presented in Table 2, amikacin t½ and, consequently, its trough concentrations increased on Day 7 compared to Day 3 in the high-dose group.
The Pearson correlation test showed a weak but significant correlation between amikacin clearance on Day 7 and ClCr on Day 10 (P=0.031, r=0.46).
None of the patients in either Group A or B developed kidney injury based on the AKIN criteria during the study period.
Discussion
Although the standard amikacin dosage is 15 mg/kg/d in most references,11,12 it is shown that higher than this standard dose (ie, 25–30 mg/kg) is necessary in critically ill patients to achieve the target Cmax/MIC value of higher than 8–10 for more resistant bacteria in ICU settings.6,13
Presently, to the best of our knowledge, our study is the first that tested the success of high-dose, once-daily amikacin for achievement of target serum level, in the elderly population.
It is shown that the peak amikacin level <40 µg/mL is associated with worse outcomes.14 In our study, almost all the patients who received standard doses of amikacin (15 mg/kg/d) had peak levels <40 µg/mL in one or both of the two measuring days. Even when the amikacin trough exceeded the threshold of toxicity (>5 µg/mL),15 in four (27%) patients of this group, the peak drug concentrations remained at <40 µg/mL. This is not surprising because the VD in critically ill patients increased largely in comparison to the normal population. In noncritically ill adult patients, the aminoglycoside VD is 0.25 L/kg,16 but the mean VD in our patients was 0.54 and 0.46 L/kg on Days 3 and 7, respectively. Some studies reported a mean value of 0.4–0.45 L/kg for VD of aminoglycosides in critically ill adult patients.13,17 Fernández de Gatta et al18 reported a mean value of 0.52±0.21 L/kg for the VD of amikacin in critically ill patients. This is in line with our study, but their patient’s mean age was 50 years while all of our patients were ≥65 years and with a mean age of 73 years. We know that aging is associated with an increase in body fat and a decrease in lean body mass and total body water; these changes will affect the apparent VD of drugs, and in the case of polar drugs VD will be decreased.19 Surprisingly, we found that in our elderly patients, the VD of amikacin increased to at least as high amount as previously reported for younger adult critically ill patients. However, variations of VD in our patients were very high (0.25–1.2 L/kg), which emphasizes the great importance of therapeutic drug monitoring in geriatric critically ill patients.
In the study of Gálvez et al,6 only 39% of patients with 25 mg/kg and 76% with 30 mg/kg of amikacin reached peak amikacin levels >60 µg/mL. White et al20 in a retrospective investigation reported that with a higher than approved dose for amikacin (ie, >15 mg/kg/d), only 63.0% and 36.9% of patients in their institution reached the target Cmax/MIC of ≥10 and AUC24/MIC of ≥75 respectively, but only 45.3% of their patients were critically ill.20 Our study revealed that with standard doses of amikacin (ie, 15 mg/kg/d), no patient reached target peak levels. Even in the high-dose group, in less than half of the patients did the amikacin peak concentrations reach the target level (>64 µg/mL).
As shown in Table 2, elimination t½ is increased in our elderly critically ill patients compared to the t½ of 2–3 hours in the normal adult population. This is partly due to the preexisting renal dysfunction due to aging, although other factors such as critical illness, decreased renal flow due to hypo-perfusion, etc., could also play a role.
There are some limitations which should be addressed in this study. The sample size is relatively small, but considering the difficulty of patient enrollment based on the inclusion and exclusion criteria and because the primary objective was to show the efficiency of two dose regimen of amikacin to achieve desired level and not a statistical comparison of a clinical outcome, the results might be less influenced by the sample size. Another limitation which could be mentioned is the ClCr estimation by the Cockcroft–Gault equation. This equation may overestimate ClCr in the elderly population because of reduced lean body mass by aging. Measuring ClCr by the method of 24-hour urine collection is the gold standard method. Also, we acknowledge that more frequent sampling during a 24-hour period would offer a more precise calculation of some pharmacokinetic parameters such as VD compared to a peak and trough level measurement.
Conclusion
It might prove reasonable to administer amikacin in high doses (at least 25 mg/kg) in elderly critically ill patients to achieve target peak levels (>64 µg/mL). Nevertheless, to reduce toxicity, the kidneys should be allowed more time to eliminate the drug. This interval in the elderly people should be more than 24 hours (based on the renal functional reserve). It should be noted that due to the great variability in the VD between patients and rapid changes in critical illness, the true interval cannot be predicted unless the drug level is measured at appropriate times and individualized treatment is available for every patient.
Disclosure
The authors report no conflicts of interest in this work.
References
Leibovici L, Vidal L, Paul M. Aminoglycoside drugs in clinical practice: an evidence-based approach. J Antimicrob Chemother. 2009;63(2):246–251. | ||
Scaglione F, Paraboni L. Pharmacokinetics/pharmacodynamics of antibacterials in the Intensive Care Unit: setting appropriate dosing regimens. Int J Antimicrob Agents. 2008;32(4):294–301. | ||
Marik PE. Aminoglycoside volume of distribution and illness severity in critically ill septic patients. Anaesth Intensive Care. 1993;21(2):172–173. | ||
Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155(1):93–99. | ||
Aminoglycosides: EUCAST clinical MIC breakpoints. Available from: http://www.eucast.org/clinical_breakpoints/. | ||
Galvez R, Luengo C, Cornejo R, et al. Higher than recommended amikacin loading doses achieve pharmacokinetic targets without associated toxicity. Int J Antimicrob Agents. 2011;38(2):146–151. | ||
Turnheim K. Drug therapy in the elderly. Exp Gerontol. 2004;39(11–12):1731–1738. | ||
Traynor AM, Nafziger AN, Bertino JS Jr. Aminoglycoside dosing weight correction factors for patients of various body sizes. Antimicrob Agents Chemother. 1995;39(2):545–548. | ||
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. | ||
Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. | ||
Chambers HF. The aminoglycosides. In: Brunton LL, editor. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. Vol 2, 11th ed. New York: McgGraw-Hill; 2006:1155–1171. | ||
Gilbert DN, Leggett JE. Aminoglycosides. In: Mandell GL, Bennett JE, Dolin R, editor. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7 ed. Philadelphia: Churchill Livingstone; 2010:359–384. | ||
Taccone FS, Laterre PF, Spapen H, et al. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care. 2010;14(2):R53. | ||
Moore RD, Smith CR, Lietman PS. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med. 1984;77(4):657–662. | ||
Tod M, Lortholary O, Seytre D, et al. Population pharmacokinetic study of amikacin administered once or twice daily to febrile, severely neutropenic adults. Antimicrob Agents Chemother. 1998;42(4):849–856. | ||
Bauer LA. Applied clinical pharmacokinetics. Vol 2, 2nd ed. New York: McGraw Hill Medical; 2008. | ||
Triginer C, Izquierdo I, Fernandez R, et al. Gentamicin volume of distribution in critically ill septic patients. Intensive Care Med. 1990;16(5):303–306. | ||
Fernandez de Gatta MM, Mendez ME, Romano S, Calvo MV, Dominguez-Gil A, Lanao JM. Pharmacokinetics of amikacin in intensive care unit patients. J Clin Pharm Ther. 1996;21(6):417–421. | ||
Schenker S. The aging liver. In: Abrams WB, Beers MH, Berkow R, Fletcher AJ, Besdine RW, editors. The Merck Manual of Geriatrics. 2nd ed. Whitehouse Station, NJ: Merck Research Laboratories Whitehouse Station; 1995:696–699. | ||
White BP, Lomaestro B, Pai MP. Optimizing the initial amikacin dosage in adults. Antimicrob Agents Chemother. 2015;59(11):7094–7096. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.