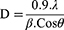Back to Journals » International Journal of Nanomedicine » Volume 19
High Antiparasitic and Antimicrobial Performance of Biosynthesized NiO Nanoparticles via Wasted Olive Leaf Extract
Authors Alghamdi SQ, Alotaibi NF, Al-Ghamdi SN, Alqarni LS, Amna T, Moustafa SMN, Alsohaimi IH, Alruwaili IA, Nassar AM
Received 10 October 2023
Accepted for publication 31 January 2024
Published 14 February 2024 Volume 2024:19 Pages 1469—1485
DOI https://doi.org/10.2147/IJN.S443965
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Samia Q Alghamdi,1 NF Alotaibi,2 Sameera N Al-Ghamdi,3 Laila S Alqarni,4 Touseef Amna,1 Shaima MN Moustafa,5 Ibrahim Hotan Alsohaimi,2 IA Alruwaili,2 AM Nassar2
1Department of Biology, Faculty of Science, Al-Baha University, Al-Baha, 65799 Saudi Arabia; 2Chemistry Department, College of Science, Jouf University, Sakaka, Saudi Arabia; 3Chemistry Department, Faculty of Science, Al-Baha University, Al-Baha, Saudi Arabia; 4Chemistry Department, College of Science, Imam Mohammad Ibn Saud Islamic University (IMSIU), Riyadh, 11623 Saudi Arabia; 5Biology Department, College of Science, Jouf University, Sakaka, Saudi Arabia
Correspondence: AM Nassar, Jouf University, Sakaka, Aljouf, 2014, Saudi Arabia, Tel +966555978016, Email [email protected]
Background: Nowadays, recycling agricultural waste is of the utmost importance in the world for the production of valuable bioactive compounds and environmental protection. Olive leaf bioactive compounds have a significant potential impact on the pharmaceutical industry. These compounds possess remarkable biological characteristics, including antimicrobial, antiviral, anti-inflammatory, hypoglycemic, and antioxidant properties.
Methods: The present study demonstrates a green synthetic approach for the fabrication of nickel oxide nanoparticles (NiO-olive) using aqueous wasted olive leaf extract. Calcination of NiO-olive at 500°C led to the fabrication of pure NiO nanoparticles (NiO-pure). Different techniques, such as thermal gravimetric analysis (TGA), Fourier-transform infrared spectra (FTIR), ultraviolet-visible spectra (UV-Vis), X-ray diffraction (XRD), scanning electron microscopy (SEM) fitted with energy-dispersive X-ray analysis (EDX), and transmission electron microscopy (TEM), were used to characterize both NiO-olive and NiO-pure. The extract and nanoparticles were assessed for antiparasitic activity against adult ticks (Hyalomma dromedarii) and antimicrobial activity against Bacillus cereus, Pseudomonas aeruginosa, Aspergillus niger, and Candida albicans.
Results: From XRD, the crystal sizes of NiO-olive and NiO-pure were 32.94 nm and 13.85 nm, respectively. TGA, FTIR, and EDX showed the presence of olive organic residues in NiO-olive and their absence in NiO-pure. SEM and TEM showed an asymmetrical structure of NiO-olive and a regular, semi-spherical structure of NiO-pure. UV–Vis spectra showed surface plasmon resonance of NPs. Antiparasitic activity showed the highest mortality rate of 95% observed at a concentration of 0.06 mg/mL after four days of incubation. The antimicrobial activity showed the largest inhibition zone diameter of 33 ± 0.2 mm against the Candida albicans strain.
Conclusion: Nanoparticles of NiO-olive outperformed nanoparticles of NiO-pure and olive leaf extract in both antiparasitic and antimicrobial tests. These findings imply that NiO-olive may be widely used as an eco-friendly and effective antiparasitic and disinfection of sewage.
Keywords: biosynthesis, olive, NiO-NP, antiparasitic, antimicrobial]
Introduction
Green nanotechnology involves the use of natural bioactive agents such as microorganisms, plant materials, and bio-waste to synthesize nanoparticles (NPs) and improves the sustainability.1,2 As a result, it is considered a crucial component of clean technologies that clean up the environment and transform waste bioactive products into green nanomaterials that are advantageous and eco-friendly.3,4 Although there are many methods for producing nanoparticles,5,6 biosynthesized nanoparticles are more suitable for use in medicine than by the chemically synthesized methods because of their higher compatibility, safety, and cost-effectiveness.7–11 Biosynthesis of NPs has been carried out by using plant sources, microbes, and biomolecules. Regarding cost and safety, plant-based sources are preferred over the other ones.8,12–14 Green nanotechnology has applications in multiple biological activities.15 For instance, the plant extracts are used in green synthesis because they contain multiple bioactive molecules, including polyphenols, terpenoids, sugars, phenolic acids, proteins, and alkaloids etc.16,17
Nickel oxide (NiO) is a p-type semiconductor transition metal oxide having a cubic lattice structure. It has a broad band gap, good thermal-chemical stability, outstanding ferromagnetic characteristics, large coercive forces, and chemical stability.18 Due to their numerous applications, NiO-NPs have attracted the attention of biologists and chemists in particular.19 NiO-NPs have shown significant antibacterial, antifungal, antioxidant, anti-inflammatory, anti-cancer, and enzyme inhibition potential. NiO-NP has been found to be toxic to a variety of microorganisms and microalgae by producing reactive oxygen species (ROS).20 In the green synthesis of NiO-NPs, various natural resources have been utilized to produce NiO-NPs.21
Evergreen olive trees have been cultivated since ancient times and have been used in traditional medicine. One of the most significant industrial crops in the world is olive.22 Olive trees have been cultivated all over the world to satisfy the great demand for table olives and olive oil, which are olive products with beneficial health benefits.23 Olive leaves are the most prevalent by-product and are produced during olive harvesting.24 Due to their high concentration of bioactive chemicals, olive leaves are well known for their beneficial pharmacological effects.25 All these compounds have remarkable biological qualities like antimicrobial, antiviral, anti-inflammatory, hypoglycemic, and antioxidant actions.26,27
Ectoparasites have a significant negative impact on animal health and the global economy. Ticks are ectoparasites that feed on the blood of vertebrate animals. They spread several contagious illnesses, such as babesiosis, anaplasmosis, ehrlichiosis, borreliosis, and rickettsiosis.28–31 Ticks not only transmit diseases but they also have a direct impact on livestock health, causing itchy bites, bleeding, skin damage, and anorexia, which slows growth.32 Saudi Arabia, which had a population of about 500,000 in 2017, is one of the nations with a recent high growth rate of the camel population, and the highest percentage occurs in the Riyadh province.33,34 The Camelus genus contains two species: Camelus dromedary (Arabian camel or dromedary), which is found in North Africa and the Middle East, and Camelus dromedarius (Bactrian camel), which is found in the deserts and chilly steppes of Central Asia.35 The dromedary camel is important to the Arab world’s economy and culture.36 The most significant tick species found in Saudi Arabia is Hyalomma dromedarii, which has an impact on cattle, camels, and goats, causes weight loss, and lowers the output of meat and milk. Chemical acaricides are usually used to control the parasites. However, the use of these drugs has a number of risks, including the development of chemical resistance in the tick’s population, contaminated food and the environment, and negative bystander effects on non-target animals, including people. In order to overcome the worries about the security and environmental effects of these chemical acaricides, there is a search for sustainable and alternative approaches to biological tick management that use compounds derived from plants.28,37 In recent times, nanotechnology has revolutionized treatment approaches. Animal parasite treatment using nanotechnology is now thought to be a unique strategy.38,39 Recent advancements in environmentally friendly metal NPs synthesis techniques have been made to solve these issues.40 In the Mediterranean region, traditional medicine frequently employs olive tree leaves (Olea europaea).41 It has been claimed to have antiparasitic properties. It was shown that metal, metal oxide, and carbon nanoparticles, especially those synthesized using environmentally benign procedures, were very effective against a variety of arthropod pests and vectors.42 New tick-control methods are required to reduce the negative effects of chemical acaricides.
In the current study, nickel oxide nanoparticles (NiO-NPs) have been synthesized via the aqueous extract of wasted olive leaves and characterized with different physicochemical tools, then checked to evaluate their possible antiparasitic effects on Hyalomma dromedarii and antimicrobial effect against Bacillus cereus, Pseudomonas aeruginosa, Aspergillus niger, and Candida albican.
Experimental
Instruments
SEM photo detection was carried out using a Thermo Scientific Quattro S system. For Fourier-transform infrared (FT-IR) measurements, a spectrophotometer IRTracer-100 SHIMADZU, was employed. XRD-7000 SHIMADZU was used to determine X-ray diffraction (XRD) patterns using a copper radiation source. The TGA-51SHIMADZU was used to detect thermal gravimetric analysis (TGA) at a heating rate of 10°C/min. Shimadzu A LABOMED-Spectro 99 UV–Vis double beam 3200, Japan was utilized to find electronic spectra in the range from 200 to 800 nm. The transmittance electron microscope (TEM) photos were captured using a JEOL GEM-1010 Transmission electron microscope operated at 80 kV.
Materials
Wasted olive leaves (Olea europaea L.) were gathered from a public garden in Al-Baha, Saudi Arabia. The plant specimen was identified by Dr Najla A. Al Shaye and deposited at the Princess Nourah bint Abdulrahman University Herbarium, voucher number 6217 PNUH. Nickel nitrate hexahydrate was procured from Sigma-Aldrich, USA, and used as received without further purification. Milli-Q water was used to prepare an aqueous solution. The synthesized materials were evaluated for antimicrobial activity against the bacterial strains: Bacillus cereus (B. cereus) (MW830387), Pseudomonas aeruginosa (P. aeruginosa) (OP890578), and the tested fungi were Asperigillus niger (A. niger) (MW596373) and Candida albicans (C. albicans) (MW534712). These isolates were acquired from the Biology Department, College of Science, at Jouf University. Adult ticks (Hyalomma dromedarii) (Acari: Ixodidae) were collected from camels (Camelus dromedarius), not treated with any pesticides, in Al-Baha, Saudi Arabia. A total of 300 ticks were kept in dry Eppendorf tubes at room temperature until used.
Preparation of Aqueous Extract of Olive Leaf
Olive leaves (Olea europaea) (Figure 1) were washed with tap and distilled water several times to remove the impurities, and then dried in the air. The aqueous extract was prepared by mixing 2 g of dried waste leaves with 200 mL of distilled water and refluxing the mixture for 2 h at 100°C. The obtained solution was cooled to room temperature. The solution was filtered using Whatman No. 1 filter paper, and the filtrate was used for further experiments.43
 |
Figure 1 Olive leaves waste (Olea europaea L.) used for preparation of aqueous extract. |
Synthesis of NiO-olive and NiO-pure
NiO-olive was synthesized using a slight modification of the green synthesis protocol for NiO-NPs [21]. One hundred milliliters of double-distilled water was used to dissolve 2 g of nickel nitrate hexahydrate [Ni(NO3)2.6H2O] before it was added to 100 mL of olive leaf extract, and the mixture was stored at 70°C and 1200 rpm for 2 hours. NiO-NPs had formed as a dark green precipitate. The solution was then allowed to cool before being centrifuged three times for 15–25 minutes at 3,000 rpm using double-distilled water. The resulting green precipitate of NiO-olive was filtered out using Whatman’s No. 1 filter paper, air-dried, and weighed (0.6 g). To obtain NiO-pure, 0.3 g of NiO-olive was heated to 500°C for 2 hours in the muffle furnace, resulting in the formation of NiO-pure (0.25 g).
Antiparasitic Activity Experiments
The olive extract, NiO-olive, and NiO-pure were subjected to antiparasitic experiments. Each sample was combined with 200 L of blood in 1.5-mL Eppendorf tubes (Eppendorf, Sigma-Aldrich, Oldenburg, Germany) and swirled for 1 minute on a vortex surface (Scientific Industries Inc, Bohemia, NY). Four hundred adult ticks were divided into four groups (NiO-olive, NiO-pure, olive extract, and control), each with three replicates (10 per replication). The antiparasitic activity was tested using different concentrations (0.01, 0.02, 0.03, and 0.06 µg/mL) for each sample for seven days to check for the minimum inhibitory concentration needed to produce acaricidal activity. The number of unfed adult individuals and the mortality rate were reported. Ticks in three groups were fed with blood mixed with natural extracts, NiO-olive and NiO-pure, while ticks in the fourth group were fed only with the blood. Adult ticks that had not received any treatment and consumed blood only served as a control. Every dish has a 2-day diet treatment. Each plate was covered with foil, and a hole was made into the top of the plastic to allow air to enter. Both the treatment and control groups of ticks were incubated at room temperature with suitable humidity (25°C, 61–75%). Mortality rate was recorded every day till 7 days. Daily observations were made according to previously reported methods44–46 with suitable modifications, and the adult tick mortality % was recorded once the mortality rate had reached 50%.
Antimicrobial Experiments
A broth macro-dilution technique was used to determine the MIC and MBC or MFC. The determination of MBC (minimum bactericidal concentration) or MFC (minimum fungicidal concentration) was performed by preparing tubes that contain 2 mL (Nutrient broth or Potato dextrose broth), 20 µL of each microbial culture, and six serial dilutions (concentrations 0.08, 0.16, 0.32, 0.64, 1.28, and 2.56 µg/mL) for olive extract, NiO-olive, and NiO-pure in each tube were prepared. After that, the tubes were incubated at 37°C for 48 h.47 The experiments were performed in duplicate. The tested bacteria were B. cereus and P. aeruginosa. The tested fungi were A. niger and C. albicans. The strains were isolated from human excreta, sewage effluents, and construction industry wastes in Sakaka, Saudi Arabia. The antimicrobial activity was determined by the agar-well diffusion method.7 The isolates were inoculated using NB for bacterial culture and PDA medium for fungal cultures, respectively. Each isolate was inoculated onto separate agar plates, and the wells (0.5-mm-diameter) were formed using a cork borer. The wells were filled with 100 µL of tested compounds (olive leaf extract, NiO-olive, and NiO-pure). The petri dishes were then incubated at 30°C in HL-340 Autoclave (vertical type). The antimicrobial activity of the samples was measured by determining the diameter of the inhibition zone. All the experiments were repeated twice.
Data Analysis
The data show the three replicates mean values and standatd deviations. SPSS and Origin Pro2016 software were used to conduct the statical analysis.
Results
In the current work, NiO-NPs were synthesized utilizing olive leaf aqueous extract. This study evidently revealed that the sustainable bioresources may work in eco-friendly NPs synthesis and enhancement of their biological applications. Figure 2 illustrates the green synthesis, characterization, and biological applications of NiO-NPs.
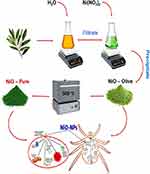 |
Figure 2 Schematic representation of synthesis, characterization, antiparasitic and antimicrobial applications of NiO-NPs. |
Chemistry
XRD Analysis
The purity and crystallinity of the NiO-olive and NiO-pure samples were examined using powder XRD, as shown in Figure 3.
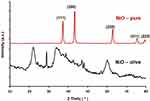 |
Figure 3 XRD patterns of NiO-olive (with phytochemicals from olive leaf extract) and NiO-pure (without phytochemicals). |
Morphological Studies
The morphology of NiO-olive and NiO-pure was investigated using SEM. Figure 4 displays the SEM and EDX images.
 |
Figure 4 SEM images of NiO-olive (a) and NiO-pure (c) and EDX spectra of NiO-olive (b) and NiO-pure (d). |
TEM Analysis
The TEM photos of NiO-pure and NiO-olive obtained are displayed in Figure 5.
 |
Figure 5 TEM images of NiO-pure (a and b), NiO-olive (c and d), SAED image of NiO-pure (e). |
TGA
The purity and thermal stability of NiO-olive and NiO-pure were evaluated using TGA, Figure 6.
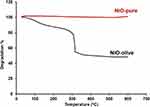 |
Figure 6 TGA curves of NiO-olive and NiO-pure. |
FT-IR Spectra
The FT-IR spectra of NiO-olive and NiO-pure are illustrated in Figure 7.
 |
Figure 7 FT-IR spectra of NiO-pure and NiO-olive. |
Electronic Spectra
The electronic spectra of olive leaf extract and NiO-NPs are detected in the range of 200–450 nm, Figure 8.
 |
Figure 8 UV-Vis spectra of olive leaf extract and nanoparticles. |
Biology
Antiparasitic Activity
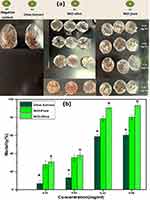
In this study, olive leaf extract, NiO-olive, and NiO-pure were tested against H. dromedarii adult ticks for the first time. The ticks were exposed to different concentrations ranging from 0 to 0.06 mg/mL as aforementioned. Three replicate experiments were performed for each concentration. The mortality % was observed as dose dependent, as shown in Figure 9. No death was observed in control samples until seven days of incubation. The highest mortality rate was observed at a concentration of 0.06 mg/mL after four days of incubation time.
Antimicrobial Activity
MIC, MBC, and MFC values at different concentrations of 0.08, 0.16, 0.32, 0.64, 1.28, and 2.56 µg/mL were tested using the standard serial dilution method against selected microbes.48 The results are summarized in Table 1. MIC values were found to be 0.64 µg/mL, 2.65 µg/mL, and 0.32 µg/mL for extract, NiO-pure, and NiO-olive, respectively, with no visible turbidity. The antimicrobial results have been provided in Table 2 and illustrated in Figure 10. As shown in the table, the results followed the following order: NiO-olive > olive leaf extract > NiO-pure.
 |
Table 1 Antimicrobial Activity of Olive Leaf Extract, NiO-pure, and NiO-olive Against Tested Microbes at 30°C in Dark |
 |
Table 2 Zone of Inhibition Diameters of Olive Leaf Extract, NiO-pure, and NiO-olive Against Tested Microbes at 30°C in Dark |
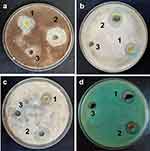 |
Figure 10 Effect of olive leaf extract (1), NiO-olive (2), NiO-pure (3), on the growth of tested microbes B. cereus (a), P. aeruginosa (b), A. Niger (c), and C. albicans (d) in dark at 30 °C. |
Discussion
Chemistry
The XRD patterns of NiO-olive show broad peaks depicting amorphous phase, which may be due to the presence of organic phytochemicals in NiO-NPs.48 The XRD pattern of NiO-pure showed a sharp peak, indicating the high purity and crystallinity of single-phase NiO-NPs. The peak positions at 2θ= 37.20, 43.20, 62.87◦, 75.20◦, and 79.38◦ can readily be indexed as (111), (200), (220), (311), and (222) crystal planes of the bulk NiO, respectively.49 All these diffraction peaks can be perfectly indexed to the face-centered cubic (FCC) crystalline structure of NiO, which is in accordance with that of the standard NiO-NPs (JCPDS, No. 04–0835).50 The XRD pattern of NiO-pure shows that the samples are single-phase, and no other impurities were detected. Using the Scherrer equation, eq. 1, the crystal size of NiO-pure was determined.7
Where θ is XRD angle, β is the half-maximum width and λ = 1.5418 Å is the wavelength of X-ray (for Cu Kα1). The NiO-olive and NiO-pure crystals’ sizes were calculated to be 32.94 nm and 13.85 nm, respectively.
The SEM image of NiO-olive shows an asymmetrical form due to the presence of phytochemicals from olive extract. The SEM image of NiO-pure shows a regular, semi-spherical, and poly dispersed structure, with a diameter ranging from 48 to 95 nm. The EDX spectrum of NiO-olive shows major peaks for the elements oxygen (O) and nickel (Ni), which can clearly be seen. Minor peaks of carbon (C), nitrogen (N), phosphorus (P), and sulfur (S) are also seen. The utilization of plant extract during the synthesis process may be the cause of the presence of C, N, P, and S.3 The EDX spectrum of NiO-pure, after the calcination of NiO-olive, demonstrates the presence of Ni and O elements, which are uniformly distributed in the sample. No traces of elemental contaminants were found. This demonstrates the formation of NiO-pure.
TEM images of NiO-pure NPs possess spherical and semi-spherical forms, which were evenly distributed, had a smooth surface, and were of uniform size. Owing to their tiny sizes and high surface energies, a few small particles may agglomerate into secondary particles.3 Because of the presence of an organic layer from olive phytochemicals, the TEM investigation of the NiO-olive revealed that the NiO-NPs are amorphous and non-crystalline form. The clear brilliant rings those are visible in the SAED pattern of NiO-pure support the preferential orientation of NiO nanocrystals.
The TGA curve of NiO-olive shows a decrease in weight throughout the temperature range of 25–490°C, due to the degradation of the organic compounds in the olive leaf extract after complete decomposition (56.50% of the original sample weight). Above 490°C, there was no degradation, which accounts for the pure weight of the NPs (43.50% of the original sample weight). On the other hand, the TGA curve of NiO-pure shows the high purity of sample, which can be attributed to the absence of any thermal degradation, indicating the absence of organic contaminants.
The NiO-olive spectrum shows bands at 3335, 2990, 1650, 1050, and 1000 cm−1. These bands are assignable to organic bioactive molecules found in the olive extract, such as flavonoids, sugars, phenolic compounds, and hydroxytyrosol.51 On the other hand, these bands were not observed in the spectrum of NiO-pure, which indicates the absence of organic phytochemicals after heat treatment of NiO-olive. Both spectra show bands around 2300 cm−1, which are attributed to the adsorbed CO2. The band at 520 cm−1 is due to the Ni–O bond.52
The absorption spectra of NiO-olive and NiO-pure show absorbance in the UV range. The band observed at ≈355 nm is due to the band gap absorption of NiO.52 The spectra showed a distinct surface plasmon resonance, which results from the interaction of incoming photons with the conduction electrons of metal nanoparticles. This is indicated by the appearance of a maximum absorption peak at ≈ 270 nm.7
Biological Applications
Antiparasitic Activity
The ticks are parasites that can result in strong allergic responses and transmit a variability of pathogens such as viruses, bacteria, rickettsia, helminth, and protozoa that can distress human beings, animals, as well as cattle. Ticks are obligate blood-sucking ectoparasites that impose skin irritability and damage and communicate infections to individuals and animals.31 Tick administration management has confronted a lot of problems in current times, for instance, quick development of resistance and non-target impression of chemical pesticides on public health as well as on the atmosphere. A variety of governing approaches are operated to deal with these ectoparasites. In this regard, nanopesticides are a comparatively novel technological approach. The application of nanomaterials prepared by means of diverse synthesis techniques such as innovative pesticides has gripped the scientific community’s attention.53 The green synthesis approach is thought to be risk-free and secure and also addresses issues with conventional chemical and physical methods. In previous studies, acaricidal impact of silver nanoparticles synthesized by green synthesis was tested on Ixodidae tick species.44 The NiO-NPs also exhibited the greatest mortality rates against adult H. dromedarii ticks. These antiparasitic NiO-NPs are efficient against larvae.54
Among all the tested samples (olive leaf extract, NiO-olive, and NiO-pure), the most effective antiparasitic material was found NiO-olive. NiO-pure also depicted good antiparasitic activity. The lowest effect was observed in olive leaf extract. Nevertheless, in all the samples, it was observed that the mortality % increased with the increase in concentration. From the obtained results, it has been concluded that the activity followed the following order: NiO-olive > NiO-pure > olive leaf extract in a dose-dependent manner. The untreated control samples (fed on blood only) depicted least mortality during the entire incubation period.
A definite understanding of the complete mechanisms of action of nanoparticles against insects and mites is imperfect. It has been anticipated that the nanopesticides act on ticks through several means, eg, altering the lipids or proteins, generating oxidative stress, or distressing the metabolism of ticks. The synthesized metal nanoparticles may bind to amino acids, proteins, and nucleic acids, lessening the membrane penetrability of cells and setting off organelle and thermal degradation, which might lead to death.55 It has been observed that silicon and aluminum nanopesticides cause cell death via absorption through the cuticle layer and cause dehydration of cells, leading to cell destruction of ticks.56 In the present study, because of its thin chitinized cuticles, this might be described as the cause of mortality. Conversely, a recent investigation of the tick’s developmental stages discovered that unfed adult H. dromedarii specimens that were fed showed no mortality. This might be due to its heavily chitinized cuticles.54
Antimicrobial Activity
The olive leaf extract and nanoparticles were tested as antimicrobial agents against some isolated water pathogens. NiO-olive exhibited the best activity and showed high activity against Gram-positive bacteria and fungi, while moderate activity was observed against Gram-negative bacteria. Gram-negative bacteria are more resistant to drugs than Gram-positive bacteria. The outer membrane of Gram-negative bacteria protects them from several drugs. Additionally, they have higher levels of transport proteins, which help antibiotics leave cells before they can do any harm. Also, Gram-negative bacteria have a slime cover that protects them.57 This explains why P. aeruginosa was the least affected by NiO-olive.
Several research studies have been conducted on the antimicrobial activity of NiO-NPs. Iqbal et al20 studied the antimicrobial activity of NiO-NPs against different microorganism strains, which were exposed to different concentrations of NiO-NPs (0.034 –1.1 µg/mL). The diameter of the inhibition zone with 1.1 µg/mL concentration was about 10, 20, and 20 mm for P. aeruginosa, A. niger, and C. albicans, respectively. However, in our study, the effect of NiO-olive was reported 20, 32, and 33 mm, respectively, at 0.64 µg/mL.
Mechanism of Bioactivity
It is suggested that NiO-NPs induce toxicity either by speeding or decelerating host cell biological pathways.21,58 Nanoparticles, in particular, are capable of creating reactive oxygen species (ROS), which have the power to destroy pathogenic microorganisms due to their high chemical reactivity.59 Figure 11 shows the way NiO interacts with nucleic acids to form complexes and then displays these complexes with nucleosides to show their antimicrobial and antiparasitic properties. The passage of sugars, proteins, and nuclear material through the damaged membrane is proof that nanoparticles changed the permeability of the membrane. Because of NiO-NPs improved adherence to the cytoplasm and cell membrane, the permeability of the cell membrane increased, which also caused the casings to break down. Adenosine triphosphate (ATP) release may be inhibited because reactive oxygen species (ROS) are produced. Moreover, NiO-Nps may alter the structure of deoxyribonucleic acid (DNA), therefore disrupting the integrity of the cell membrane. NiO-NPs have the ability to cause denaturation of cytoplasmic ribosomal elements and may allow for the effective halting of protein synthesis. Because of their small size, NiO-NPs could penetrate cell walls and cause denaturation of cell membranes, which can inhibit microbial and parasite growth. The membrane’s intracellular and extracellular components could separate because of denaturation of the cell membrane, which also causes cell lysis in bacteria, fungi, and ticks.59–62
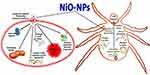 |
Figure 11 Proposed mechanism of antiparasitic and antimicrobial activities of NiO-NPs. |
For comparison, the synthesized nanoparticles were compared to similar reported works Table 3.
 |
Table 3 Comparison of the Bioactivity of NiO and the Previously Reported Antiparasitic and Antimicrobial Nanoparticles |
Conclusion
In this study, we succeeded in achieving the intended goals of employing olive leaf wastes for the green synthesis of NiO-NPs for using as effective, eco-friendly, and sustainable biomedical materials. Various physicochemical techniques were used to characterize the synthesized NPs thoroughly. Gram-positive bacteria, Gram-negative bacteria, and fungi were highly responsive to NiO-olive antimicrobial tests. The adult ticks of Camelus dromedaries, Hyalomma dromedarii, have been assessed for antiparasitic activity for the first time in the current work. The present study’s findings imply that NiO-olive may be a promising antiparasitic agent. Before practical application, thorough in vivo research studies are required to assess the antiparasitic activity. Also, NiO-olive showed a high inhibition level of pathogenic microbes isolated from human wastes, which may be applied as an eco-friendly material for use in sewage disinfection.
Data Sharing Statement
The authors confirm that the article contains all the data produced during the study.
Acknowledgment
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia, for funding this research work through project number 223202.
Disclosure
The authors declare no competing interests.
References
1. Salem SS. A mini review on green nanotechnology and its development in biological effects. Arch Microbiol. 2023;205(4):128. doi:10.1007/s00203-023-03467-2
2. Alzubaidi AK, Al-Kaabi WJ, Ali AA, et al. Green synthesis and characterization of silver nanoparticles using flaxseed extract and evaluation of their antibacterial and antioxidant activities. Appl Sci. 2023;13(4):2182. doi:10.3390/app13042182
3. Alqarni LS, Alghamdi MD, Alshahrani AA, Nassar AM. Green nanotechnology: recent research on bioresource-based nanoparticle synthesis and applications. J Chem. 2022;2022. doi:10.1155/2022/4030999
4. Alghamdi MD, Nazreen S, Ali NM, Amna T. ZnO nanocomposites of Juniperus procera and Dodonaea viscosa extracts as antiproliferative and antimicrobial agents. Nanomaterials. 2022;12(4):664. doi:10.3390/nano12040664
5. Salem SS, Fouda A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res. 2021;199(1):344–370. doi:10.1007/s12011-020-02138-3
6. Al-Zahrani FA, Salem SS, Al-Ghamdi HA, Nhari LM, Lin L, El-Shishtawy RM. Green synthesis and antibacterial activity of Ag/Fe2O3 nanocomposite using Buddleja lindleyana extract. Bioengineering. 2022;9(9):452. doi:10.3390/bioengineering9090452
7. Hussein S, Mahmoud AM, Elgebaly HA, et al. Green synthesis of trimetallic nanocomposite (Ru/Ag/Pd)-Np and Its In Vitro antimicrobial and anticancer activities. J Chem. 2022;2022:1–14. doi:10.1155/2022/4593086
8. Venkatalakshmi N, Kini HJ, Naik HB. Green-synthesized nickel oxide nanoparticles: magnetic and biomedical applications. Inorg Chem Commun. 2023;151:110490. doi:10.1016/j.inoche.2023.110490
9. Sankar AUR, Kiran Y, Mohan VM, Kumar AK, Varalakshmi MV. Green synthesis of cotton flower shaped nickel oxide nanoparticles: anti-bacterial and tribological studies. Inorg Chem Commun. 2023;157:111239. doi:10.1016/j.inoche.2023.111239
10. Mathekga K, Hintsho-Mbita NC. Synthesis of C. Benghanlensis NiO NPs for the degradation of malachite green and the removal of bacteria. Chem Physics Impact. 2023;7:100277. doi:10.1016/j.chphi.2023.100277
11. Baytar O, Ekinci A, Şahin Ö, Kutluay S. Green synthesis of NiO from watermelon seed shell extract for the evaluation of H2 production from NaBH4 hydrolysis and photocatalytic reduction of methylene blue. Mat Sci Eng. 2023;296:116704. doi:10.1016/j.mseb.2023.116704
12. Khudier MA, Hammadi HA, Atyia HT, et al. Antibacterial activity of green synthesized selenium nanoparticles using Vaccinium arctostaphylos (L.) fruit extract. Cogent Food Agric. 2023;9(1):2245612.
13. Alsohaimi IH, Alotaibi NF, Albarkani AM, et al. Photocatalytic wastewater treatment and disinfection using green ZnO-NP synthesized via cera alba extract. Alexandria Eng J. 2023;83:113–121. doi:10.1016/j.aej.2023.10.037
14. Naik J, David M. ROS mediated apoptosis and cell cycle arrest in human lung adenocarcinoma cell line by silver nanoparticles synthesized using Swietenia macrophylla seed extract. J Drug Delivery Sci Technol. 2023;80:104084. doi:10.1016/j.jddst.2022.104084
15. Abdelghany TM, Al-Rajhi AM, Yahya R, et al. Phytofabrication of zinc oxide nanoparticles with advanced characterization and its antioxidant, anticancer, and antimicrobial activity against pathogenic microorganisms. Biomass Convers Biorefin. 2023;13(1):417–430. doi:10.1007/s13399-022-03412-1
16. Soliman MK, Salem SS, Abu-Elghait M, Azab MS. Biosynthesis of silver and gold nanoparticles and their efficacy towards antibacterial, antibiofilm, cytotoxicity, and antioxidant activities. Appl Biochem Biotechnol. 2023;195(2):1158–1183. doi:10.1007/s12010-022-04199-7
17. Alqarni LS. Green creation of CoFe2O4 nanosorbent for superior toxic Cd ions elimination. Z Naturforschung A. 2023;2023:1.
18. Ahmad W, Bhatt SC, Verma M, Kumar V, Kim H. A review on current trends in the green synthesis of nickel oxide nanoparticles, characterizations, and their applications. Environ Nanotechnol Monit Manage. 2022;18:100674. doi:10.1016/j.enmm.2022.100674
19. Zeid EA, Nassar A, Hussein M, et al. Mixed oxides CuO-NiO fabricated for selective detection of 2-aminophenol by electrochemical approach. J Mater Res Technol. 2020;9(2):1457–1467. doi:10.1016/j.jmrt.2019.11.071
20. Iqbal J, Abbasi BA, Ahmad R, et al. Phytogenic synthesis of nickel oxide nanoparticles (NiO) using fresh leaves extract of Rhamnus triquetra (wall.) and investigation of its multiple in vitro biological potentials. Biomedicines. 2020;8(5):117. doi:10.3390/biomedicines8050117
21. Berhe MG, Gebreslassie YT. Biomedical applications of biosynthesized nickel oxide nanoparticles. Int j Nanomed. 2023;Volume 18:4229–4251. doi:10.2147/IJN.S410668
22. Talhaoui N, Taamalli A, Gómez-Caravaca AM, Fernández-Gutiérrez A, Segura-Carretero A. Phenolic compounds in olive leaves: analytical determination, biotic and abiotic influence, and health benefits. Food Res Int. 2015;77:92–108. doi:10.1016/j.foodres.2015.09.011
23. Yang L, Ma J, Hou C, et al. Evolution of phenotypic traits and main functional components in the fruit of ‘Chenggu-32’olives (Olea europaea L.) cultivated in longnan (China). Journal of Oleo Science. 2020;69(9):973–984. doi:10.5650/jos.ess19329
24. Žugčić T, Abdelkebir R, Alcantara C, et al. From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends Food Sci Technol. 2019;83:63–77. doi:10.1016/j.tifs.2018.11.005
25. Pasković I, Lukić I, Žurga P, et al. Temporal variation of phenolic and mineral composition in olive leaves is cultivar dependent. Plants. 2020;9(9):1099. doi:10.3390/plants9091099
26. Lama-Muñoz A, Del Mar Contreras M, Espínola F, Moya M, Romero I, Castro E. Content of phenolic compounds and mannitol in olive leaves extracts from six Spanish cultivars: extraction with the Soxhlet method and pressurized liquids. Food Chem. 2020;320:126626. doi:10.1016/j.foodchem.2020.126626
27. Alcántara C, Žugčić T, Abdelkebir R, et al. Effects of ultrasound-assisted extraction and solvent on the phenolic profile, bacterial growth, and anti-inflammatory/antioxidant activities of Mediterranean olive and fig leaves extracts. Molecules. 2020;25(7):1718. doi:10.3390/molecules25071718
28. Abdel-Shafy S, Allam NA, Mediannikov O, Parola P, Raoult D. Molecular detection of spotted fever group rickettsiae associated with ixodid ticks in Egypt. Vector-Borne Zoonotic Dis. 2012;12(5):346–359. doi:10.1089/vbz.2010.0241
29. Hassan MI, Gabr HS, Abdel-Shafy S, Hammad KM, Mokhtar MM. Molecular detection of Borrelia sp. in ornithodoros savignyi and Rhipicephalus annulatus by FlaB gene and babesia bigemina in R. annulatus by 18S rRNA gene. J Egyptian Soc Parasi. 2017;47(2):403–414. doi:10.21608/jesp.2017.77795
30. Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a one health perspective. Trends Parasitol. 2012;28(10):437–446. doi:10.1016/j.pt.2012.07.003
31. Guglielmone AA, Beati L, Barros-Battesti DM, et al. Ticks (Ixodidae) on humans in South America. Exp Appl Acarol. 2006;40(2):83–100. doi:10.1007/s10493-006-9027-0
32. Jabbar A, Abbas T, Z-u-D S, Saddiqi HA, Qamar MF, Gasser RB. Tick-borne diseases of bovines in Pakistan: major scope for future research and improved control. Parasites Vectors. 2015;8(1):1–13. doi:10.1186/s13071-015-0894-2
33. Faye B. Role, distribution and perspective of camel breeding in the third millennium economies. Emirates Journal of Food and Agriculture. 2015;27(4):318–327. doi:10.9755/ejfa.v27i4.19906
34. Faostat F Agriculture organization of the united nations statistics division. Economic and social development department, Rome, Italy Available from 2016. http://faostat3faoorg/home/E.
35. Faye B. The camel today: assets and potentials. Anthropozoologica. 2014;49(2):167–176. doi:10.5252/az2014n2a01
36. Alanazi AD, Nguyen VL, Alyousif MS, et al. Ticks and associated pathogens in camels (Camelus dromedarius) from Riyadh Province, Saudi Arabia. Parasites Vectors. 2020;13(1):1–9. doi:10.1186/s13071-020-3973-y
37. Massoud A, Kutkat M, El-Khateeb R, Labib I, Labib IM. Acaricidal efficacy of myrrh (Commiphora molmol) on the fowl tick argas persicus (Acari: argasidae). J Egyptian Soc Parasi. 2005;35(2):667–686.
38. Irache JM, Esparza I, Gamazo C, Agüeros M, Espuelas S. Nanomedicine: novel approaches in human and veterinary therapeutics. Vet Parasitol. 2011;180(1–2):47–71. doi:10.1016/j.vetpar.2011.05.028
39. Rai M, Ingle A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl Microbiol Biotechnol. 2012;94(2):287–293. doi:10.1007/s00253-012-3969-4
40. Sharmila G, Thirumarimurugan M, Muthukumaran C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem J. 2019;145:578–587. doi:10.1016/j.microc.2018.11.022
41. El SN, Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 2009;67(11):632–638. doi:10.1111/j.1753-4887.2009.00248.x
42. Benelli G, Beier JC. Current vector control challenges in the fight against malaria. Acta Trop. 2017;174:91–96. doi:10.1016/j.actatropica.2017.06.028
43. Minjas J, Sarda R. Laboratory observations on the toxicity of Swartzia madagascariensis (Leguminosae) extract to mosquito larvae. Trans Royal SocTropi Med Hyg. 1986;80(3):460–461. doi:10.1016/0035-9203(86)90345-7
44. Marimuthu S, Rahuman AA, Rajakumar G, et al. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol Res. 2011;108(6):1541–1549. doi:10.1007/s00436-010-2212-4
45. Leckie BM, Ownley BH, Pereira RM, Klingeman WE, Jones CJ, Gwinn KD. Mycelia and spent fermentation broth of beauveria bassiana incorporated into synthetic diets affect mortality, growth and development of larval helicoverpa zea (Lepidoptera: Noctuidae). Biocontrol Sci Technol. 2008;18(7):697–710. doi:10.1080/09583150802262906
46. Abdel-Ghany HS, Abdel-Shafy S, Abuowarda MM, El-Khateeb RM, Hoballah EM, Fahmy MM. Acaricidal efficacy of biosynthesized zinc oxide nanoparticles against hyalomma dromedarii (acari: Ixodidae) and their toxic effects on Swiss albino mice. Acta Parasi. 2022;67(2):878–891. doi:10.1007/s11686-022-00530-8
47. Meskini M, Esmaeili D, Ghorbani M, Alizadehgan M. Comparison of micro-dilution and macro-dilution methods for mic determination of plant extracts; 2017.
48. Lohrasbi S, Kouhbanani MAJ, Beheshtkhoo N, Ghasemi Y, Amani AM, Taghizadeh S. Green synthesis of iron nanoparticles using plantago major leaf extract and their application as a catalyst for the decolorization of azo dye. BioNanoScience. 2019;9:317–322. doi:10.1007/s12668-019-0596-x
49. Bonomo M. Synthesis and characterization of NiO nanostructures: a review. J Nanopart Res. 2018;20(8):1–26. doi:10.1007/s11051-018-4327-y
50. Kuang C, Zeng W, Ye H, Li Y. A novel approach for fabricating NiO hollow spheres for gas sensors. Physica E Low Dimens Syst Nanostruct. 2018;97:314–316. doi:10.1016/j.physe.2017.12.006
51. Khemakhem I, Abdelhedi O, Trigui I, Ayadi MA, Bouaziz M. Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int J Biol Macromol. 2018;106:425–432. doi:10.1016/j.ijbiomac.2017.08.037
52. Bashir A, Razanamahandry LC, Nwanya AC, et al. Biosynthesis of NiO nanoparticles for photodegradation of free cyanide solutions under ultraviolet light. J Phys Chem Solids. 2019;134:133–140. doi:10.1016/j.jpcs.2019.05.048
53. Kong B, Seog JH, Graham LM, Lee SB. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine. 2011;6(5):929–941. doi:10.2217/nnm.11.77
54. Abdel-Ghany HS, Abdel-Shafy S, Abuowarda MM, et al. In vitro acaricidal activity of green synthesized nickel oxide nanoparticles against the camel tick, Hyalomma dromedarii (Ixodidae), and its toxicity on Swiss albino mice. Exp. Appl. Acarol. 2021;83(4):611–633. doi:10.1007/s10493-021-00596-5
55. Foldbjerg R, Jiang X, Miclăuş T, Chen C, Autrup H, Beer C. Silver nanoparticles–wolves in sheep’s clothing? Toxic Res. 2015;4(3):563–575. doi:10.1039/C4TX00110A
56. Zaheer T, Ali MM, Abbas RZ, et al. Insights into nanopesticides for ticks: the superbugs of livestock. Oxid Med Cell Longev. 2022;2022:1–18. doi:10.1155/2022/7411481
57. Sager T, Wolfarth M, Keane M, Porter D, Castranova V, Holian A. Effects of nickel-oxide nanoparticle pre-exposure dispersion status on bioactivity in the mouse lung. Nanotoxicology. 2016;10(2):151–161. doi:10.3109/17435390.2015.1025883
58. Rice A, Rooney MT, Greenwood AI, Cotten ML, Wereszczynski J. Lipopolysaccharide simulations are sensitive to phosphate charge and ion parameterization. J Chem Theory Com. 2020;16(3):1806–1815. doi:10.1021/acs.jctc.9b00868
59. Dolati M, Tafvizi F, Salehipour M, Komeili Movahed T, Jafari P. Biogenic copper oxide nanoparticles from Bacillus coagulans induced reactive oxygen species generation and apoptotic and anti-metastatic activities in breast cancer cells. Sci Rep. 2023;13(1):3256. doi:10.1038/s41598-023-30436-y
60. Mickymaray S, Al Aboody MS, Eraqi MM, et al. Chitosan-encapsulated nickel oxide, tin dioxide, and farnesol nanoparticles: antimicrobial and anticancer properties in breast cancer cells. Int J Biol Macromol. 2023;248:125799. doi:10.1016/j.ijbiomac.2023.125799
61. Bhosale SR, Shinde SB, Bhosale RR, et al. Antibacterial efficacy of NiO composites with CuO nanoclusters via co-precipitation method. Inorg Chem Commun. 2023;155:111059. doi:10.1016/j.inoche.2023.111059
62. Ullah S, Akbar Jan F, Ullah N, Aziz T. Photocatalytic and therapeutic applications of the synthesized nickle oxide (NiO) nanoparticles. J Iran Chem Soc. 2023;6:1–13.
63. Sazgarnia A, Taheri AR, Soudmand S, Parizi AJ, Rajabi O, Darbandi MS. Antiparasitic effects of gold nanoparticles with microwave radiation on promastigotes and amastigotes of Leishmania major. Int j Hyperthermia. 2013;29(1):79–86. doi:10.3109/02656736.2012.758875
64. Saad AHA, Soliman MI, Azzam AM, Mostafa AB. Antiparasitic activity of silver and copper oxide nanoparticles against Entamoeba histolytica and Cryptosporidium parvum cysts. J Egyptian Soc Parasi. 2015;45(3):593–602. doi:10.12816/0017920
65. Nayak AP, Tiyaboonchai W, Patankar S, Madhusudhan B, Souto EB. Curcuminoids-loaded lipid nanoparticles: novel approach towards malaria treatment. Colloids Surf. 2010;81(1):263–273. doi:10.1016/j.colsurfb.2010.07.020
66. Al-Zaqri N, Umamakeshvari K, Mohana V, et al. Green synthesis of nickel oxide nanoparticles and its photocatalytic degradation and antibacterial activity. J Mater Sci Mater Electron. 2022;33:11864–11880.
67. Imran Din AGN M, Rani A, Aihetasham A, Mukhtar M, Mukhtar M. Single step green synthesis of stable nickel and nickel oxide nanoparticles from Calotropis gigantea: catalytic and antimicrobial potentials. Environ Nanotechnol Monit Manage. 2018;9:29–36. doi:10.1016/j.enmm.2017.11.005
68. Anwar S, Iqbal J. Green synthesis of nickel oxide nanoparticles from berberis balochistanica stem for investigating bioactivities. Molecules. 2021;26(6):1548. doi:10.3390/molecules26061548
69. Imran Din AR M, Rani A. Recent advances in the synthesis and stabilization of nickel and nickel oxide nanoparticles: a green adeptness”. Int j Analytical Chemi. 2016;2016(3512145):14. doi:10.1155/2016/3512145
70. Hafeez M, Shaheen R, Akram B, et al. Green synthesis of nickel oxide nanoparticles using populus ciliata leaves extract and their potential antibacterial applications. South Afr J Chem. 2021;75:168–173. doi:10.17159/0379-4350/2021/v75a21
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.