Back to Journals » OncoTargets and Therapy » Volume 16
HER2-Directed Therapy in Advanced Breast Cancer: Benefits and Risks
Authors Mandó P , Waisberg F , Pasquinelli R, Rivero S, Ostinelli A, Perazzo F
Received 23 November 2022
Accepted for publication 20 January 2023
Published 19 February 2023 Volume 2023:16 Pages 115—132
DOI https://doi.org/10.2147/OTT.S335934
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Pablo Mandó,1 Federico Waisberg,2 Rosario Pasquinelli,1 Sergio Rivero,2 Alexis Ostinelli,2 Florencia Perazzo1
1Clinical Oncology Department, Centro de Educación Médica e Investigaciones Clínicas “Norberto Quirno” (CEMIC), Ciudad Autónoma de Buenos Aires, Argentina; 2Clinical Oncology Department, Instituto Alexander Fleming, Ciudad Autónoma de Buenos Aires, Argentina
Correspondence: Pablo Mandó, Centro de Educación Médica e Investigaciones Clínicas “Norberto Quirno” (CEMIC), Galvan 4102, Ciudad Autónoma de Buenos Aires, 1431, Argentina, Tel +54 11 61204997, Email [email protected]
Abstract: Around 20% of breast cancers are associated with amplification or overexpression of human epidermal growth factor receptor 2 (HER2). In this setting, anti-HER2-targeted agents are the cornerstone of cancer therapeutic strategies. This includes monoclonal antibodies, tyrosine kinase inhibitors (TKIs) and, recently, antibody–drug conjugates (ADCs). With the advent of these new alternatives, the decision-making process has become more complex, especially with regard to the treatment sequence possibilities. In spite of the fact that overall survival has significantly improved accordingly, resistance to treatment remains a challenge in HER2-positive breast cancer. The introduction of new agents has created awareness regarding new potential specific adverse events, and consequently, their increasing application pose major challenges in daily patient care. This review describes the therapeutic landscape for HER2-positive advanced breast cancer (ABC) and evaluates its benefits and risks in the clinical setting.
Keywords: breast cancer, HER2, monoclonal antibodies, tyrosine kinase inhibitors, antibody–drug conjugates
Introduction
It has been reported that 15% to 20% of the invasive breast cancers (BCs) present overexpression of human epidermal growth factor receptor 2 (HER2) protein, a tyrosine kinase receptor belonging to the epidermal growth factor receptor family.1 HER2 amplification promotes cell proliferation and invasion. Therefore, HER2-positive BC has been traditionally related to elevated recurrence rates after local treatment and, generally, to worse prognosis.2
The treatment paradigm in HER2-positive advanced BC (ABC) has been increasingly transformed since the development of several additional HER2-targeted drugs. HER2-targeting represents the treatment cornerstone in this setting, including monoclonal antibodies, tyrosine kinase inhibitors and, recently, antibody–drug conjugates (ADCs). With the advent of these new alternatives, the decision-making process has become more complex, especially with regard to the treatment sequence possibilities available in each clinical context. Even though overall survival has significantly improved through the new anti-HER2 therapies, most patients ultimately succumb to progressive disease. Moreover, survival outcomes, especially in the real-world setting, for each treatment sequence are unknown.
Besides playing a vital role in tumor progression, HER2 receptors are also responsible for healthy cell evolution and the preservation of bodily systems. In consequence, its off-target actions may lead to specific adverse events. The broad spectrum of side effects linked with the expanding number of anti-HER2 therapies is not well characterized. Consequently, their increasing application and possible treatment combinations pose major challenges in daily patient care. This review describes the therapeutic landscape for HER2-positive ABC and evaluates its benefits and risks in the clinical setting.
Current HER2-Targeted Agents in Clinical Practice
Monoclonal Antibodies
Trastuzumab is a humanized recombinant monoclonal antibody (mAb) that binds with high affinity and specificity, to the juxtamembrane subdomain (IV) of HER2 receptor, resulting in receptor downregulation. Hence, trastuzumab inhibits cell proliferation in tumor models that overexpress HER2. In addition, trastuzumab has antitumor activity by antibody-dependent cellular cytotoxicity (ADCC).3
In 1998, US Food and Drug Administration (FDA) approved trastuzumab for ABC. It was first studied as a second-line treatment after initial disease progression with chemotherapy. The authors reported an objective response rate (ORR) of 15% in the total sample and a median duration survival of 13 months.4 In 2001, a Phase 3 randomized clinical trial evaluated its combination with chemotherapy, resulting in a prolonged median progression-free survival (PFS; 7.4 vs 4.6 months; p<0.001) and overall survival (OS; 25.1 vs 20.3 months; p=0.046) in the study arm that received trastuzumab.5 Since then, it has become the mainstay of treatment of HER2-positive BC.
Pertuzumab is a humanized IgG1 recombinant mAb that prevents HER2 heterodimerization by attaching to the extracellular subdomain II of HER2. HER2 signaling impairment leads to a low activity of MAP and PI3K kinase pathways, which may promote apoptosis.6 Pertuzumab is also associated with ADCC.7
The efficacy of pertuzumab was demonstrated in the Phase III randomized clinical trial CLEOPATRA, which evaluated pertuzumab or placebo added to trastuzumab and docetaxel as first-line treatment of HER2-positive ABC.8 Patients who received the experimental arm had a significantly increased OS and PFS, with medians of 57.1 and 18.7 months for the pertuzumab-based arm and 40.8 and 12.4 months for the control group, respectively. ORRs were 80.2% and 69.3%, with a significant improvement in the pertuzumab arm. A complete response rate was observed in 5.5% of the patients that were assigned to pertuzumab.9
Regulatory agencies, such as the European Medicines Agency (EMA) and the FDA, approved pertuzumab in the first-line advanced setting, including patients with de-novo metastatic presentation or disease relapse at least 12 months after completion of neoadjuvant or adjuvant HER2-based therapy.
Margetuximab is a mAb based on the murine antibody 4D5, the forerunner in which trastuzumab was developed.10 Margetuximab joins to the same subdomain as trastuzumab, with comparable affinity and anti-proliferative activity in vitro. Through engineering technology platforms, five amino acids were changed in the margetuximab IgG1 Fc domain, increasing the binding capacity by activating isoforms of FcγRIIIa and reducing its interplay with inhibitory FcγRIIb.11 It has been proposed, that this modification may enhance anti-HER2 immune response. The Phase III trial SOPHIA randomized 538 HER2-positive ABC patients and compared investigator-defined chemotherapy (capecitabine, eribulin, gemcitabine, or vinorelbine) in addition to trastuzumab or margetuximab every 3 weeks.12 All the included population had received prior trastuzumab and pertuzumab, and over 90% were previously treated with T-DM1. Margetuximab improved PFS over trastuzumab (Median PFS: 5.8 vs 4.9 months; HR=0.76, p=0.03). ORR was higher with margetuximab: 25.2% vs 13.7%. In a planned exploratory analysis by FcRIII genotype, treatment benefit was enriched in the population with FcγIIIa 158F allele. In this subgroup, a reduction of 32% was evidenced in disease progression. In addition, in this population, the ORR was numerically higher with margetuximab/chemotherapy in comparison to trastuzumab/chemotherapy, 22.1% vs 16.0% (p=0.06).
Antibody Drug Conjugates (ADCs)
Trastuzumab emtansine (T-DM1) is an ADC containing trastuzumab covalently bound to DM1, a microtubule polymerization inhibitor via a stable linker.13 When trastuzumab binds to HER2, it is introduced by endocytosis into the tumor cytosol, which promotes the release of the cytotoxic drug. As with other classical ADCs, this effect increases the therapeutic index of DM1 by minimizing its off-target effects.
In February 2013, FDA approved the use of T-DM1 for the second-line treatment of HER2-positive ABC, after prior treatment with trastuzumab and a taxane, given the results of the phase III trials TH3RESA and EMILIA.14,15
In the TH3RESA trial, 602 HER2-positive ABC patients, with at least two prior systemic treatments for this setting were randomized to T-DM1 or treatment of physician’s choice. A significant increase in OS was observed in the experimental arm (22.7 months vs 15.8 months; HR 0.68, p=0.0007).
On the other hand, EMILIA was a phase III open-label clinical trial which included a total of 991 patients, with only one prior systemic treatment for the metastatic setting, to evaluate T-DM1 against capecitabine plus lapatinib. The superiority of T-DM1 was reported with an improvement in OS of near 6 months (30.9 months vs 25.1 months; HR 0.68, p<0.001).
Trastuzumab–Deruxtecan (T-DXd) displays deruxtecan, a topoisomerase inhibitor, that conjugated with trastuzumab suffers internalization and further cleavage of the drug linker in the lysosomes, being consequently released in the cytosol.16 After accessing the nucleus, deruxtecan causes DNA damage, which leads to apoptosis. Besides, T-DXd has the added benefit of targeted delivery of the cytotoxic payload. When the payload is released due to it high membrane permeability, it diffuses and enters neighboring tumor cells. This bystander effect particularly distinguishes T-DXd from the previously approved T-DM1.
Accelerated approval of T-DXd was granted by the FDA for the population of HER2-positive ABC previously treated with at least two regimens with HER2-targeted agents, based on the DESTINY Breast02 study. This phase III randomized trial included patients with prior treatment with TDM-1. Patients received either T-DXd or physician’s choice of HER2-targeted therapy (trastuzumab/lapatinib + capecitabine).17
T-DXd was evaluated against T-DM1 in the phase III DESTINY-Breast03 randomized clinical trial with 524 HER2-positive ABC patients who had previously received trastuzumab and a taxane.18 The primary endpoint was PFS, with a clear benefit of T-DXd (HR 0.28, p=7.8 × 10−22). Median PFS, per investigator assessment, was improved by 17.9 months in the experimental arm (25.1 months vs 7.2 months; HR 0.26, p=6.5 × 10−24). Treatment benefit was observed in the specified subgroup analysis, including the subpopulation with brain involvement (HR 0.38). Results are still immature for OS.
Tyrosine Kinase Inhibitors (TKIs)
Lapatinib is a reversible, oral, small molecule that targets HER2 and the epidermal growth factor receptor (EGFR).19 It exerts its activity intracellularly blocking tyrosine kinase phosphorylation by ATP competition at the receptor binding domain. This impacts signal transduction activity with detrimental effects on cell survival and multiplication.
In March 2007, the FDA approved lapatinib combined with capecitabine in HER2-positive ABC for patients previously treated with systemic therapy, including anthracyclines, taxanes, and trastuzumab. This combination was approved after the publication of a multicenter, open-label, randomized clinical trial that compared lapatinib and capecitabine vs capecitabine alone.20 A total of 399 patients were selected for inclusion. Median PFS favored the experimental arm (27.1 weeks versus 18.6 weeks; HR 0.57, p=0.00013).
It was then studied in 2010 the role of lapatinib added to trastuzumab in patients with HER2 ABC who experienced disease progression on prior trastuzumab-containing regimens.21 A total of 296 patients were enrolled to randomly receive lapatinib added to trastuzumab versus lapatinib as a single therapy. The combination of lapatinib with trastuzumab supplied a significantly prolonged PFS (12 weeks vs 8.1 weeks; HR 0.73, p=0.008). ORR did not differ between treatment arms.
Neratinib binds to the tyrosine kinase domain of EGFR, HER2, and HER4, reducing its autophosphorylation capacity, and therefore blocking downstream signaling pathways.22 The impairment of RAS/RAF/MEK and PI3K/AKT/mTOR pathways leads to cell cycle arrest. Another proposed antineoplastic mechanism is the reversal of ATP transporters-mediated multidrug resistance mechanism, which may improve the cytotoxic effects of chemotherapy. This supports the rationale of combining neratinib with standard cytotoxic agents for patient treatment.
In 2020, FDA approved neratinib in ABC based on the NALA study, a randomized open-label, Phase III clinical trial.23 This study included 621 ABC HER2-positive patients previously treated with at least two HER2-targeted therapies. Neratinib combined with capecitabine was compared to lapatinib and capecitabine. A statistically significant improvement in PFS was evidenced (HR 0.76, p=0.0059), although no relevant differences in OS were observed.
Tucatinib is an orally bioavailable inhibitor of HER2 tyrosine kinase.24 It selectively binds to and inhibits HER2/3 phosphorylation, preventing HER2 signal transduction pathways activation, with the resultant growth inhibition and HER2-expressing tumor cell death. In contrast to other TKIs, it exerts minimal anti-EGFR activity.
In April 2020, FDA approved tucatinib in HER2-positive ABC, for patients who experienced disease progression to other chemotherapy regimens, after the publication of the HER2CLIMB study.25 This Phase 2 clinical trial randomized patients with HER2-positive ABC who had previously received trastuzumab, pertuzumab, and T-DM1to receive trastuzumab and capecitabine, in addition to either tucatinib or placebo. Patients with brain involvement were allowed for inclusion. Median PFS and OS were 7.8 months vs 5.6 months, and 21.9 months vs 17.4 months for the experimental and control arm, respectively. Among the patients with brain involvement at inclusion, PFS at 12 months was around 25% in the tucatinib-based arm group and 0% for the subgroup that received trastuzumab, capecitabine and placebo (Median PFS: 7.6 months vs 5.4 months; HR 0.48, p<0.001).
Pyrotinib is a small molecule that irreversibly inhibits EGFR, HER2, and HER4 by binding to the tyrosine kinase domain.26 It was evaluated in the open-label randomized phase 3 PHOEBE clinical trial. In this study, a total of 267 patients with HER2-positive ABC who had previously received trastuzumab combined with taxanes, and a maximum of two prior systemic therapy lines in the metastatic setting.27 Patients were randomized to treatment with capecitabine with either pyrotinib or lapatinib. PFS was significantly longer with pyrotinib plus capecitabine (12.5 months vs 6.8 months, HR 0.39; one-sided p<0.0001). An important limitation was that other standard HER2-targeted treatments, such as pertuzumab or T-DM1 were not approved in China during study enrollment. Consequently, this clinical trial is unable to evaluate the role of pyrotinib in patients after these therapies.
Main findings of pivotal trials for approved anti-HER2 therapies are summarized in Table 1.
 |
Table 1 Pivotal Trials in Advanced HER2-positive Disease |
Therapeutic Algorithms for HER2 ABC
Due to the dynamic and fast development of multiple anti-HER2 drugs, patients have increased their survival, but defining a one-size-fits-all therapeutic algorithm has become more complex. Therapeutic decisions should be tailored according to several factors, including previous treatments, disease evolution, and sites of metastases.
For patients with de-novo HER2-positive ABC or with disease relapse 12 months after completing HER2-based adjuvant treatment, the combination of pertuzumab and trastuzumab with a single-agent chemotherapy is usually the standard of choice following the CLEOPATRA study approach.
While this strategy remains the strongest option, several important points need to be approached. First, since then, other microtubule-targeted drugs, such as paclitaxel, nab-paclitaxel, or vinorelbine have been evaluated in this setting, with comparable results. All these agents represent currently accepted treatment alternatives.28,29
Second, around 90% of the included population in the CLEOPATRA trial had not undergone treatment with trastuzumab in the early disease setting.30 However, other studies support the efficacy of this regimen in patients who had already received prior anti-HER2 agents. In the randomized phase III PHEREXA trial, pertuzumab showed consistent activity added to a capecitabine and trastuzumab in patients who had already received another trastuzumab-based therapy in the advanced setting.31
In the CLEOPATRA trial, maintenance endocrine therapy was not permitted. For this reason, the role of hormone therapy in this setting is unclear. Nonetheless, it is reasonable to recommend maintenance with endocrine therapy, together with trastuzumab and pertuzumab after chemotherapy discontinuation if no disease progression has been evidenced.32
Finally, in patients with complete tumor response, there is no consensus regarding the optimal duration of treatment with pertuzumab and trastuzumab. Stopping anti-HER2 mAbs may be an attractive approach for selected patients. However, the possibility of treatment rechallenge reimbursement in case of further disease progression needs to be carefully evaluated.33
T-DM1 has been largely recognized as the standard second-line after the publication of the EMILIA trial.34 The recently presented DESTINYBreast-03 clinical trial resulted in a significant PFS improvement of T-DXd versus T-DM1, demonstrating the activity of this ADC in in this setting.18 Consequently, it is expected that T-DXd will become the standard second-line therapy in most countries.
The scenario after disease progression with T-DXd is particularly challenging, and no specific approach has been established in this setting. Optimizing treatment sequencing requires an understanding of mechanisms of resistance to this drug and cross-resistance patterns among ADCs, which still need to evolve in the next years. In the absence of such data, factors such as toxicity profiles, comorbidities, prior therapies, metastatic sites, drug availability, treatment costs are key elements of the decision-making process.
Drugs with anti-HER2 activity should always be considered throughout the entire disease course. Different alternatives, including trastuzumab, ADCs and tyrosine kinase inhibitors have been explored in pretreated patients. Particularly, the scenario of disease progression after TDM-1, trastuzumab and pertuzumab was the target setting of the phase 2 HER2CLIMB trial. The OS improvement in the subgroup that received tucatinib-based therapy supports this strategy as an attractive third-line approach, especially if T-DXd is not available.
Other studies evaluated the role of HER2-directed therapies after at least two systemic treatment lines, including the phase 3 TH3RESA clinical trial. T-DM1 was associated with OS improvement in patients who had received at least two anti-HER directed agents for advanced disease. A second open-label Phase 3 clinical trial, PRECIOUS, revealed restrained effectivity from rechallenge of pertuzumab, trastuzumab added to chemotherapy in patients that had already been exposed to this mAbs.35 Other novel directed agents combined with chemotherapy were evaluated in the SOPHIA and NALA clinical trials.12,23 Although PFS was improved in both trials, none of them were associated with OS benefit in their final analysis.
Lately, another ADC, [vic]trastuzumab duocarmazine was compared to investigator’s treatment choice, in the third-line setting or after T-DM1 in the Phase III SYD985.002/TULIP clinical trial.36 This interesting design gave more insights to analyze the effectiveness of ADCs in patients who already received another ADC. In this trial, an improvement of PFS was observed favoring the experimental arm (Median PFS was 7.0 months vs 4.9 months; HR 0.64, p=0.002). At the time of the presentation, a trend towards benefit in OS was observed in the [vic]trastuzumab arm, although it did not achieve statistical significance (HR 0.83, 95% CI 0.62–1.09, p=0.153).
A definitive treatment sequence after second-line treatment cannot be established based on direct evidence. Standard recommendations in this setting include chemotherapy combined with neratinib, lapatinib, trastuzumab or margetuximab. However, T-DXd, T-DM1, or the combination of tucatinib, trastuzumab and capecitabine represent more effective strategies, if they were not used in previous lines.
The preferred treatment option for patients who present disease relapse during adjuvant treatment or within 6 months of its finalization is T-DXd. This recommendation is derived from results of the DESTINYBreast-03 trial.18 Similarly, if the adjuvant treatment regimen included pertuzumab, in patients with disease relapse between 6 and 12 months after adjuvant treatment discontinuation, T-DXd is the treatment of choice. Although both populations were specifically included in DESTINYBreast-03, different studies also support a possible role of the combination of pertuzumab, trastuzumab and a taxane in this setting. Interestingly, a retrospective real-world cohort of patients that experienced early disease relapse evidenced an increase of PFS and OS in patients treated with pertuzumab, trastuzumab and a taxane compared to T-DM1, including the subgroups less than 6 months or between 6 and 12 months of recurrence-free survival.37
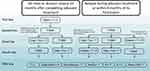
More data are needed in the subgroup of patients with early disease relapse for decision-making. In the patient subgroup with relapse after 6–12 months following exposure to single HER2 blockage (T-DM1 or trastuzumab alone), we endorse THP as a first-line treatment alternative. A proposed clinical algorithm for treatment decisions inHER2-positive ABC is presented in Figure 1.
Specific Clinical Situation: HER2-positive ABC with Brain Metastases (BM)
In HER2-positive early breast cancer (EBC) patients, BM 10-year risk is approximately 12%,38 and in the advanced setting, 50% of the patients develop BM during their disease.39 In the HER2-positive ABC scenario, observational studies like registHER40 and SystHER41 have evidenced a cumulative incidence of BM of 37.3% and 30.6%, respectively, which was associated with worse OS. Furthermore, brain involvement has been classically considered as an exclusion criterion in clinical trials, especially in the subgroup with symptomatic lesions or progressive disease after local therapy.42 Nowadays, the development of new drugs with greater penetrance in CNS has allowed for improving antitumor activity in patients with brain involvement. The growing efficacy of this new drugs should never exclude the important role of local treatments with modern radiation techniques, as stereotactic radiosurgery, or surgery, especially for certain subgroup of patients as those with oligometastatic disease.
In patients with CNS progressive disease and extracranial disease control, guidelines prioritize optimizing local treatment of BM and recommend the continuation of the previous systemic therapy.43
Regarding systemic treatment, initial experiences included the evaluation of TKIs, because of their low molecular mass and their predicted ability to penetrate the blood–brain barrier (BBB). Nevertheless, BBB is typically replaced due to metastatic involvement, by poorly cohesive endothelial cells, associated with higher penetrability for larger drugs.44 Importantly, contrasting information has been obtained regarding anti-HER2 agents in this setting, which may lead to specific treatment decisions for this patient subgroup.
Trastuzumab
Adjuvant trastuzumab has been associated with a higher incidence of BM involvement as the first recurrence site.45 This finding has been explained by the low CNS penetration of this agent. Nevertheless, in registHER trial, treatment with trastuzumab was associated with a median of 6.5 months of higher time to diagnosis of CNS metastases among patients without a history of CNS involvement. Authors also reported an improvement of OS after BM diagnosis (17.5 versus 3.7 months).40 Similarly, in a retrospective cohort reported by Dawood and coauthors, trastuzumab was associated with a more delayed BM occurrence (HR 2.13, p<0.001).46 In summary, trastuzumab has changed HER2-positive natural evolution improving extracranial control and delaying CNS metastatic progression.
Pertuzumab
In the CLEOPATRA trial, patients with BM were excluded.9 Importantly, pertuzumab was not associated with lower BM incidence, although a more prolonged time to the occurrence of CNS metastases was evidenced (15 vs 11.9 months, p=0.005).47 In the single-arm Phase II PATRICIA clinical trial, a cohort of 40 patients with progressive BM after local radiotherapy, received pertuzumab 840 mg and then 420 mg every 3 weeks plus a higher-dose regimen of trastuzumab, which was administrated 6 mg/kg weekly.48 Intracranial benefit rate (CBR) at 6 months was 51%, and total ORR was 11%. Adequate maximum and mean observed through concentrations were evidenced with high-dose trastuzumab in the steady state. In this trial, the authors concluded that pertuzumab added to high-dose trastuzumab was associated with clinical utility in patients with HER2-positive ABC and CNS disease progression.
T-DM1
T-DM1 has been evaluated in this population in the phase IIIb KAMILLA study.49 A total of 398 patients with prior CNS involvement history were included in the trial. ORR was 21.4% among 126 participants with measurable disease. Median times of obtained PFS and OS were 5.5 and 18.9 months, respectively. Noteworthily, a median interval to treatment failure of 8.8 months was evidenced in patients that continued with T-DM1 after initial evidence of new brain lesions. Furthermore, a cohort of 95 patients who had previously received local treatment for CNS metastases was enrolled in the EMILIA trial. Among this subgroup, no difference in PFS or intracranial progression rate was evidenced.14,34 In conclusion, T-DM1 is associated with intracranial activity, with similar ORR and CBR to TKI and chemotherapy combination.
Lapatinib
Lapatinib given as a single agent was associated with limited intracranial efficacy in patients with progressing CNS lesions in the EGF105804 clinical trial.50 Tellingly, 20% of the 50 patients who received capecitabine in addition to lapatinib, after disease progression with lapatinib in this trial, achieved partial tumor response. Comparatively, Blackwell et al reported in a small randomized clinical trial that lapatinib and trastuzumab were associated with a numerically lower incidence of BM disease progression compared to trastuzumab.21 Lapatinib plus capecitabine was associated with partial response in 29 (66%) out of 45 patients with CNS involvement and no previous treatment, in the single-arm LANDSCAPE trial.51 Petrelli et al reported ORR in 29.2% of the patients with treated CNS metastases with this combination.52 It should be concluded that lapatinib is associated with clinical activity in patients with BM and ABC when given in combination with other therapies, such as capecitabine. This approach could be of benefit to patients with untreated CNS involvement.
Neratinib
Neratinib was evaluated in HER2-positive ABC patients, with BM in the Phase II TBCRC 022 trial.53 The cohort assigned to the combination of neratinib and capecitabine showed an ORR of 49%, including an ORR of 33% among the subpopulation previously exposed to lapatinib. In the phase III NALA trial, a demonstrated intracranial activity was evidenced in the arm that received capecitabine and neratinib, with a significantly lower cumulative incidence of BM, 22% versus 29.2%, and fewer requirements for symptomatic CNS metastases intervention.23 Under these circumstances, the combination of capecitabine and neratinib is an attractive approach in patients with CNS involvement.
Tucatinib
In the pivotal HER2CLIMB clinical trial, a subgroup of 291 patients with stable or active BM was included for randomization.54 One year-PFS was 24.9% and 0% for the experimental and control arm, respectively (HR 0.48, p<0.001), with a median estimated PFS of 7.6 months vs 5.4 months. Importantly, intracranial PFS and ORR were increased in the tucatinib arm (PFS: 9.9 months vs 4.2 months; HR 0.32, p<0.0001; ORR: 47.3% vs 20.0%; p=0.03). Importantly, in this typically excluded patient subpopulation, a statistically significant increase in OS was evidenced (18.1 vs 12.0 months; HR 0.58, p=0.005).54 Moreover, as allowed per protocol, 30 patients continued treatment beyond progression and in that patient subgroup, median time to second disease progression or death was prolonged favoring the tucatinib arm (15.9 months vs 9.7 months; HR 0.33). The activity of this modern TKI was also evidenced added to T-DM1 or trastuzumab in Phase I studies.55,56 Tucatinib became the first small agent associated with intracranial tumor response in patients with untreated HER2-positive ABC in a phase 3 clinical trial, favoring the consideration of this TKI in patients of this subgroup.
Pyrotinib
Pyrotinib was evaluated added to capecitabine in patients with HER2-positive ABC and BM in the single-arm, Phase 2, PERMEATE trial.57 Intracranial ORR was 74.6% in untreated patients and 42.1% in patients that experienced disease progression after local radiotherapy. Although response rates are extremely encouraging, it is important to highlight that unlike HER2CLIMB study, these patients were not previously treated with pertuzumab or T-DM1.
T-DXd
A cohort of HER2-positive ABC patients with BM, and prior local treatment were included in the Phase 2 registration DESTINY Breast 01 clinical trial.58 Fourteen out of the 24 included patients (58.3%) had tumor response, and the median PFS in this subgroup was 18.1 months. Importantly, intracranial disease progression was only evidenced in 2 patients (8.3%). Parallelly, in the DESTINY-Breast 03 study, among the subgroup of patients with treated BM, intracranial ORR was 63.9%.59
Data coming from small cohorts of phase 2 trials strengthen the role of T-DXd for BM treatment. The DEBBRAH trial evaluated T-DXd activity in a subgroup of patients with ABC and BM.60 ORR was 80% in the subgroup with disease control after local treatment, and 50% in patients with asymptomatic untreated CNS involvement. Additionally, 66.7% of the patients with disease progression after local therapy experienced tumor response.
Moreover, the TUXEDO-1 cohort recruited 15 patients with active BM, and evidenced a total ORR of 73.3%, including 13.3% with complete response, 60% cases of partial response, and 20% with disease stabilization.61
Under this evidence, T-DXd became an attractive option in this setting, although more robust evidence is needed. The single-arm, Phase 3b/4 DESTINY-Breast12 (NCT04739761) will include patients with and without BM to assess the efficacy of T-DXd in different patient subgroups.
Margetuximab
Although patients with BM and stable lesions after local treatment could be included in the SOPHIA trial, no specific CNS-related endpoints were prospectively evaluated.12 The group assigned to margetuximab was associated with demonstrated improved PFS, although clinical relevance remains controversial. No BM-related specific endpoints have been reported so far. More information is needed to determine the clinical activity of margetuximab in this setting.
The management of HER2-positive ABC with CNS involvement is challenging and requires a multidisciplinary team. Considering that HER2CLIMB was the only randomized trial that included patients with active CNS metastases and that survival benefit was observed in this subpopulation, the combination of tucatinib, capecitabine and trastuzumab is a preferred strategy after disease progression on trastuzumab, pertuzumab, and a taxane.
When possible, local treatment should always be considered, especially in symptomatic patients. In this situation, prior systemic treatment may be continued when there is adequate extracranial disease control. This treatment sequence is subject to constant modifications. The high ORR observed in patients who received modern ADCs, may lead to the consideration of postponing local treatment for CNS control in even a larger group of patients with BM.
Toxicities Associated with the Treatment and Patient-Reported Outcomes (PROs)
HER2-directed therapies have been available for its use for more than 20 years. Although their safety profile can be characterized as predictable and manageable, knowing their adverse effects thoroughly allow practitioners to prevent and diagnose adequately specific drug-related toxicities. A summary of the most frequent toxicities related to each drug is presented in Figure 2.
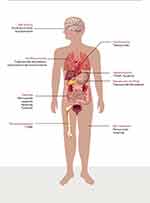 |
Figure 2 Main adverse events of anti-HER2 drugs according to compromised system. |
Cardiotoxicity
Congestive heart failure (CHF) is an important adverse reaction to trastuzumab. Common signs and symptoms may include dyspnea, orthopnea, cough, pulmonary edema, and the decrease of left ventricular ejection fraction (LVEF).62 In a pooled analysis of adjuvant trastuzumab trials, in which the vast majority (97.4%) of included patients had received anthracycline-based chemotherapy, 452 patients (11.3%) had a cardiovascular adverse event. The most common toxicity, evidenced in 8.7% of the total included population, was asymptomatic or mildly symptomatic LVEF decrease.63 Severe CHF was relatively more observed in patients who received trastuzumab (2.3 vs 0.8%). Cardiovascular toxicity was the leading cause of treatment discontinuation (10.0%), and the majority of cardiac events were evidenced during the treatment period (78.1%). Authors highlighted that a number of baseline risk factors were associated with a higher incidence of cardiac events, including age equal to or higher than 60, body mass index higher than 25, non-Caucasian ethnicity, prior history of hypertension, and initial LVEF below 60%.
LVEF decrease has been characterized as reversible and its incidence remains low in patients that were not previously or concomitantly treated with anthracyclines.64 Concomitantly, low cardiac adverse event rates were observed when pertuzumab was added to trastuzumab, as well as in clinical trials that evaluated margetuximab, T-DM1, tucatinib, and T-DXd.65–67 Specifically, no cardiac events were considered related to tucatinib in the HER2CLIMB trial and only asymptomatic LVEF decrease was observed in 2.3% of the patients who received T-DXd in the DESTINY-Breast03 clinical trial.18,67
Current guidelines recommend LVEF monitoring with a baseline echocardiogram every 3 months during treatment.65 According to the 2022 European Society of Cardiology Guidelines on Cardio-Oncology, a multidisciplinary team should guide clinical decisions in this setting.66 Drug interruption is indicated in cases of severe cardiac dysfunction (LVEF <40%), even in asymptomatic patients. While moderate symptomatic dysfunction cases should be managed with treatment interruption, in patients only presenting mild symptomatic dysfunction, treatment continuity should be discussed within a multidisciplinary team. Cardioprotective therapy, including ARB/ACE-I and/or beta-blockers, together with frequent cardiac monitoring, are recommended in patients with asymptomatic moderate or mild cardiac dysfunction. In this scenario, HER2-targeted therapy might be continued. Moderate dysfunction corresponds to cases with LVEF between 40% and 49%, and mild dysfunction is defined by a significant reduction of the global longitudinal strain or the increase in cardiac biomarkers, in patients with LVEF equal to or higher than 50%.
Nevertheless, a LVEF dysfunction history is not necessarily an exclusion criterion to considerate treatment with trastuzumab in patients with ABC. The SAFE-HEaRt trial evaluated the safety of HER2-targeted agents in patients with reduced LVEF, after initiating cardioprotective therapy and frequent cardiac monitoring visits.67 At 3.5-year follow-up, there was only one new cardiac event, no cardiac deaths, and 89% of the patients with metastatic disease remained on therapy beyond 1 year. There was an improvement in overall mean LVEF, including in those patients who were maintained on HER2-targeted therapies and in patients with prior anthracycline use. This observed improvement in LVEF is consistent with the effect of cardioprotective agents in addition to lifestyle modifications.
The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines strengthens that lifestyle modifications contribute to decreasing the risk of cardiac events during and after trastuzumab therapy.
Diarrhea
Diarrhea was evidenced in 68.4% of ABC patients who received pertuzumab. Most events occurred during the first treatment cycles and were categorized as mild or moderate. Pivotal trials reported an incidence of grade 3–4 diarrhea in 9.3% of the patients who received pertuzumab,9 with an adequate response to anti-diarrhea agents.
Diarrhea is also a common adverse event of all anti-HER2 TKI. Its incidence has been reported in around 60% of the patients treated with lapatinib combined with a second agent, including letrozole, capecitabine, or trastuzumab.20,21,68 Similarly, most reported cases were grade 1 or 2 and responded adequately with regular antidiarrheals, such as loperamide.
In the HER2CLIMB trial,25 diarrhea was evidenced in 80% of the patients who received tucatinib. Nonetheless, most events were self-limiting and median duration was 8 days. Prophylactic antidiarrheal agents are not recommended in this setting.
Around 83% of the patients who received neratinib in the NALA trial experienced one or more episodes of diarrhea.23 Grade 3 diarrhea was reported in 24.4% of the patients, with 83.6% of patients reporting this toxicity in the first week. It was the leading cause of therapy discontinuation in this clinical trial. Common recommendations for its management include neratinib dose escalation (Week 1: 120 mg daily, Week 2: 160 mg daily; Week 3 and thereafter: 240 mg per day) or loperamide prophylaxis. Current recommendations suggest that during the first 2 weeks loperamide dose should be 4 mg three times a day and, 4 mg twice a day for weeks 3–8, and as needed from week 9 onwards. A maximum dose of 16 mg per day should not be exceeded.
In PHOEBE trial of pyrotinib, 95% of the patients presented with diarrhea, of which 31% were grade 3. Importantly, grade 3 diarrhea was mostly observed in the first treatment cycle.
Diarrhea is a common adverse event in HER2-directed therapies and its occurrence might also be aggravated by the chemotherapy backbone regimens used in HER2-positive ABC. For this reason, early intervention and patient education are necessary elements to preserve the quality of life during treatment.
Nausea and Vomiting
In the DESTINY-Breast 03 trial, 76% of the patients reported nausea and 49% vomiting.18 Nevertheless, most nausea and vomiting events were grade 1 or 2. Only 6.6% and 1.6% of the included patients presented grade 3 nausea and vomiting, respectively. The incidence of these adverse events was highest in the first treatment cycle. NCCN Guidelines® for Antiemesis considers that TDXd is associated with moderate emetic risk and recommends several prophylactic antiemetic regimens to help decrease potential vomiting, including 5HT inhibitors and corticosteroids.69
Skin Reactions
Rash is a common adverse event in most clinical trials of pertuzumab and is generally of low-grade.70 Common presentations include papulopustular or acneiform lesions. In this setting, common recommendations for prophylaxis include the use of sunblocks of at least SPF 15 and frequent skin moisturization. Tetracyclines, including minocycline or doxycycline, and topical corticosteroids are indicated for the treatment of grade 2 or 3 rash.70 Hand-foot syndrome was commonly observed in patients treated with tucatinib, being described in 65% of the cases, of which 14% were grade ≥3.25
Thrombocytopenia
A decreased platelet count has been reported in 28% of the patients in ABC clinical trials with TDM-1, being the most common toxicity leading to drug discontinuation (2.6%).14 Dose reduction is the recommended strategy.
Hepatotoxicity
T-DM1 is associated with transaminase elevations, and guidelines suggest therapy continuation without dose delay or reduction for grade 1–2 events.71 Drug interruption should be indicated for grade 3 AST or ALT elevation, and treatment might be reinstalled at a lower dose after recovery to grade 2 or less. In grade 4 AST or ALT elevation (more than 20 times upper limit of normal) developed at any time during treatment, T-DM1 should be permanently discontinued, regardless of the treatment setting.
Nine percent of the patients treated with tucatinib required dose reduction due to ALT, AST or bilirubin elevations.25 Consequently, regular liver function tests should be offered in patients undergoing treatment with T-DM1 or tucatinib.
Interstitial Lung Disease (ILD) or Pneumonitis
T-DXd was evaluated in clinical studies that enrolled patients with different tumor models. In this population, the overall reported incidence of drug-related ILD or pneumonitis reached 15.4%. Alarmingly, 2.2% of these cases were fatal.72
Most ILD/pneumonitis events (77.4%) were categorized as grade 1 or 2 and were reported during the first year of treatment. Authors reported that risk factors for ILD or pneumonitis included age >65 years, baseline oxygen saturation lower than 95%, kidney function impairment, concomitant respiratory comorbidities dose over 6.4 mg/kg and a time after the first diagnosis higher than 4 years. Regular monitoring, which may include periodic chest computed tomography scans and early intervention with steroids are important measures to improve outcomes. It is important to find and study the potential contributors and rule out other potential causes of ILD/pneumonitis. Involving a respiratory team for ILD/pneumonitis in patients under treatment with T-DXd is recommended, as it was done in the trials taskforce.
Hypersensitivity Reactions
This common therapeutic adverse event commonly presents with dyspnea, bronchospasm, tachycardia, and oxygen saturation decrease. Other symptoms may include headache, nausea, and rash. Symptom management is recommended with antihistamines and/or steroids.
Eye Toxicity
EGFR pathway is associated with corneal and conjunctival cell migration and proliferation. Due to the higher expression of HER2 on superficial cells, HER2-directed therapy is associated with alterations in the turnover process of both conjunctival and corneal epithelial cells. Therefore, common adverse events from HER2-directed drugs include conjunctivitis, keratitis, epiphora, dry-eye syndrome, and blurred vision, which may be evidenced in around 5% of the patients.73
Importantly, conjugated antibodies may have an increased risk of ocular toxicity, given the potential alteration caused by the cytotoxic payload. Particularly, dry eye syndrome was reported in 11.4% of patients who received T-DXd in the DESTINY-Breast 01 trial.58 Notably, 78.1% and 21.3% of all grade and grade 3 ocular adverse events were described with trastuzumab duocarmazine, including conjunctivitis and dry-eye syndrome. Ocular adverse events led to drug discontinuation in 20.8% of the included population, and dose reduction in 22.9% of the patients who received this therapy.74 Eye toxicity was evidenced even though different risk mitigation recommendations were performed in the trial, including lubricating eye drops and regular ocular examinations.
Ocular toxicity is a potential adverse event of HER2-directed therapy that may affect quality of life. Prompt referral to an ophthalmologist and therapies including eye lubricants, artificial tears, and topical serum may be recommended in accordance with the specific diagnosis. Dose reduction and drug discontinuation may be required for toxicity resolution.
Patient-Reported Outcomes (PROs)
The necessity of addressing PROs as an essential endpoint of clinical trials has been increasingly emphasized by international societies and guidelines.75 QoL questionnaires have been the most common tool implemented in decision-changing studies to evaluate PROs. Specifically, the Functional Assessment of Cancer Therapy-Breast (FACT-B) has been one of the most widely administered questionnaires in HER2 ABC clinical trials.
While in many clinical trials no significant differences have been observed regarding QoL, in some cases, a statistically significant delay in clinically meaningful symptom worsening has been evidenced.76
Patients who received pertuzumab in the CLEOPATRA trial were associated with a median of 8.4 weeks more time without clinical deterioration than the placebo arm.76 In the EMILIA trial, T-DM1 treatment, compared to capecitabine plus lapatinib, delayed clinically meaningful symptom worsening (7.1 months versus 4.6 months).77
Recent presentations have addressed upcoming information regarding PROs in new agents. For T-DXd, an association with better emotional functioning and pain management has been recently presented considering the results of the DESTINY-Breast 03 trial.78 In the HER2CLIMB clinical trial, the risk of clinical deterioration according to the visual analog scale was significantly lower in patients with BM that received tucatinib. Importantly, PROs according to the FACT-B scale have been identified as an independent prognostic factor for OS and PFS, considering a pooled analysis of some of the largest HER2-positive ABC clinical trials, such as CLEOPATRA, EMILIA, and MARIANNE.75
Nevertheless, it should be stated that HER2-positive ABC population represents a particular subgroup of breast cancer patients. Disease evolution, the high incidence of CNS involvement, and particular targeted therapy-related toxicities support the necessity of implementing more specific tools to evaluate PROs. An attractive example is the PRO-CTCAE tool,79 which can be tailored according to expected drug-related adverse events, and disease characteristics. More efforts should be made to address patient heterogeneity at the moment of evaluating clinical trial outcomes.80
Selected Ongoing Trials and Future Perspectives
HER2 targeted therapy is currently being evaluated in different treatment settings and patient subgroups. Modern approaches have been proposed since the emerging effectiveness of ADCs in the advanced setting.
New Settings for Approved Targets
In HER2-positive ABC, T-DXd is currently being explored for the first-line setting in the Destiny-Breast 09 trial (NCT04784715). In this potentially practice-changing study, T-DXd will be combined with or without pertuzumab and compared to trastuzumab, pertuzumab, and docetaxel. The Phase Ib/II clinical trial Destiny-Breast 07 (NCT04538742) is also exploring the combination of T-DXd with other agents for second or later treatment lines in the advanced setting. Drugs that will be partnered with T-DXd include durvalumab, paclitaxel, pertuzumab, tucatinib, and the combination of durvalumab and paclitaxel. The combination of tucatinib and T-DXd is also being studied in the Phase II HER2CLIMB-04 clinical trial (NCT04539938). Moreover, the addition of pembrolizumab to this ADC is also being evaluated (NCT04042701).
Of note, for the HER2-low and ultralow (>0 and <1+) subgroups the Phase III randomized Destiny-Breast 06 clinical trial is comparing T-DXd versus chemotherapy in patients with HR+/HER2- ABC after prior endocrine therapy (NCT04494425).
Tucatinib is also being evaluated in the first-line setting. The Phase III placebo-controlled HER2CLIMB-05 clinical trial is recruiting ABC HER2-positive patients that have completed 4 to 8 cycles of trastuzumab, pertuzumab, and a taxane (NCT05132582). Importantly, patients with asymptomatic untreated brain metastases are allowed for inclusion. For the second-line setting, tucatinib is also being compared to placebo, in addition to TDM-1 in the randomized Phase III HER2CLIMB-02 clinical trial (NCT03975647). Unfortunately, patients that have been previously exposed to T-DXd are excluded from participation.
The randomized Phase III PHILA clinical trial, which evaluated the introduction of pyrotinib to a backbone of trastuzumab and chemotherapy, was presented in ESMO 2022. It met its primary endpoint, confirming a significant improvement in PFS. A number of clinical trials are exploring the role of pyrotinib in different treatment settings, including the concurrent treatment of pyrotinib, capecitabine, and brain radiotherapy for patients with CNS metastases (NCT04582968), and the combination of pyrotinib and vinorelbine for subjects with disease progression after trastuzumab with or without pertuzumab (NCT04605575).
Innovative Combinations
Mutations throughout the PI3K/AKT/mTOR pathway have been classically characterized as a mechanism of resistance to HER2-targeted therapy.81 Additionally, around 30% of the HER2 breast cancer harbor PIK3CA mutations. Consequently, the combination of PI3K inhibitors and HER2-targeted agents is a strategy of increasing interest. A Phase Ib/II clinical trial will determine if alpelisib and tucatinib, plus fulvestrant for the HR+ subgroup is an effective approach in HER2-positive ABC patients with disease progression after two HER2-targeted agents (NCT05230810). In the same molecular subgroup, but as a maintenance treatment after first-line trastuzumab, pertuzumab, and chemotherapy treatment, two clinical trials are studying agents directed to this pathway. While the phase IB IPATHER clinical trial is evaluating the addition of ipatasertib, the phase III EPIK-B2 (NCT04208178) clinical trial is exploring the role of alpelisib as a component of the maintenance strategy.
A collaborative study conducted by the SOLTI group is evaluating the role of CDK inhibitors together with trastuzumab and endocrine therapy for the HR+/HER2+ subgroup.82 The authors reported initial results of the 71 patients recruited for cohorts A, B1, and B2. Cohort B2, which evaluated the activity of the combination of the three drugs, had a PFS rate of 44.4% at 6 months. Given that more benefit was observed in the luminal intrinsic subtype according to PAM50, this clinical trial was amended to explore the role of this therapeutic strategy in this specific population (NCT02448420). In an interesting approach, the combination of pyrotinib and dapiciclib together with fulvestrant or inetetamab will be explored for the first-line setting in patients with HR+/HER2+ ABC (NCT05328440).
Immune checkpoint inhibitors have been associated with modest benefits in HER2-positive ABC. For example, the Phase III KATE-2 trial that evaluated the addition of atezolizumab to TDM-1 was prematurely closed due to futility.83 Subgroup analysis led to the consideration that patients with PDL-1 positive tumors might have been associated with more benefit from the experimental arm. Consequently, the currently ongoing Phase III KATE-3 clinical trial has been designed with identical treatment arms, but it will only include patients with HER2-positive PDL-1 positive ABC in the second-line setting (NCT04740918).
It can be argued that optimal biomarkers to define the subgroup with the most benefits from these agents are urgently needed. The SOLTI collaborative group is also addressing these questions in the Phase II ATREZZO study, which will evaluate the combination of atezolizumab, trastuzumab, and vinorelbine in patients with non-luminal ABC according to PAM50 (NCT04759248). Another combination strategy is being analyzed in the randomized Phase II AVIATOR study which is evaluating the addition of the CD137 agonist utomilumab, to avelumab, trastuzumab, and vinorelbine in patients who had disease progression with trastuzumab, pertuzumab and TDM-1 (NCT03414658).
Preclinical studies have shown activity of combining PARP inhibitors and HER2-directed therapies.84 Two clinical trials will determine the clinical activity of the combination of niraparib and trastuzumab (NCT03368729) or trastuzumab-duocarmazine (NCT04235101) in previously treated HER2-positive ABC patients. In another attractive approach, the combination of trastuzumab-duocarmazine and paclitaxel is being investigated in a Phase I/Ib clinical trial that is incorporating patients with different tumor models, including HER2-positive, HER2-low ABC (NCT04602117).
Modern Agents for HER2-positive ABC
ARX788 is a novel ADC that combines a fully humanized monoclonal antibody directed to HER2, which is conjugated with a tubulin inhibitor (AS269) towards a synthetic amino acid: para-acetylphenylalanine.85 This specific drug design prevents early payload detachment. In 19 heavily pretreated ABC patients, with HER2 overexpressing tumors, the reported overall response was 74%. The ACE-Breast 03 Phase II study is recruiting patients with HER2-positive metastatic breast cancer, previously treated with T-DXd (NCT04829604). This study may contribute to evaluating the role of HER2-targeted agents after modern ADCs. Another specific open-label Phase II clinical trial is testing ARX788 activity in patients with untreated brain metastases (NCT05018702).
Inetetamab is a novel anti-HER antibody that binds to the domain III of HER2. Results of the combination of inetetamab, camrelizumab (anti-PD1 inhibitor), and utidelone have been reported in ASCO 2022.86 An ORR of 30% was evidenced in the 27 included patients, which had a median number of prior systemic therapies for ABC of 3. The authors highlighted that these results supported enrolling patients in this Phase II trial for Part 2 (NCT04681287). Concomitantly, a Phase II trial is exploring the combination of inetetamab, pyrotinib, and chemotherapy selected by the investigator (NCT04681911), and a Phase III clinical trial is evaluating the combination of inetetamab, rapamycin, and chemotherapy in comparison to pyrotinib and chemotherapy (NCT04736589).
KN026 is a biparatopic HER2 inhibitor, which targets the same receptor domains as pertuzumab and trastuzumab.87 KN026 monotherapy has been associated with an ORR of 28.3% in a cohort of 63 patients that experienced disease progression with other HER2 inhibitors. CDK12 was characterized as a predictive response biomarker in this population. For HER2-positive ABC, a Phase II trial is currently recruiting patients to evaluate KN026 in combination with palbociclib and fulvestrant (NCT04778982).
Finally, disitamab vedotin (RC48-ADC) is a HER2 ADC that combines RC48, which targets HER2 with better molecular affinity than trastuzumab, a cleavable linker, and the synthetic anti-mitotic monomethyl auristatin E (MMAE).88 The pooled analysis of the Phase 1 and Phase 1b C001 and C003 CANCER clinical trials evidenced an ORR were 40% and 30.9% for HER2-positive and HER2-low ABC patients.89 A Phase 2/3 trial is currently comparing disitamab vedotin to capecitabine and lapatinib in HER2-positive breast cancer patients who had disease progression to no more than 2 systemic therapies in the advanced setting.
Conclusion
In conclusion, valuable clinical research has built an important set of therapeutical tools for patients with advanced HER2-positive breast cancer. Advances in targeting HER2 has included new technology engineered monoclonal antibodies, TKIs with more capabilities and ADCs with variable linkers, payload or antibody scaffold to optimize efficacy. New molecules have impacted immensely in survival outcomes in multiple trials, paving the way for future technology advances and innovative combinations that will surely improve efficacy results and PROs. Nevertheless, as we obtain more benefits of anti-HER treatment, we also assume risks due to new toxicities. Clinical oncologists have the responsibility to approach patient management with profound knowledge of possible adverse events and generate information regarding strategies to prevent them. A multi-disciplinary approach involving other specialists has become paramount in this field for high-quality medical care. Furthermore, there is still an unmet need to define a precise sequence of HER2-directed agents and to ensure access to these drugs for patients worldwide.
Acknowledgments
We would like to thank Angeles Baeck for preparing manuscript graphs and figures.
Funding
No sponsor was involved in any of the stages from study design to submission of the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Eroles P, Bosch A, Pérez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012;38(6):698–707. doi:10.1016/j.ctrv.2011.11.005
2. Hwang KT, Kim J, Jung J, Chang JH, Chai YJ, Oh SW. Impact of breast cancer subtypes on prognosis of women with operable invasive breast cancer: a population-based study using SEER database. Clin Cancer Res. 2018;17:100.
3. Gall VA, Philips AV, Qiao N, et al. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells trastuzumab increases HER2 cross-presentation. Cancer Res. 2017;77(19):5374–5383. doi:10.1158/0008-5472.CAN-16-2774
4. Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639. doi:10.1200/JCO.1999.17.9.2639
5. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi:10.1056/NEJM200103153441101
6. Franklin MC, Carey KD, Vajdos FF, Leahy DJ, De Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5(4):317–328. doi:10.1016/S1535-6108(04)00083-2
7. Tóth G, Szöőr Á, Simon L, Yarden Y, Szöllősi J, Vereb G. The combination of trastuzumab and pertuzumab administered at approved doses may delay development of trastuzumab resistance by additively enhancing antibody-dependent cell-mediated cytotoxicity.
8. Baselga J, Cortés J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. doi:10.1056/NEJMoa1113216
9. Swain SM, Miles D, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530. doi:10.1016/S1470-2045(19)30863-0
10. Nordstrom JL, Gorlatov S, Zhang W, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res. 2011;13(6):1–14. doi:10.1186/bcr3069
11. Stavenhagen JB, Gorlatov S, Tuaillon N, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcγ receptors. Cancer Res. 2007;67(18):8882–8890. doi:10.1158/0008-5472.CAN-07-0696
12. Rugo HS, Im S-A, Cardoso F, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(4):573–584. doi:10.1001/jamaoncol.2020.7932
13. Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16(2):1–12. doi:10.1186/bcr3621
14. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi:10.1056/NEJMoa1209124
15. Krop IE, Kim S-B, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–699. doi:10.1016/S1470-2045(14)70178-0
16. Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18(11):1512–1522. doi:10.1016/S1470-2045(17)30604-6
17. André F, Shahidi J, Lee C, Wang K, Krop I. Phase III study of [fam-] trastuzumab deruxtecan vs investigator’s choice in T-DM1-pretreated HER2+ breast cancer. Ann Oncol. 2019;30:iii63. doi:10.1093/annonc/mdz100.048
18. Cortés J, Kim S-B, Chung W-P, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–1154. doi:10.1056/NEJMoa2115022
19. Scaltriti M, Verma C, Guzman M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28(6):803–814. doi:10.1038/onc.2008.432
20. Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi:10.1056/NEJMoa064320
21. Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–1130. doi:10.1200/JCO.2008.21.4437
22. Tiwari SR, Mishra P, Abraham J. Neratinib, a novel HER2-targeted tyrosine kinase inhibitor. Clin Breast Cancer. 2016;16(5):344–348. doi:10.1016/j.clbc.2016.05.016
23. Saura C, Oliveira M, Feng Y-H, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138. doi:10.1200/JCO.20.00147
24. Kulukian A, Lee P, Taylor J, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther. 2020;19(4):976–987. doi:10.1158/1535-7163.MCT-19-0873
25. Curigliano G, Mueller V, Borges V, et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33(3):321–329. doi:10.1016/j.annonc.2021.12.005
26. Li X, Yang C, Wan H, et al. Discovery and development of pyrotinib: a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharma Sci. 2017;110:51–61. doi:10.1016/j.ejps.2017.01.021
27. Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(3):351–360. doi:10.1016/S1470-2045(20)30702-6
28. Miles D, Ciruelos E, Schneeweiss A, et al. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Ann Oncol. 2021;32(10):1245–1255. doi:10.1016/j.annonc.2021.06.024
29. Perez EA, López-Vega JM, Petit T, et al. Safety and efficacy of vinorelbine in combination with pertuzumab and trastuzumab for first-line treatment of patients with HER2-positive locally advanced or metastatic breast cancer: VELVET Cohort 1 final results. Breast Cancer Res. 2016;18(1):1–13. doi:10.1186/s13058-016-0773-6
30. Swain SM, Kim S-B, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi:10.1016/S1470-2045(13)70130-X
31. Urruticoechea A, Rizwanullah M, Im S-A, et al. Randomized phase III trial of trastuzumab plus capecitabine with or without pertuzumab in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer who experienced disease progression during or after trastuzumab-based therapy. J Clin Oncol. 2017;35(26):3030–3038. doi:10.1200/JCO.2016.70.6267
32. Rimawi M, Ferrero J-M, de la Haba-Rodriguez J, et al. First-line trastuzumab plus an aromatase inhibitor, with or without pertuzumab, in human epidermal growth factor receptor 2–positive and hormone receptor–positive metastatic or locally advanced breast cancer (PERTAIN): a randomized, open-label phase II trial. J Clin Oncol. 2018;36(28):2826–2835. doi:10.1200/JCO.2017.76.7863
33. Butterbaugh S, Patel R, Romond E, Mathew A. Trastuzumab use in patients with durable complete response in HER2-amplified metastatic breast cancer: to continue or not to continue. Ann Oncol. 2017;28(12):3098–3099. doi:10.1093/annonc/mdx532
34. Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–742. doi:10.1016/S1470-2045(17)30312-1
35. Yamamoto Y, Iwata H, Taira N, et al. Pertuzumab retreatment for HER2-positive advanced breast cancer: a randomized, open-label phase III study (PRECIOUS). Cancer Sci. 2022;113(9):3169–3179. doi:10.1111/cas.15474
36. Manich CS, O’Shaughnessy J, Aftimos P, et al. LBA15 Primary outcome of the phase III SYD985. 002/TULIP trial comparing [vic-] trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol. 2021;32:S1288. doi:10.1016/j.annonc.2021.08.2088
37. Schettini F, Conte B, Buono G, et al. T-DM1 versus pertuzumab, trastuzumab and a taxane as first-line therapy of early-relapsed HER2-positive metastatic breast cancer: an Italian multicenter observational study. ESMO Open. 2021;6(2):100099. doi:10.1016/j.esmoop.2021.100099
38. Arvold ND, Oh KS, Niemierko A, et al. Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat. 2012;136(1):153–160. doi:10.1007/s10549-012-2243-x
39. Pestalozzi BC, Zahrieh D, Price K, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. 2006;17(6):935–944. doi:10.1093/annonc/mdl064
40. Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. doi:10.1158/1078-0432.CCR-10-2962
41. Hurvitz SA, O’Shaughnessy J, Mason G, et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from SystHERsCNS metastasis and HER2-positive MBC from SystHERs. Clin Cancer Res. 2019;25(8):2433–2441. doi:10.1158/1078-0432.CCR-18-2366
42. Deshpande K, Buchanan I, Martirosian V, Neman J. Clinical perspectives in brain metastasis. Cold Spring Harb Perspect Med. 2020;10(6):a037051. doi:10.1101/cshperspect.a037051
43. Stavrou E, Winer E, Lin N. How we treat HER2-positive brain metastases. ESMO Open. 2021;6(5):100256. doi:10.1016/j.esmoop.2021.100256
44. Percy DB, Ribot EJ, Chen Y, et al. In vivo characterization of changing blood-tumor barrier permeability in a mouse model of breast cancer metastasis: a complementary magnetic resonance imaging approach. Invest Radiol. 2011;46(11):718. doi:10.1097/RLI.0b013e318226c427
45. Olson E, Abdel-Rasoul M, Maly J, Wu C, Lin N, Shapiro C. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24(6):1526–1533. doi:10.1093/annonc/mdt036
46. Dawood S, Broglio K, Esteva F, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19(7):1242–1248. doi:10.1093/annonc/mdn036
47. Swain S, Baselga J, Miles D, et al. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. 2014;25(6):1116–1121. doi:10.1093/annonc/mdu133
48. Lin NU, Kumthekar P, Sahebjam S, et al. Abstract P1-18-03: pertuzumab (P) plus high-dose trastuzumab (H) for the treatment of central nervous system (CNS) progression after radiotherapy (RT) in patients (pts) with HER2-positive metastatic breast cancer (MBC): primary efficacy analysis results from the phase II PATRICIA study. Cancer Res. 2020;80(4_Supplement):P1-18-03-P11-18–03.
49. Montemurro F, Ellis P, Anton A, et al. Safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced breast cancer: primary results from the KAMILLA study cohort 1. Eur J Cancer. 2019;109:92–102. doi:10.1016/j.ejca.2018.12.022
50. Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–1459. doi:10.1158/1078-0432.CCR-08-1080
51. Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. doi:10.1016/S1470-2045(12)70432-1
52. Petrelli F, Ghidini M, Lonati V, et al. The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: a systematic review and pooled analysis. Eur J Cancer. 2017;84:141–148. doi:10.1016/j.ejca.2017.07.024
53. Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2–positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081. doi:10.1200/JCO.18.01511
54. Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610. doi:10.1200/JCO.20.00775
55. Metzger Filho O, Leone J, Li T, et al. Phase I dose-escalation trial of tucatinib in combination with trastuzumab in patients with HER2-positive breast cancer brain metastases. Ann Oncol. 2020;31(9):1231–1239. doi:10.1016/j.annonc.2020.05.014
56. Borges VF, Ferrario C, Aucoin N, et al. Tucatinib combined with Ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial. JAMA Oncol. 2018;4(9):1214–1220. doi:10.1001/jamaoncol.2018.1812
57. Yan M, Ouyang Q, Sun T, et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 2022;23(3):353–361. doi:10.1016/S1470-2045(21)00716-6
58. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621. doi:10.1056/NEJMoa1914510
59. Hurvitz S, Kim S-B, Chung W-P, et al. Abstract GS3-01: trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03. Cancer Res. 2022;82(4_Supplement):GS3-01-GS03–01. doi:10.1158/1538-7445.SABCS21-GS3-01
60. Pérez-García JM, Batista MV, Cortez P, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro-Oncology. 2022;2022:215.
61. Bartsch R, Berghoff AS, Furtner J, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022;2022:1–8.
62. Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Safety. 2008;31(6):459–467. doi:10.2165/00002018-200831060-00002
63. de Azambuja E, Ponde N, Procter M, et al. A pooled analysis of the cardiac events in the trastuzumab adjuvant trials. Breast Cancer Res Treat. 2020;179(1):161–171. doi:10.1007/s10549-019-05453-z
64. Ewer MS, Vooletich MT, Durand J-B, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23(31):7820–7826. doi:10.1200/JCO.2005.13.300
65. Davis CC, Zelnak A, Eley JW, Goldstein DA, Switchenko JM, McKibbin T. Clinical utility of routine cardiac monitoring in breast cancer patients receiving trastuzumab. Ann Pharmacothe. 2016;50(9):712–717. doi:10.1177/1060028016654160
66. Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur Heart J Cardiovasc Imaging. 2022;23(10):e333–e465. doi:10.1093/ehjci/jeac106
67. Khoury K, Lynce F, Barac A, et al. Long-term follow-up assessment of cardiac safety in SAFE-HEaRt, a clinical trial evaluating the use of HER2-targeted therapies in patients with breast cancer and compromised heart function. Breast Cancer Res Treat. 2021;185(3):863–868. doi:10.1007/s10549-020-06053-y
68. Johnston S, Pippen JJ, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor–positive metastatic breast cancer. J Clin Oncol. 2009;27(33):5538–5546. doi:10.1200/JCO.2009.23.3734
69. Gradishar W, Moran M, Abraham J. NCCN clinical practice guidelines in oncology: breast cancer, version 2.2022. 2022:1.
70. Drucker AM, Wu S, Dang CT, Lacouture ME. Risk of rash with the anti-HER2 dimerization antibody pertuzumab: a meta-analysis. Breast Cancer Res Treat. 2012;135(2):347–354. doi:10.1007/s10549-012-2157-7
71. Raedler LA. Kadcyla (Ado-trastuzumab Emtansine): first antibody-drug conjugate approved for the treatment of HER2-positive metastatic breast cancer. 2022.
72. Powell C, Modi S, Iwata H, et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO open. 2022;7(4):100554. doi:10.1016/j.esmoop.2022.100554
73. Canino F, Omarini C, Cerma K, et al. Ocular toxicity in breast cancer management: manual for the oncologist. Clin Breast Cancer. 2022;22:289–299. doi:10.1016/j.clbc.2022.02.002
74. Banerji U, van Herpen CM, Saura C, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124–1135. doi:10.1016/S1470-2045(19)30328-6
75. Modi N, Danell N, Perry R, et al. Patient-reported outcomes predict survival and adverse events following anticancer treatment initiation in advanced HER2-positive breast cancer. ESMO Open. 2022;7(3):100475. doi:10.1016/j.esmoop.2022.100475
76. Cortés J, Baselga J, Im Y-H, et al. Health-related quality-of-life assessment in CLEOPATRA, a phase III study combining pertuzumab with trastuzumab and docetaxel in metastatic breast cancer. Ann Oncol. 2013;24(10):2630–2635. doi:10.1093/annonc/mdt274
77. Welslau M, Diéras V, Sohn JH, et al. Patient‐reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T‐DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2–positive locally advanced or metastatic breast cancer. Cancer. 2014;120(5):642–651. doi:10.1002/cncr.28465
78. Curigliano G, Dunton K, Rosenlund M, et al. 163O patient-reported outcomes (PROs) from DESTINY-Breast03, a randomized phase III study of trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (pts) with HER2-positive (HER2+) metastatic breast cancer (MBC). Ann Oncol. 2022;33:S196–S197. doi:10.1016/j.annonc.2022.03.182
79. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051–1059. doi:10.1001/jamaoncol.2015.2639
80. Federico W, Carlos L, Diego E, et al. Assessing the methodological quality of quality-of-life analyses in first-line non-small cell lung cancer trials: a systematic review. Crit Rev Oncol Hematol. 2022;103747. doi:10.1016/j.critrevonc.2022.103747
81. Rasti AR, Guimaraes-Young A, Datko F, Borges VF, Aisner DL, Shagisultanova E. PIK3CA mutations drive therapeutic resistance in human epidermal growth factor receptor 2–positive breast cancer. JCO Precis Oncol. 2022;6:e2100370. doi:10.1200/PO.21.00370
82. Ciruelos E, Villagrasa P, Pascual T, et al. Palbociclib and trastuzumab in HER2-positive advanced breast cancer: results from the phase II SOLTI-1303 PATRICIA TrialPalbociclib and trastuzumab in HER2-positive breast cancer. Clin Cancer Res. 2020;26(22):5820–5829. doi:10.1158/1078-0432.CCR-20-0844
83. Emens LA, Esteva FJ, Beresford M, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21(10):1283–1295. doi:10.1016/S1470-2045(20)30465-4
84. Dokter WH, Dijcks FA, Groothuis PG, et al. SYD985 synergy with PARP inhibitors (PARPi) in human tumor cell lines and patient-derived xenografts (PDXs). Cancer Res. 2020;80(16_Supplement):4043. doi:10.1158/1538-7445.AM2020-4043
85. Hurvitz SA, Park H, Frentzas S, et al. Safety and Unique Pharmacokinetic Profile of ARX788, a Site-Specific ADC, in Heavily Pretreated Patients with HER2-Overexpresing Solid Tumors: Results from Two Phase 1 Clinical Trials. Wolters Kluwer Health; 2021.
86. Yan M, Lv H, Niu L, et al. Anti-HER2 Antibody Inetetamab Plus Camrelizumab and Utidelone for Pretreated HER2-Positive Advanced Breast Cancer: A Single-Arm, Multicenter, Phase 2 Study. American Society of Clinical Oncology; 2022.
87. Zhang J, Ji D, Cai L, et al. First-in-human HER2-targeted bispecific antibody KN026 for the treatment of Patients with HER2-positive metastatic breast cancer: results from a phase I StudyHER2-targeted bispecific antibody KN026 in MBC. Clin Cancer Res. 2022;28(4):618–628. doi:10.1158/1078-0432.CCR-21-2827
88. Shi F, Liu Y, Zhou X, Shen P, Xue R, Zhang M. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv. 2022;29(1):1335–1344. doi:10.1080/10717544.2022.2069883
89. Wang J, Liu Y, Zhang Q, et al. RC48-ADC, a HER2-Targeting Antibody-Drug Conjugate, in Patients with HER2-Positive and HER2-Low Expressing Advanced or Metastatic Breast Cancer: A Pooled Analysis of Two Studies. Wolters Kluwer Health; 2021.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.