Back to Journals » Infection and Drug Resistance » Volume 15
Herpetic Keratitis Following Corneal Crosslinking for Keratoconus: A Case Series
Authors Wang L, Deng Y, Ma K, Yin H, Sun C, Tang J
Received 14 September 2022
Accepted for publication 2 November 2022
Published 9 November 2022 Volume 2022:15 Pages 6555—6562
DOI https://doi.org/10.2147/IDR.S389920
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Lixiang Wang, Yingping Deng, Ke Ma, Hongbo Yin, Chengshu Sun, Jing Tang
Department of Ophthalmology, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China
Correspondence: Jing Tang, Department of Ophthalmology, West China Hospital of Sichuan University, No.37, Guoxue Alley, Sichuan, People’s Republic of China, Tel +86 18980603835, Email [email protected]
Background: Corneal crosslinking is widely applied to enhance corneal biomechanical properties and delay the progression of keratoconus. The surgical procedure and application of ultraviolet A irradiation (UVA) during corneal crosslinking have been recognized to induce the reactivation of simplex herpes virus (HSV) but are rarely reported and poorly analyzed.
Case Presentation: We report the first case series of herpetic keratitis in 4 keratoconus patients undertaking corneal crosslinking, who were all clinically diagnosed at routine follow-up visits 3 days to 1 month after the surgery. Different from the typical new onset of secondary herpetic keratitis that mainly presents with epithelial lesions and severe eye pain, these patients all presented with stromal infiltrates and were generally asymptomatic except for vision blurring in 2 patients. All patients responded well to antiviral therapy, topical steroids, and epithelial nourishment medication, leaving corneal macula or nebula at the last follow-up visit.
Conclusion: Close follow-up is essential and the most effective way to diagnose herpetic keratitis after corneal crosslinking due to the lack of subjective symptoms. The prophylactic use of antiviral therapy on asymptomatic patients is controversial and should be evaluated based on long-term prognosis.
Keywords: simplex herpes virus, herpetic keratitis, keratoconus, corneal crosslinking, prophylactic antiviral therapy
Introduction
Corneal crosslinking has become a standard procedure to halt the progression of corneal thinning and bulging in keratoconus patients, which has been introduced in the recent decade. The application of corneal crosslinking is found to reduce the demand for corneal transplantation by 25% and prevent surgical complications and long-term reliance on immunosuppressive therapy of keratoplasty.1 Currently, many different techniques of corneal crosslinking have been explored and introduced, but generally, they all apply a photo-reactive dye called riboflavin, which forms covalent crosslinks by interacting with carbonyl groups of corneal stromal collagens under ultraviolet A (UVA) exposure for several minutes.2 To enhance the penetration of riboflavin across the cornea, the corneal epithelium is conventionally scrapped prior to the procedure. However, novel modified techniques such as transepithelial corneal crosslinking and accelerated corneal crosslinking can avoid the removal of the corneal epithelium.3 The procedure results in the strengthening of corneal collagen and its biomechanical property.4
The ocular surface is naturally inhabited by a microbiota system in healthy people.5 However, simplex herpes virus (HSV) −1 as a pathogenic virus primarily responsible for herpetic keratitis was found to be extremely prevalent in adults and over 90% of individuals over 40 years old were seropositive.6 The virus may reside in trigeminal nerve ganglions after primary infection for years before leading to secondary infection under suitable conditions, which is usually symptomatic and even destructive.7 In clinics, previous episodes of herpetic keratitis can be clinically diagnosed based on patients’ typical symptoms and signs. Serum test for anti-HSV antibodies can also provide useful information on past or present infections. Recently, novel molecular test methods including shotgun sequencing and metagenomics next-generation sequencing can provide both taxonomic information and higher sensitivity.8–10 However, due to the high prevalence of latent infection and the possibility of reactivation of the residing virus, negative results in diagnostic tests before surgery cannot rule out the onset of herpetic keratitis after surgery, which is challenging for corneal surgeons.
Reactivation of HSV after corneal crosslinking has been reported sporadically, which is considered a rare complication of the procedure.11–14 Since the introduction of corneal crosslinking surgery in June 2021 at our center, 4 cases of herpetic keratitis in keratoconus patients with no history of herpetic eye diseases have been diagnosed out of approximately 300 patients undertaking corneal crosslinking surgery, who had no typical symptoms of eye pain or even no subjective symptom of vision blurring due to low initial vision of keratoconus, who may easily get ignored if not followed properly.
We herein discuss the unmet clinical challenge of patient identification and management after corneal crosslinking and emphasize the need for prophylactic antiviral therapy in patients at risk.
Case Series
A total of 4 young patients (2 females and 2 males) undertaking corneal crosslinking surgery for the treatment of keratoconus at the ophthalmology department of West China Hospital from February to July 2022 were clinically diagnosed with new onset of herpetic keratitis after surgery. The age of patients ranged from 14 to 31 years old. Except for patient 3 who received subsequent corneal crosslinking surgeries on 2 eyes with a gap time of 1 week, other patients underwent surgeries only on the eye with more advanced keratoconus. The basic information, clinical history, and eye examination results before surgery are summarized in Table 1. All patients denied any history of previous herpetic eye diseases, ocular trauma, previous eye surgery, or other ocular or systemic diseases. All Patients presented with Vogt line, Fleischer ring, and Munson sign on slit lamp examination, which were typical signs of keratoconus. No signs of stromal scarring or clouding indicating past episodes of viral keratitis were observed. Preoperative serum antibody tests were positive for the anti-herpes simplex virus (HSV) I/II IgG, but negative for anti-HSV I/II IgM in patients 1, 2, and 4 (not available for patient 3). Tear secretion, tear breakup time, and corneal fluorescein staining were tested to rule out the comorbidity of dry eye disease, which all turned negative in these patients.
 |
Table 1 Patients’ Demographic and Clinical Information at the Initial Evaluation Before Corneal Crosslinking Surgery |
All crosslinking surgeries were conducted in the same environmental setting in a sterile operation room by the same surgeon following a consistent protocol. Patients were informed about surgical benefits and risks and signed written consents before surgery. Briefly, the surgical eye was anesthetized with topical proparacaine eye drops and manually held open with an eye speculum. Thirty percent ethanol solution was then applied to the central 9mm area for 20 seconds of the corneal surface and the corresponding corneal epithelium was debrided by a spatula. Riboflavin sodium phosphate solution (1%) was applied to the corneal surface for 10 minutes before crosslinking. Ultraviolet A irradiation (UVA) at the wavelength of 370±5 μm was applied to the central deepithelized area for 4 minutes at a power of 30 mW/cm2, with a total energy dose of 7.2 J. For patients 1 and 3 who had a thin cornea, a stromal lenticule extracted from patients undertaking small incisional lenticule extraction surgery (SMILL) was applied to the corneal surface and increased the overall corneal thickness before the application of riboflavin. After surgery, a corneal bandage contact lens was applied to the surgical eyes for 3~5 days. Topical antibiotics or steroids were prescribed as needed. The detailed surgical parameters and post-operational management of each patient are summarized in Table 2.
 |
Table 2 Summary of Surgical Details and Post-Operational Management |
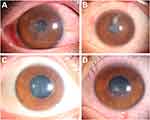
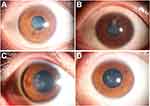
The 4 patients were clinically diagnosed with herpetic keratitis 3 days to 1 month after surgery based on their typical presentations of corneal stromal infiltrates with/without epithelial involvement and response to anti-viral therapy (Figure 1). Two of them (patient 1 and patient 2) had subjective symptoms of reduced visual acuity and the other 2 patients were asymptomatic at onset, who were all diagnosed at the routine follow-up visits. All 4 patients denied eye pain, photophobia, or foreign body sensation during the whole disease course. Full-dose topical ganciclovir and systemic acyclovir were given for at least 1 month until full remission of active keratitis. Topical steroids (prednisone, dexamethasone, or fluorometholone) were applied to relieve corneal stromal edema and reduce scarring and were tapered until full remission. In addition, deproteinized calf serum was used 4 times daily to promote reepithelization. All 4 patients responded well to the therapy and the corneal lesions turned under control after treatment. Except for patient 3 who had a sustained disease course of over 2 months, the other 3 patients recovered within 2 weeks. Nevertheless, all patients had stromal involvement and presented with corneal stromal nebula or macular at the last follow-up visit (Figure 2). The detailed clinical summary of these 4 patients are available in Table 3.
 |
Table 3 Summary of Clinical Manifestations of Patients Diagnosed with Herpetic Keratitis After Corneal Crosslinking (CXL) |
Discussion
Corneal crosslinking has been demonstrated to be an efficient therapy to enhance the corneal biomechanical strength and slow down the progression of keratoconus.15 However, corneal crosslinking surgery may be a potential trigger for the reactivation of the herpes simplex virus (HSV), which can lead to vision-threatening complications if not properly treated. In this study, we presented 4 cases of newly-onset herpetic keratitis on young keratoconus patients following corneal crosslinking surgery in a single eye center, who all denied any previous episodes of herpetic eye diseases. As reviewed in the literature, several cases of herpetic keratitis after corneal crosslinking had been reported, which is regarded as a very rare complication of corneal crosslinking surgery.11–14 However, our study as the first case series warns of the potentially higher incidence of HSV reactivation (4 cases out of 300 patients receiving corneal crosslinking surgeries during the period) that indicates higher attention on its prophylaxis and diagnosis. Currently, no consensus on the prevention and surveillance of herpetic keratitis after corneal crosslinking is available, which needs further study and discussion.
HSV belongs to the human herpes virus family. HSV type 1 is mainly associated with ocular and oral lesions, which is ubiquitous and leads to lifelong latency after primary infection. Epidemiological studies indicate that approximately 80% of adults are seropositive for HSV type 1 antibody.16 HSV primarily invades humans by close contact through tiny oral or ocular surface lesions, which usually occurs early in life and is generally asymptomatic. After the initial infection, HSV moves to dorsal root ganglions through sensory nerve endings and establishes lifelong latency.7 During the latency, intermittent shedding of virus DNA into tears and saliva has been noted.17 The secondary infection of HSV, which can present with either corneal epithelial, stromal, or endothelial involvement, tends to be relapsing and may cause permanent vision impairment due to stromal scarring or endothelial dysfunction in patients who respond poorly to antiviral therapy.7 As the 2nd leading cause of blindness in the developed world, herpetic keratitis was estimated to cause newly acquired vision impairment in 230,000 people worldwide in 2016.18 Previous studies have found several risk factors which may induce the reactivation of ocular HSV in humans and animal models, including eye surgery, ultraviolet exposure, latanoprost, topical steroids, and immunocompetent state. Therefore, patients with a history of herpetic eye diseases should be carefully evaluated before any eye surgeries, including corneal crosslinking. Patients with recent episodes of active herpetic keratitis are contraindicated for corneal crosslinking to prevent reactivation. However, due to the high prevalence of latent infection, patients denying a history of herpetic eye disease are also subject to viral reactivation, which tends to be a major challenge for clinical management. In addition, secondary herpetic keratitis can also be asymptomatic as the neurotrophic HSV is also the leading cause of neurotrophic keratitis, which adds difficulty to prompt diagnosis.19 In our case series, patients 1 and 2 got aware of the abnormal condition after noticing decreased vision after surgery. However, as patients with advanced keratoconus generally has a low visual acuity, the vision impairment associated with herpetic keratitis can be easily ignored, which can lead to advanced damage before diagnosis. Fortunately, all patients were promptly diagnosed and managed in the routine follow-up visits, leaving no permanent visual impairment. Above all, the challenge to recognize patients at risk of HSV reactivation and diagnose patients promptly indicates the need to closely follow up with the patients after corneal crosslinking as scheduled to prevent missing any cases.
HSV detection methods have been continuously explored to aid the prompt diagnosis of herpetic keratitis in clinics. Although herpetic keratitis can be basically diagnosed by its typical signs and symptoms, it can also be hard to differentiate from other common postoperative complications of corneal surgery in the beginning. Detection of HSV-1 in corneal scrapping or swabbing samples provides direct evidence of infection. Generally, the presence of virus at the lesion site can be detected by microscopy imaging, agglutination assay, Western blot assay, and different methods of polymerase chain reaction (PCR).20 In addition, systemic detection of viral antibodies can also assist diagnosis, which can employ different techniques including hemagglutination assay, Western blot assay, enzyme-linked immunosorbent assay (ELISA), fluorescence immunoassay, multiplexed flow immunoassay, and luciferase immunoprecipitation assay.20 Novel detection methods aimed to improve the sensitivity and provide more useful information including the taxonomic and functional profiles. Recently, metagenomics next-generation sequencing and shotgun sequencing have become emerging techniques that aid fast detection of HSV-1 at the lesion site.8–10
To reduce the risk of HSV reactivation, some authors have proposed the application of prophylactic antiviral therapy during the perioperative period of ocular surgeries such as laser corneal refractive surgery, keratoplasty, and corneal crosslinking. However, no consensus on the appropriate candidates, drug regimen, or time frame for antiviral therapy has been reached. Sound evidence of the efficacy of prophylactic antiviral therapy to prevent viral reactivation in patients with previous episodes of herpetic keratitis has been established.21 In addition, compared to topical acyclovir, systemic acyclovir therapy more efficiently reduced the recurrence of herpetic keratitis following penetrating keratoplasty and increased the survival rate of the corneal graft.22 For patients undergoing corneal refractive surgery who had previous episodes of herpetic keratitis, oral acyclovir 400 mg twice daily or valacyclovir 500mg daily during the perioperative period has been suggested to prevent the new onset of viral infection.23,24 But for patients with no history of herpetic keratitis, the prophylactic use of antiviral therapy is controversial. Chronic use of systemic acyclovir is associated with a higher incidence of adverse effects, including vomiting, nausea, and impairment of liver function, and the benefits and risks should be balanced.25 In addition, long-term use of prophylactic acyclovir leads to the selection of resistant viral isolates from the cornea and increases the difficulties of drug management of future recurrence.26 In our cases, patients all responded well to the initial anti-viral therapy and the infection generally subsided within several weeks. However, due to the short follow-up time, whether corneal crosslinking can lead to recurrent episodes of herpetic keratitis in these patients in the future remains unknown. Future studies may focus on the long-term prognosis of patients with newly-onset herpetic keratitis after surgery and provide evidence on the use of prophylactic antiviral therapy on these patients.
As far as we know, our study is the first case series of herpetic keratitis after corneal crosslinking surgery. Reactivation of HSV after corneal surgery is not that rare and needs close attention during the post-operational period. However, our study also has several limitations. First of all, due to the limited laboratory detection methods available, all cases of herpetic keratitis were diagnosed based on typical clinical signs and symptoms, which were also supported by patients’ responses to anti-viral therapy. Lack of confirmation tests by laboratory methods may not provide definite evidence of viral infection. Second, all patients were followed up for only several weeks or months and their long-term prognosis needs to be explored in the future due to the relapsing feature of herpetic keratitis.
Above all, our case series suggest that corneal crosslinking is a trigger for the reactivation of HSV, even in patients with no previous episodes of herpetic eye diseases. Close follow-up is essential and the most effective way to diagnose herpetic keratitis due to the lack of subjective symptoms. The prophylactic use of antiviral therapy on asymptomatic patients is controversial and should be evaluated based on long-term prognosis.
Data Sharing Statement
Data sharing does not apply to this article because no datasets were generated or analyzed during the current study.
Institutional Review Board Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of West China Hospital. A parent or legal guardian of the patients under 18 years of age provided informed consent for the case details and images to be published.
Informed Consent Statement
The patients whose case reports are given in this manuscript provided their written informed consent for publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Postdoctoral Research Funding of West China Hospital (No. 2020HXBH044), the Science & Technology Department of Sichuan Province (China) funding project (No. 2021YFS0221), and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. 2022HXFH032, ZYJC21058, 2021-023, 2022-014).
Disclosure
The authors declare no conflict of interest.
References
1. Godefrooij DA, Gans R, Imhof SM, et al. Nationwide reduction in the number of corneal transplantations for keratoconus following the implementation of cross-linking. Acta Ophthalmol. 2016;94(7):675–678. doi:10.1111/aos.13095
2. Subasinghe SK, Ogbuehi KC, Dias GJ. Current perspectives on corneal collagen crosslinking (CXL). Graefe’s Arch Clin Experiment Ophthalmol. 2018;256(8):1363–1384. doi:10.1007/s00417-018-3966-0
3. Angelo L, Gokul Boptom A, McGhee C, et al. Corneal crosslinking: present and future. Asia Pacific j Ophthalmol. 2022;11(5):441–452. doi:10.1097/APO.0000000000000557
4. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi:10.1016/S0002-9394(02)02220-1
5. Borroni D, Paytuví-Gallart A, Sanseverino W, et al. Exploring the healthy eye microbiota niche in a multicenter study. Int J Mol Sci. 2022;23(18):10229. doi:10.3390/ijms231810229
6. Chen CY, Shen JH, Huang YC. Seroepidemiology of Epstein-Barr virus and herpes simplex virus-1 in Taiwan. Int J Antimicrob Agents. 2013;42:S135. doi:10.1016/S0924-8579(13)70535-1
7. Toma HS, Murina AT, Areaux RG, et al. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008;23(4):249–273. doi:10.1080/08820530802111085
8. Parekh M, Romano V, Franch A, et al. Shotgun sequencing to determine corneal infection. Am J Ophthalmol Case Rep. 2020;19:100737. doi:10.1016/j.ajoc.2020.100737
9. Borroni D. Granulicatella adiacens as an unusual cause of microbial keratitis: a metagenomic approach. Ocul Immunol Inflamm. 2021;1–2. doi:10.1080/09273948.2021.1933066
10. Borroni D, Romano V, Kaye SB, et al. Metagenomics in ophthalmology: current findings and future prospectives. BMJ Open Ophthalmol. 2019;4(1):e000248. doi:10.1136/bmjophth-2018-000248
11. Kymionis GD, Portaliou DM, Bouzoukis DI, et al. Herpetic keratitis with iritis after corneal crosslinking with riboflavin and ultraviolet A for keratoconus. J Cataract Refract Surg. 2007;33(11):1982–1984. doi:10.1016/j.jcrs.2007.06.036
12. Sitaula S, Singh SK, Gurung A. Bilateral viral keratitis following corneal collagen crosslinking for progressive keratoconus. J Ophthalmic Inflamm Infect. 2019;9(1). doi:10.1186/s12348-019-0185-8
13. Al-Qarni A, Alharbi M. Herpetic keratitis after corneal collagen cross-linking with riboflavin and ultraviolet-A for keratoconus. Middle East Afr J Ophthalmol. 2015;22(3):389–392. doi:10.4103/0974-9233.159777
14. Yuksel N, Bilgihan K, Hondur AM. Herpetic keratitis after corneal collagen cross-linking with riboflavin and ultraviolet-A for progressive keratoconus. Int Ophthalmol. 2011;31(1):1–3. doi:10.1007/s10792-011-9489-x
15. Santodomingo-Rubido J, Carracedo G, Suzaki A, et al. Keratoconus: an updated review. Contact Lens Anterior Eye. 2022;45(3). doi:10.1016/j.clae.2021.101559
16. Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186(SUPPL.1):S3–S28. doi:10.1086/343739
17. Kaufman HE, Azcuy AM, Varnell ED, et al. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46(1):241–247. doi:10.1167/iovs.04-0614
18. McCormick I, James C, Welton NJ, et al. Incidence of herpes simplex virus keratitis and other ocular disease: global review and estimates. Ophthalmic Epidemiol. 2021;29(4):353–362. doi:10.1080/09286586.2021.1962919
19. Gabison EE, Saad S, Doan S, et al. Epidemiology of neurotrophic keratitis: prevalence, etiologies, outcomes and clinical management. Invest Ophthalmol Vis Sci. 2018;59(9):1800.
20. Nath P, Kabir MA, Doust SK, et al. Diagnosis of herpes simplex virus: laboratory and point-of-care techniques. Infect Dis Rep. 2021;13(2):518–539. doi:10.3390/idr13020049
21. Van Rooij J, Rijneveld WJ, Remeijer L, et al. Effect of oral Acyclovir after penetrating keratoplasty for herpetic keratitis: a placebo-controlled multicenter trial. Ophthalmology. 2003;110(10):1916–1919. doi:10.1016/S0161-6420(03)00798-X
22. Ghosh S, Jhanji V, Lamoureux E, et al. Acyclovir therapy in prevention of recurrent herpetic keratitis following penetrating keratoplasty. Am J Ophthalmol. 2008;145(2):198–202.e192. doi:10.1016/j.ajo.2007.10.005
23. De Rojas Silva MV, Díez-Feijóo E, Javaloy J, et al. Prophylactic perioperative antiviral therapy for LASIK in patients with inactive herpetic keratitis. J Refract Surg. 2006;22(4):404–406. doi:10.3928/1081-597x-20060401-19
24. Moshirfar M, Milner DC, Baker PA, et al. Corneal refractive surgery in patients with a history of herpes simplex keratitis: a narrative review. Clin Ophthalmol. 2020;14:3891–3901. doi:10.2147/OPTH.S282070
25. O’Brien JJ, Campoli-Richards DM. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1989;37(3):233–309. doi:10.2165/00003495-198937030-00002
26. Van Velzen M, Van De Vijver DAMC, Van Loenen FB, et al. Acyclovir prophylaxis predisposes to antiviral-resistant recurrent herpetic keratitis. J Infect Dis. 2013;208(9):1359–1365. doi:10.1093/infdis/jit350
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.