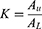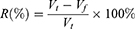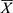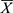Back to Journals » Drug Design, Development and Therapy » Volume 15
Hepatoprotective Effects of Polysaccharide from Anoectochilus roxburghii (Wall.) Lindl. on Rat Liver Injury Induced by CCl4
Authors Yu X, Huang L , You C , Huang L
Received 9 March 2021
Accepted for publication 9 June 2021
Published 6 July 2021 Volume 2021:15 Pages 2885—2897
DOI https://doi.org/10.2147/DDDT.S310263
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Video abstract of "ARPS on rat liver injury induced by CCl4" [ID 310263].
Views: 510
Xiaoling Yu,1,2 Lingyi Huang,1 Chen You,2 Liying Huang1
1School of Pharmacy, Fujian Medical University, Fuzhou, Fujian, People’s Republic of China; 2Department of Pharmacy, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, People’s Republic of China
Correspondence: Liying Huang
School of Pharmacy, Fujian Medical University, No.1 Xuefu North Road University Town, Fuzhou, Fujian, 350122, People’s Republic of China
Tel +86 13860635139
Fax +86 591 22862016
Email [email protected]
Purpose: The polysaccharide of Anoectochilus roxburghii (wall.) Lindl. (ARPS) is one of its important active ingredients. Hepatoprotective effects of ARPS on rat liver injury induced by CCl4 were studied.
Methods: ARPS was extracted using the ultrasonic method and successfully purified by high-speed counter-current chromatography (HSCCC) with a two-phase aqueous system composed of 12.5% PEG 1000– 20% K2HPO4:KH2PO4 (1:1). The HSCCC conditions were optimized, and the structure of ARPS was characterized. The hepatoprotective effects of ARPS against CCl4-induced chronic hepatic injury in SD rats were evaluated.
Results: The results showed that ARPS was a water-soluble polysaccharide with a molecular weight of 28,518 Da. It was composed of mannose, ribose, glucose, and arabian sugar; its monosaccharide molar ratio was glucose:ribose:arabinose:mannose = 54.24:13.20:1.09:1.00. The purity of ARPS was determined by HPLC to be 96.93%. The intervention effects of ARPS on CCl4-induced hepatic damage model in rats showed that ARPS could effectively reduce the activity of alanine amino transferase and aspartate amino transferase, decrease the content of malondialdehyde and nitric oxide synthesis, and increase the content of glutathione. Pathology revealed that liver plate order, liver cell degeneration, and edema were improved; inflammatory cell infiltration was not observed after ARPS intervention.
Conclusion: ARPS had the function of antioxidant for protecting CCl4-induced injured liver, and the mechanisms were related to anti-lipid peroxidation, which could eliminate oxygen-free radicals and protect liver cells from attacks by free radicals.
Keywords: Anoectochilus roxburghi, polysaccharide, CCl4-induced hepatic damage, hepatoprotective effects, rat
Introduction
Anoectochilus roxburghii (Wall.) Lindl. (A. roxburghii), also known as Jin Xian Lian in Chinese and “the king of medicine”,1,2 is widely distributed in Southern China,3 especially in Fujian and Guangdong.4 In recent decades, many studies have shown that A. roxburghii, as a Chinese folk medicine, has diverse pharmacological effects and medicinal properties, such as hepatoprotection,5–9 anti-diabetes,10,11 antifatigue,12 antiosteoporosis,9,13,14 vascular protective,15 antioxidation,16,17 and antitumor.18,19 Some scholars have applied advanced and efficient technologies in the extraction, separation, and structural characterization of the active ingredients from A. roxburghii because of its curative effects. In recent years, the research team of the authors has conducted an in-depth exploration of the active components of A. roxburghii. The results showed that the components contained alkaloid,20 nucleosides,21 nucleotide,21 sterol,22 triterpenoids,23 flavonoid,24,25 and gastrodine.26 In addition, the pharmacological effects and mechanism of A. roxburghii were also studied.10,27,28 For example, the polysaccharide from artificially cultivated A. roxburghii has been reported to possess antitumor effects.19
However, little information has been published in terms of purification and structural characterization of the polysaccharide from A. roxburghii and its effects on CCl4-induced liver injury by in rats thus far. Some reports are available on the hepatoprotective effects of A. roxburghii from the different genera of the same family. For instance, Shih et al confirmed that the aqueous extract from Anoectochilus formosanus could significantly improve the liver fibrosis caused by dimethylnitrosamine in mice.29 Wu et al reported that the aqueous extract from A. formosanus could improve the hepatic fibrosis induced by thioacetamide and inhibit the activity of Kupffer cells.30 Furthermore, a study by Yang et al revealed that preparation of ARPT from A. roxburghii has anti-inflammatory, antioxidative, and antiapoptotic effects on hepatic injury in carbon tetrachloride (CCl4)-induced mice.8
The present study aimed to find the best method to separate polysaccharides from A. roxburghii by using high-speed counter-current chromatography (HSCCC) and preliminarily identify their structure, which is the fundamental research direction for the further investigation of their pharmacological effects and mechanism. The structure of ARPS was characterized, and it confirmed that ARPS was a water-soluble polysaccharide with increased purity. However, to the knowledge of the authors, little information is known about the hepatoprotective activities of such polysaccharides on CCl4-induced liver injury. Therefore, this study also aimed to investigate the hepatoprotective activities and possible mechanisms underlying the hepatoprotective effects of polysaccharides from A. roxburghii.
Materials and Methods
Reagents and Materials
Anoectochilus roxburghii (Wall.) Lindl. (A. roxburghii), also known as Jin Xian Lian in Chinese, was purchased from Fujian Wuyishan Luzhou Group (Fujian, China) and identified by Professor Zhang Yonghong (Department of Pharmaceutical Chemistry, Fujian Medical University). A. roxburghii is planted in Moso bamboo forest. It was cultivated from February to September under the conditions of 800 m altitude, approximately 80% shade rate, and 75% relative humidity. Dialysis tubing MD44 with a molecular weight cutoff of 8000–14,000 Da was purchased from Beijing Solaibao Technology Co., Ltd. (Beijing, China). All standard substances were supplied by Sigma–Aldrich Reagent Co., Ltd. (St. Louis, MO, USA). Methanol and acetonitrile of chromatographic grade were provided by Merck (Merck KGaA, Germany). SD rats (SPF grade) were obtained from the Experimental Animal Centre of Chinese Academy of sciences (Shanghai, China. SCXK 2012–0002). Reagent kits for measuring alanine amino transferase (ALT), aspartate amino transferase (AST), glutathione (GSH), nitric oxide synthesis (NOS) and malondialdehyde (MDA) were purchased from Nanjing Jiancheng Institute of Bioengineering and Technology (Nanjing, China). Mannose, ribose, rhamnose, glucose, arabinose, xylose, and galacturonic acid were obtained from Sigma Chemical Co (St. Louis, USA). All other chemical reagents were of analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Distilled water was acquired from a Milli-Q purification system (Millipore, USA). This study was approved by the Institutional Ethical Committee of the Faculty of Medicine, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fujian, China (No. 2020_051_01).
Instruments
The HSCCC (TBE-300A, Shanghai Tauto Biological Company, China) was equipped with a temperature and rotary-speed controlling system, a BSZ-100 automatic fraction collector (Shanghai Huxi Analytical Instrument Factory), a SpectraMax M5 (Molecular Devices, USA), an LC-1100 high-performance liquid chromatograph (Agilent, USA), a Nicolet iS50 FTIR spectrometer (ThermoFisher Scientific Inc., USA), and an LC-1260 HPLC instrument (Agilent, USA).
Extraction of Polysaccharides
A. roxburghii Preprocessing
The harvested A. roxburghii required pretreatment. It was washed cleanly with water and then dried below 60 °C until reaching a constant weight. Afterwards, the fresh A. roxburghii became dried herbs, which were smashed into dried homogenized powder that was screened through a mesh size of 80 with a high-speed disintegrator.
Extraction of Crude Polysaccharide
The dried powder of A. roxburghii (100 g) was extracted three times with distilled water (2 L) at 60 °C for 30 min under ultrasonic wave. After centrifugation at 4200 rpm for 10 min, the supernatant was collected and concentrated. The concentrated solution was deproteinized by Sevag method (chloroform: n-butanol = 4:1). The mixtures were shocked three times at 250 rpm for 20 min and then centrifuged at 4200 rpm for 10 min. Afterwards, metaprotein and organic solvents were discarded, and the supernatants were reserved and precipitated by anhydrous ethanol at a ratio of 1:4 under 4 °C overnight. Finally, after centrifugation at 4200 rpm was performed for 10 min, the precipitates were collected using the evaporator at 30 °C under the vacuum condition to drive away the ethanol and then dried at −20 °C using a vacuum-freezing dryer until constant weight and dried powders were achieved; these powders were the crude polysaccharide of A. roxburghii (CARPS, 16 g).
Purification of CARPS
Selection of Aqueous Biphasic System of HSCCC
In accordance with the recommendation and rules of two-phase solvent system,31 the influences of PEG with different molecular weights (600, 800, and 1000 Da) and phosphates (K2HPO4 and KH2PO4) with different proportions of the partition coefficient (K) were studied. Phosphates were thoroughly dissolved in distilled water and then equally mixed together with dissolved PEG. After equilibrium was achieved, the miscible mixtures turned into supernatant liquid as the upper layer (the stationary phase) and a subnatant liquid as the lower layer (the mobile phase), whose volumes were recorded. CARPS (5 mg) was added to the two mutually equilibrated solvent phases (2 mL each) in a stoppered test tube and completely mixed with the vortex to balance the contents. After stratification, an equal volume of the upper and lower layers (500 µL) each was transferred into a separate test tube. Then, the polysaccharide concentration of CARPS in both phases was measured using a spectrophotometer at an appropriate wavelength to obtain the AU and AL values via phenol-sulfuric acid method. K was expressed as follows:
where Au and AL are the absorbance of polysaccharide by the phenol-sulfuric acid method in the upper and lower layers, respectively.
Meanwhile, 2 mL of each phase was transferred into a test tube (total volume of 4 mL). This tube was plugged and inverted several times and then immediately placed in an upright position to determine the layered time called for the two phases to become a distinct interface.
Flow Rate of Mobile Phase and Revolution Speed Effect on Retention Ratio of Stationary Phase
The effect of different flows of the mobile phase and different revolution speeds on the stationary phase was explored. First, the column of HSCCC was entirely filled with the stationary phase until its flow from the column outlet to ensure that no air was present in the column. Then, by testing various revolution speeds, the mobile phase was pumped into the column at different flow rates. Finally, the mobile phase constantly flowed from the column outlet until the two-phase solvent system reached hydrodynamic equilibrium in the column of HSCCC where the stationary phase stopped to flow. During the whole process, the stationary phase flowing out of the column was collected in a measuring cylinder, and its volume was measured. The retention ratio of the stationary phase (R) was calculated as follows:31
where Vt is the total volume pumped into the column of the stationary phase, and Vf is the volume flowing from the column.
Separation and Purification of CARPS by HSCCC
As mentioned, a two-phase solvent system reached hydrodynamic equilibrium in the column of HSCCC at a selected flow rate of the mobile phase and desired revolution speed. Then, the CARPS (200 mg) dissolved in the mobile phase (20 mL) was injected into the column. Afterwards, the effluents from the outlet of the column were continuously collected (5 mL, each tube, every 5 min), and their color change was monitored using the phenol-sulfuric acid method.
After fractions were collected, the effluents belonging to the same polysaccharide component were combined and evaporated into concentrated solutions in a vacuum. The concentrated solutions were dialyzed with a dialysis bag, which cut off 8000–14,400 molecular weight (Mw) to remove phosphate. After small molecular substances, especially phosphate, were removed completely, all solutions were freeze-dried at −20 °C in a vacuum freeze dryer until their weight was constant to that of dried powder, which was the purified ARPS, and then stored in vacuum dryer at normal temperature for subsequent use.
Characterization of ARPS
Analysis of Homogeneity and Determination of Average Molecular Weight
The ARPS (2 mg) dissolved in distilled water (5 mL) was detected with an ultraviolet and visible spectrophotometer and then scanned in the range of 200–800 nm. ARPS (5 mg) was dissolved in distilled water (1 mL), analyzed via high-performance gel-permeation chromatography on an LC instrument equipped with the column of tiger kin ODS (5 µm, 10×250 mm), and eluted with 0.7% Na2SO4 solutions32 at a flow rate of 1.0 mL/min through monitoring via refractive index detector. A series of known molecular mass Dextrans (MW of 150, 80, 50, 12, and 5 KDa) were used for standard substances, and a calibration curve was created to calculate the molecular weight of ARPS following the literature reported by Alosp and Vlachogiannis.33
Infrared Spectra Analysis
Spectrum Two FTIR was used to detect the functional groups of ARPS (2 mg), which was evenly mixed with moderate KBr and pressed into slices. The mixture was then analyzed and scanned in the range of 400–4000 cm−1.
Determination of Composition
LC analysis was applied for quantification and identification of ARPS. The monosaccharide derivatization of 1-phenyl-3-methyl-5-pyrazolone (PMP) was implemented. ARPS (3 mg) was hydrolyzed with 0.02 mmol/L of trifluoroacetic acid at 100 °C for 12 h and then cooled. After centrifugation at 8000 rpm was conducted for 10 min, the pH of supernatants was mediated by adding 0.1 mol/L of NaOH as hydrolyzed sample solutions. Then, 0.5 mol/L 100 µL PMP and 0.1 mol/L of 30 µL NaOH were added into the hydrolyzed samples of ARPS and various standard monosaccharides (mannose, ribose, rhamnose, glucose, arabinose, xylose, and galacturonic acid). The mixture was mixed and reacted in a bath at 70 °C. Through the above procedure of hydrolysis and derivatization, the reaction solution was extracted with chloroform to remove PMP, and then the aqueous layer was filtered through a 0.45 µm membrane for HPLC analysis. This analysis was performed using an HPLC instrument equipped with RP-C18 column (4.6 × 250 mm, 5 μm, Waters) at a wavelength of 254 nm. The eluotropic procedure was as follows: the concentrations of acetonitrile were 7%, 19%, 25%, and 45%; the other solvent system was distilled water; the time changes were 0, 4, 13, and 28 min; and the flow rate was 0.8 mL/min.
Hepatoprotective Effects of ARPS on CCl4-Induced Liver Injury in Rats
The hepatoprotective effects of ARPS against CCl4-induced chronic hepatic injury in rats were evaluated. Specific pathogen-free grade SD rats (male, 6 weeks old) with body weights (BW) of 250 ± 10 g were kept in standard conditions with controlled temperature (23 °C ± 1 °C) and humidity (55% ± 10%) under a 12-h light/dark cycle and with free access to a standard laboratory pellet diet and water. The procedures involving animals strictly complied with Chinese law, specifically the use and care of laboratory animals.
After 1 week of adjustment, the rats were randomly divided into five groups (three for each group) in accordance with the preliminarily experimental results: (I) normal control group (NC group), (II) model control group (MC group), (III) low-dose group of ARPS (100 mg/kg/d, LD group), (IV) medium-dose group of ARPS (200 mg/kg/d, MD group), and (V) high-dose group of ARPS (400 mg/kg/d, HD group). All rats except those in the NC group were administered with intraperitoneal injection of CCl4 (50%, 1 mL/kg, BW) for 10 weeks until CCl4-induced liver injury models were successfully achieved. Then, the rats were administered with corresponding doses of ARPS. Blank blood samples were collected before administration, and then 4, 8, 12, 24, and 48 h were set for taking blood spots. Cell Counting Kit-8 (CCK-8) experiment was carried out to determine the dose of ARPS on CCl4-induced liver injury in rats and the time of blood sampling.
Meanwhile, the rats were randomly divided into four groups (five for each group): (I) normal control group (NC group), (II) CCl4-induced model control group (MC group), (III) lentinan treatment group (positive control group, PC group), and (IV) ARPS treatment group (ARPS group). All rats except those in the NC group were administered with intraperitoneal injection of CCl4 (50%, 1 mL/kg, biw) for 10 weeks, while the NC group received physiological saline. After CCl4-induced liver injury models were successfully established, the rats in the ARPS group underwent ARPS treatment; the dose depended on the screening of CCK-8 experiment, while those in the PC group received lentinan (200 mg/kg, dissolved in physiological saline). All treatments were administered via intragastric gavage, once a day for 14 days in each group. After the last experimental medication was administered (at 24 h), all rats were weighed. Blood samples were collected by retro-orbital bleeding, and the rats were sacrificed via cervical dislocation. The blood samples were separated into serum at 4 °C. The activities of serum ALT and AST were determined using commercial reagent kits in accordance with the manufacturer’s instructions via dinitrophenyl-hydrazine method. The levels of NOS and MDA were estimated according to the manufacturers’ instructions by Thiobarbituric Acid Reactive Substance (TBARS) and spectrophotometry using kits obtained from Nanjing Jiancheng Institute of Bioengineering and Technology. The activities of GSH were measured using GSH Quantification kits acquired from Nanjing Jiancheng Institute of Bioengineering and Technology according to the manufacturers’ instructions. The hepar from rats in the different groups was excised and fixed in 10% neutral formalin solution once, dehydrated in graded alcohol, and embedded in paraffin. Slices with a thickness of 4–5 µm were cut and stained with hematoxylin and eosin (H&E) for photomicroscopic observation.
Results
Optimization of Aqueous Biphasic System
The aqueous biphasic system composed of PEG-K2HPO4-KH2PO4-H2O34,35 with different molecular weights and proportions for HSCCC was introduced in the present study to obtain the optimum K values. In accordance with the experiment, the proportion of PEG with different molecular weights (600, 800, and 1000) exceeded 12.5%, while the solubility of ARPS in the upper phase decreased as the K values declined. Meanwhile, the viscosity of the solution gradually increased with the increase in the amount of polymer, and then the layer time of the two-phase solvent system was gradually prolonged. On the contrary, the proportion below 12.5% PEG was difficult for the two-phase solvent system. Therefore, a proportion of 12.5% PEG was chosen to further optimize the aqueous biphasic system.
The effect of different molecular weights of 12.5% PEG and mass fractions of K2HPO4 - KH2PO4 (1:1) on K and layer time was surveyed. When the mass fractions of K2HPO4-KH2PO4 in PEG 600 and 800 were below 16% and 18%, respectively, the two-phase solvent system was difficult to separate. At PEG 1000 and 20% mass fraction of K2HPO4-KH2PO4 (1:1), the K value was optimum and the layer time was the shortest. Therefore, PEG 1000-K2HPO4-KH2PO4-H2O (1.25:1:1:6.75, w/w) was the optimum aqueous biphasic system for purifying CARPS by HSCCC, of which the K value was 0.868 and the layer time was 20 s.
Retention of Stationary Phase
Successful separation in HSCCC widely relies on the ratio of the stationary phase reserved in the column. The higher the retention of the stationary phase, the better the resolution of the peak.36 Besides the separating time of the two-phase solvent system, the optimum flow rate of the mobile phase and the suitable revolution speed of HSCCC apparatus considerably influence the retention ratio of the stationary phase in the column.
In this study, to protect the rotated gear composed of polytetrafluoroethylene materials in the HSCCC instrument, the temperature of the circulating water was set to 25 °C to cool the large amount of heat caused by the high rotational speed. The flow rate of the stationary phase was 15 mL/min, which could reduce the waiting time. The following effects on the retention of the stationary phase were studied: flow rate of the mobile phase (0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mL/min) and revolution speed (700, 750, 800, 850, and 900 rpm). As a result, the lower flow rate generally generated a higher retention ratio of the stationary phase and improved the peak resolution but required a longer separation time than the higher flow rate (as shown in Figure 1A). The higher the revolution speed, the higher the retention ratio of the stationary phase (Figure 1B). The high revolution speed simultaneously caused the immoderate solute band to broaden because of severe vibration under high pressure and too much heat to damage the instrument. However, the lower speed reduced the volume of the stationary phase reserved in the column and led to lowered peak resolution. In conclusion, the flow rate for the mobile phase was 1.0 mL/min, the flow rate for the stationary phase was 15.0 mL/min, and the revolution speed was 850 rpm, with 25 °C as the temperature of circulating water. These parameters were the optimal for the best retention ratio of the stationary phase (32.59%). Satisfactory separation could still be obtained at a high K value, although retention of over 50% is perfect.36
 |
Figure 1 Effect of the flow rate of the mobile phase (A) and the revolution speed (B) on the retention of the stationary phase. |
Separation CARPS by HSCCC
In accordance with the above experimental parameters, the aqueous biphasic system consisted of PEG 1000-K2HPO4-KH2PO4-H2O (1.25:1:1:6.75, w/w), and separation was performed at a flow rate of 1.0 mL/min for the mobile phase and 15.0 mL/min for the stationary phase, with a revolution speed of 850 rpm and 25 °C as the temperature of circulating water. The effluents were monitored at 482 nm using phenol-sulfuric acid method. The effluents from the outlet of the column were continuously collected (5 mL, each tube, every 5 min), and the fractions (37–57 tubes) that belonged to the same polysaccharide components were combined and monitored at 482 nm using the same method, as depicted in Figure 2. On the basis of this method, the ARPS contained 96.93% carbohydrate, while the ARPS in CARPS was 67.09%.
 |
Figure 2 Spectrogram of ARPS collected via HSCCC and detected using phenol-sulfuric acid. |
Characterization of ARPS
As indicated in Figure 3A, the ARPS purified by HSCCC had no absorption at 260 and 280 nm and no chemical reaction with Folin-phenol reagent, thus revealing the free protein and nucleic acid in ARPS.
 |
Figure 3 Characterization of ARPS. (A) UV-visible spectrum of ARPS in the range of 200–800 nm; (B) Profile of ARPS in HPGPC; (C) FT-IR spectrum of ARPS in the range of 400–4000 cm−1. |
The molecular weight of polysaccharides is usually determined using high-performance gel –permeation chromatography (HPGPC).37 The formula of the calibration curve correlating the molecular weight of ARPS was lgMW = −0.2382 t + 6.5932, R2 = 0.9902, where MW stands for the average molecular weight of ARPS. HPGPC analysis of ARPS displayed a symmetrical and single sharp peak (Figure 3B), whose retention time was 11.959 min. Its molecular weight was calculated to be 28,518 Da.
The infrared spectra in Figure 3C showed that the band of 3370.38 cm−1 had a strong and wide stretching peak for O-H stretching vibrations (in the range of 3650–3200 cm−1). The weak absorption peak at 2928 cm−1 was caused by C-H stretching vibrations (in the range of 2950–2850 cm−1), and the band in the region of 1627 cm−1 was in connection with bound water. The absorptive peak at 1408 cm−1 was related to C-O stretching vibrations (in the range of 1440–1395 cm−1). The absorptions at 1153, 1078, and 1028 cm−1 indicated the corresponding sugar residues because both ring vibrations overlapped with the stretching vibrations of C-OH and the glycosidic band vibration of C-O-C dominated in the range of 1000–1200 cm−1. The peak at 934 cm−1 manifested the pyranose form of sugar, which needed to be further determined.38
The compositions of monosaccharides were detected via HPLC, with various known monosaccharides as standard substances (Figure 4A). As shown in Figure 4B, ARPS consisted of glucose, ribose, arabinose, and mannose at a molar ratio of 54.24:13.20:1.09:1.00. In accordance with the HPLC method in the previous study, the purity of ARPS was determined to reach 96.93%.
In-vivo Hepatoprotective Effects of ARPS
Drug Concentration Value of Pre-Experimental Results
CCK-8 was used to detect cell proliferation, and the absorbance value (OD) was measured at 450 nm to determine the number of viable cells. The greater the OD value is, the stronger the cell activity. As shown in Table 1 and Figure 5, the OD value of CCK-8 cell in rats with CCl4-induced liver injury in different dose groups was statistically analysed. The OD of the HD group was significantly higher than that of the MD group and the LD group. The OD value of the HD group increased gradually with the prolongation of blood withdrawal time (0, 4, 8, 12, and 24 h), the OD value of the HD group gradually increased, reached the maximum at 24 h, and then slightly decreased at 48 h. This trend was compared with that of the MD and LD groups at different time points. According to the above cell growth curve and statistical results, the OD value of HD group was higher than that of the MD and LD groups at 24 h blood collection time point, indicating that the cell activity was the highest at this time point. Therefore, HD group (400 mg/kg/d) was selected for drug intervention in liver injury model induced by CCl4. Blood samples were collected at 24 h after ARPS intervention for the detection of various indicators.
 |
Table 1 The OD Value of CCK-8 Cell in Rats with CCl4-Induced Liver Injury in Different Dose Groups (n=3, |
 |
Figure 5 The growth curve of CCK-8 cell in rats with CCl4-induced liver injury in different dose groups. |
Effects of ARPS on the Activity of Serous ALT and AST
As illustrated in Table 2, the activities of serous ALT and AST in the MC group significantly increased (P < 0.05) compared with those in the NC group. Intervention by either lentinan or ARPS significantly decreased (P < 0.05) the activities of ALT and AST compared with the MC group. ARPS administration reduced the activities of ALT and AST 51.16% and 54.60%, respectively, while lentinan, a standard drug, decreased these activities by 64.76% and 54.78%, respectively.
 |
Table 2 The Activity of ALT and AST in Rats with CCl4-Induced Liver Injury in Different Groups (n = 5, |
Effects of ARPS on the Content of Serous MDA, GSH and NOS
As described in Table 3, the contents of serous MDA and NOS in rats with CCl4-induced liver injury in the MC group significantly increased (P < 0.05) compared with those in the NC group. However, intervention by either lentinan or ARPS could be reversed in the opposite direction. Compared with the NC group, the MC group showed sharply reduced hepatic GSH levels (P < 0.05) after intragastric administration of CCl4. However, the contents of serous GSH in the ARPS group considerably increased compared with those in the MC group. By contrast, ARPS administration reduced the contents of MDA and NOS by 41.62% and 46.99%, respectively. These values were close to those obtained with the standard drug lentinan (43.97% and 54.81%, respectively). Moreover, compared with the MC group, the ARPS group showed increased hepatic GSH levels of 264.15%, which was quite comparable to the levels from lentinan treatment (277.36%).
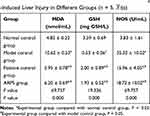 |
Table 3 The Content of MDA, GSH, and NOS in Rats with CCl4-Induced Liver Injury in Different Groups (n = 5, |
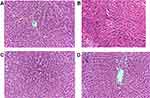
Histopathological Results
As shown in Figure 6, the rats in the NC group had a normal liver lobular architecture and cell structure, the liver plate was neatly arranged, and no inflammatory infiltration was found (Figure 6A). On the contrary, extensive areas of necrosis with loss of hepatocytes architecture around the blood vessels, a change in hepatic cell cytoplasmic acid, and dissoluted and necrotized partial hepatic cells with a small amount of inflammation were obviously observed in the livers of rats in the MC group (Figure 6B). However, intervention by either lentinan or ARPS could reduce the injured area, edematous liver cells, and CCl4-induced inflammatory infiltration (Figure 6C and D).
Discussion
In this experiment, the ultrasonic-assisted water extraction method was adopted in accordance with the polarity of the polysaccharide. The extracted crude polysaccharides were removed from the protein by Sevage method and purified by HSCCC. HSCCC has been widely applied in the separation and purification of polysaccharides.35,39 Selecting a suitable diphase solvent system is a crucial step of HSCCC.40 Systematic search for an appropriate two-phase solvent system depends on the suitable K, which is the ratio of solute distributed between the mutually balanceable two solvent phases and satisfactory retention of the stationary phase in the column. The proper K values to the analyte for HSCCC are 0.5 < K < 1.0.36 The effect of the mass fraction of phosphate in the range of 12–26% on K was investigated. When the amount of phosphate ≥16%, the two phases began to separate. Increasing the amount of salt could make the two phases separate and make partially dissolved ARPS change from the lower phase to the upper phase. According to the “similar miscibility” principle, ARPS is excessively enriched in the polar phosphate phase (mobile phase), which is not conducive to the separation of ARPS in HSCCC. Increasing the amount of phosphate could change the phosphate aqueous solution from an unsaturated state to a saturated state. When the hydrophobicity and ionic strength of the solution have changed, the polysaccharides could not be combined with water molecules in the phosphate phase, and parts of polysaccharides are dissolved in the polymer phase (stationary phase), which increases K. The optimum mass fraction of K2HPO4-KH2PO4 (1:1) is 20%. This experiment adopted PEG 1000-K2HPO4-KH2PO4-H2O (1.25:1:1:6.75, w/w) as the solvent system with ARPS purified by HSCCC, which obtained K = 0.868. Vu:VL = 35:47, the layered time was 2.1 min, and the retention rate of the stationary phase was 32.59%.
The purity of lentinan was identified via gel chromatography, whose operation processes were tedious and time consuming.41 In reference to relevant literature,42 the purity of ARPS was analyzed by HPGPC in the present study. Oneness and symmetry analysis of the chromatograms preliminarily verified that ARPS was a single component with high purity. The average molecular weight of ARPS was 28,518 Da, as measured by HPGPC. Different plant polysaccharides have different molecular weights. For example, the molecular weight of Glycyrrhiza polysaccharide was 5000,43 while the relative molecular weight of tremella polysaccharide was 1.26 × 106–1.39 × 106.44 Polysaccharides with different molecular weights have different biological activities. Thus, the molecular weight of polysaccharides is obtained via HPGPC in this study.
In the present study, the monosaccharide composition of ARPS hydrolysis product was determined via HPLC, and five kinds of monosaccharide, including mannoses, ribose, glucoses, arabinoses and glucoses, were successfully separated. The composition of polysaccharide with wild A. roxburghii was composed of fucose: mannose: galactose: arabinose: rhamnose = 1.7: 1.3: 1.2: 1.1: 1.0 and the mole ratio of difference was bigger,45 indicating that artificially planted wild A. roxburghii has a different monosaccharide composition.
CCl4 is a common hepatotoxic and classic exogenous compound that could induce a chemical liver injury model.46 CCl4-induced liver injury animal models are widely used in various liver drug screenings. Pharmacological studies have found that many Chinese medicines have an enhanced therapeutic effect on liver disease.47 In the present study, the activities of serum ALT and AST were significantly increased (P < 0.05) after CCl4 intervention, thus suggesting CCl4-induced hepatotoxicity. However, the activities of serum ALT and AST dramatically declined (P < 0.05) in rats who underwent ARPS treatment, indicating that ARPS relieved CCl4-induced liver injury. Oxidative stress has been considered as a major mechanism of CCl4 toxicity. This study suggested that oxidative stress markers MDA and NOS increased and the antioxidant enzyme GSH decreased after CCl4 injection, indicating that oxidative stress was caused by inflammatory response factors and inflammatory mediators in the injured liver induced by CCl4 in rats. In rats intervened with ARPS, these effects were attenuated, thereby suggesting that the hepatoprotective effects of ARPS were, at least in part, caused by their radical scavenging and antioxidant activities. However, the exact molecular mechanisms and signaling pathways remain to be further clarified. The pathological section of the rat liver tissue after H&E staining showed that the liver plate of the MC group was slightly disordered. The liver cells were obviously edematous, with some showing balloon-like changes, and the liver tissue around the central vein had obvious edema and fat-like changes, which may be caused by the metabolism of CCl4 first passing through the central vein and then entering the liver tissue. In the ARPS group, the liver plates of the rats returned to a regular arrangement, the hepatocyte degeneration and edema improved, and no inflammatory cell infiltration was found. These findings were comparable to those in the PC group and tended to be normal. The results of histopathological morphology and various liver function indicators were consistent, suggesting that ARPS could reduce CCl4-induced liver injury and enhance the regeneration ability of liver cells.
Conclusion
This study provided a detailed description of the concrete procedure and essential prerequisite for purification of polysaccharide from A. raxburghii by HSCCC. The results confirmed that ARPS was a water-soluble polysaccharide mainly composed of glucose, ribose, arabinose, and mannose at a molar ratio of 54.24:13.20:1.09:1.00 and molecular mass of 28,518 Da. Meanwhile, ARPS showed hepatoprotective effects on rat liver injury induced by CCl4. The underlying mechanisms of these effects may be connected to the attenuation of oxidative stress. One limitation of this study is that it did not delve into the spatial conformation of sugar chains. Therefore, further studies are needed to detect the concrete structure of ARPS. Despite its preliminary characterization, this study clearly provided a useful foundation for further pharmacological and mechanical research on ARPS.
Abbreviations
A. roxburghii, Anoectochilus roxburghii(wall.) Lindl.; ARPS, polysaccharide of Anoectochilus roxburghii (wall.) Lindl.; HSCCC, high-speed counter current chromatography; CCl4, carbon tetrachloride; ALT, alanine amino transferase; AST, aspartate amino transferase; GSH, glutathione; NOS, nitric oxide synthesis; MDA, malondialdehyde; CARPS, crude polysaccharide of A. roxburghii; Mw, molecular weight; PMP, 1-phenyl-3-methyl-5-pyrazolone; BW, body weights; NC group, normal control group; MC group, model control group; LD group, low-dose group of ARPS; MD group, medium-dose group of ARPS; HD group, high-dose group of ARPS; CCK-8, Cell Counting Kit-8; PC group, positive control group; ARPS group, ARPS treatment group; H&E, hematoxylin and eosin; HPGP, high-performance gel –permeation chromatography.
Ethics Approval and Consent to Participate
The ethical approval of this study was provided by the Institutional Ethical Committee of the Faculty of Medicine, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fujian, China (No. 2020_051_01).
Acknowledgments
The authors wish to express their gratitude to Bin Wang (Department of Pathology, Mengchao Hepatobiliary Hospital of Fujian Medical University, China), Juxiang Zeng (Department of Infectious Diseases, Mengchao Hepatobiliary Hospital of Fujian Medical University, China), and Wenqian Jiang (Department of Infectious Diseases, Mengchao Hepatobiliary Hospital of Fujian Medical University, China) for the support on the manuscript.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval for the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by the Social Development Project of Fuzhou Science and Technology Bureau, P. R. C. [Grant numbers 2019-SZ-42]; the Special Project Scientific Research of the Fujian Provincial Department of Finance, China [Grant numbers 2019 B029]; and the Natural Fund Project of Fujian Province, China [Grant numbers 2020J011155, 2020J01623).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Du XM, Irino N, Furusho N, Hayashi J, Shoyama Y. Pharmacologically active compounds in the anoectochilus and goodyera species. J Nat Med. 2008;62(2):132–148. doi:10.1007/s11418-007-0169-0
2. Liu SS, Chen J, Li SC, Zeng X, Meng ZX, Guo SX. Comparative transcriptome analysis of genes involved in GA-GID1-DELLA regulatory module in symbiotic and asymbiotic seed germination of Anoectochilus roxburghii (Wall.) Lindl. (Orchidaceae). Int J Mol Sci. 2015;16(12):30190–30203. doi:10.3390/ijms161226224
3. Magassouba FB, Diallo A, Kouyate M, et al. Ethnobotanical survey and antibacterial activity of some plants used in Guinean traditional medicine. J Ethnopharmacol. 2007;114(1):44–53. doi:10.1016/j.jep.2007.07.009
4. Huang L, Cao Y, Xu H, Chen G. Separation and purification of ergosterol and stigmasterol in Anoectochilus roxburghii (wall) Lindl by high-speed counter-current chromatography. J Sep Sci. 2015;34(4):385–392. doi:10.1002/jssc.201000577
5. Zhang X, Jin M, Liu Y, et al. Oral bioavailability of kinsenoside in beagle dogs measured by LC-MS/MS: improvement of ex vivo stability of a lactone-containing compound. Pharmaceutics. 2018;10(3):87. doi:10.3390/pharmaceutics10030087
6. Zeng B, Su M, Chen Q, Chang Q, Wang W, Li H. Protective effect of a polysaccharide from Anoectochilus roxburghii against carbon tetrachloride-induced acute liver injury in mice. J Ethnopharmacol. 2017;200:124–135. doi:10.1016/j.jep.2017.02.018
7. Zeng B, Su M, Chen Q, Chang Q, Wang W, Li H. Antioxidant and hepatoprotective activities of polysaccharides from Anoectochilus roxburghii. Carbohydr Polym. 2016;153:391–398. doi:10.1016/j.carbpol.2016.07.067
8. Yang Z, Zhang X, Yang L, et al. Protective effect of Anoectochilus roxburghii polysaccharide against CCl4-induced oxidative liver damage in mice. Int J Biol Macromol. 2017;96:442–450. doi:10.1016/j.ijbiomac.2016.12.039
9. Qi CX, Zhou Q, Yuan Z, et al. Kinsenoside: a promising bioactive compound from anoectochilus species. Curr Med Sci. 2018;38(1):11–18. doi:10.1007/s11596-018-1841-1
10. Ye S, Shao Q, Zhang A. Anoectochilus roxburghii: a review of its phytochemistry, pharmacology, and clinical applications. J Ethnopharmacol. 2017;209:184–202. doi:10.1016/j.jep.2017.07.032
11. Guo Y, Ye Q, Yang S, et al. Therapeutic effects of polysaccharides from Anoectochilus roxburghii on type II collagen-induced arthritis in rats. Int J Biol Macromol. 2019;122:882–892. doi:10.1016/j.ijbiomac.2018.11.015
12. Ikeuchi M, Yamaguchi K, Nishimura T, Yazawa K. Effects of Anoectochilus formosanus on endurance capacity in mice. J Nutr Sci Vitaminol (Tokyo). 2005;51(1):40–44. doi:10.3177/jnsv.51.40
13. Masuda K, Ikeuchi M, Koyama T, et al. Suppressive effects of Anoectochilus formosanus extract on osteoclast formation in vitro and bone resorption in vivo. J Bone Miner Metab. 2008;26(2):123–129. doi:10.1007/s00774-007-0810-8
14. Hsiao HB, Lin H, Wu JB, Lin WC. Kinsenoside prevents ovariectomy-induced bone loss and suppresses osteoclastogenesis by regulating classical NF-kappaB pathways. Osteoporos Int. 2013;24(5):1663–1676. doi:10.1007/s00198-012-2199-z
15. Liu ZL, Zhang JG, Liu Q, Yi LT, Li YM, Li Y. The vascular protective effects of Anoectochilus roxburghii polysaccharose under high glucose conditions. J Ethnopharmacol. 2017;202:192–199. doi:10.1016/j.jep.2017.03.012
16. Thong NM, Vo QV, Huyen TL, Bay MV, Tuan D, Nam PC. Theoretical study for exploring the diglycoside substituent effect on the antioxidative capability of isorhamnetin extracted from Anoectochilus roxburghii. ACS Omega. 2019;4(12):14996–15003. doi:10.1021/acsomega.9b01780
17. Xu M, Shao Q, Ye S, et al. Simultaneous extraction and identification of phenolic compounds in Anoectochilus roxburghii using microwave-assisted extraction combined with UPLC-Q-TOF-MS/MS and their antioxidant activities. Front Plant Sci. 2017;8:1474. doi:10.3389/fpls.2017.01474
18. Yang LC, Hsieh CC, Lu TJ, Lin WC. Structurally characterized arabinogalactan from Anoectochilus formosanus as an immuno-modulator against CT26 colon cancer in BALB/c mice. Phytomedicine. 2014;21(5):647–655. doi:10.1016/j.phymed.2013.10.032
19. Yu X, Lin S, Zhang J, Huang L, Yao H, Li S. Purification of polysaccharide from artificially cultivated Anoectochilus roxburghii (wall.) Lindl. by high-speed counter current chromatography and its antitumor activity. J Sep Sci. 2017;40(22):4338–4346. doi:10.1002/jssc.201700340
20. Hua ZT, Ying HL, Yong W. Extraction and determination of the alkaloids in anoectochilus formosanus. Chem Res. 2006.
21. Chen X, Wu Y, Huang L, et al. Magnetic dispersive solid-phase micro-extraction combined with high-performance liquid chromatography for determining nucleotides in Anoectochilus roxburghii (Wall.) Lindl. J Pharm Biomed Anal. 2019;174:432–440. doi:10.1016/j.jpba.2019.06.010
22. Huang L, Zhong T, Chen T, Ye Z, Chen G. Identification of beta-sitosterol, stigmasterol and ergosterin in A. roxburghii using supercritical fluid extraction followed by liquid chromatography/atmospheric pressure chemical ionization ion trap mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(18):3024–3032. doi:10.1002/rcm.3181
23. Huang L, Chen T, Ye Z, Chen G. Use of liquid chromatography-atmospheric pressure chemical ionization-ion trap mass spectrometry for identification of oleanolic acid and ursolic acid in Anoectochilus roxburghii (wall.) Lindl. J Mass Spectrom. 2007;42(7):910–917. doi:10.1002/jms.1228
24. Huang L, Cao Y, Chen G. Purification of quercetin in Anoectochilu roxburghii (wall) Lindl using UMAE by high-speed counter-current chromatography and subsequent structure identification. Sep Purif Technol. 2008;64(1):101–107. doi:10.1016/j.seppur.2008.07.021
25. Huang L, Cao Y, Xu H, Chen G. Separation and purification of ergosterol and stigmasterol in Anoectochilus roxburghii (wall) Lindl by high-speed counter-current chromatography. J Sep Sci. 2011;34(4):385–392. doi:10.1002/jssc.201000577
26. Hui-Fang C, Pin-Pin W, Li-Ying H, et al. Analysis of gastrodine and 4-hydroxybenzyl alcohol in A. roxburghii by HPLC-DAD. Anal Test Technol Instrum. 2015.
27. Lin L, Huang L, Zheng Y. Extraction in polysaccharide of Anoectochilus roxburghii and research of its anti-oxidative. J Fujian Trad Chin Med Coll. 2006;16:37–40.
28. Zhong T, Huang L. Comparison of tumor proteomics of aqueous extraction in Anoectochilus roxburghii. Chin J Hosp Pharm. 2011;31:623–626.
29. Shih CC, Wu YW, Hsieh CC, Lin WC. Effect of Anoectochilus formosanus on fibrosis and regeneration of the liver in rats. Clin Exp Pharmacol Physiol. 2004;31(9):620–625. doi:10.1111/j.1440-1681.2004.04062.x
30. Wu JB, Chuang HR, Yang LC, Lin WC. A standardized aqueous extract of Anoectochilus formosanus ameliorated thioacetamide-induced liver fibrosis in mice: the role of Kupffer cells. Biosci Biotechnol Biochem. 2010;74(4):781–787. doi:10.1271/bbb.90824
31. Ito Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J Chromatogr A. 2005;1065(2):145–168. doi:10.1016/j.chroma.2004.12.044
32. Li C, Cai J, Geng J, Li Y, Wang Z, Li R. Purification, characterization and anticancer activity of a polysaccharide from Panax ginseng. Int J Biol Macromol. 2012;51(5):968–973. doi:10.1016/j.ijbiomac.2012.06.031
33. Alsop RM, Vlachogiannis GJ. Determination of the molecular weight of clinical dextran by gel permeation chromatography on TSK PW type columns. J Chromatogr A. 1982;246(2):227–240. doi:10.1016/s0021-9673(00)95862-x
34. Xavier L, Freire MS, Vidal-Tato I, González-álvarez J. Recovery of phenolic compounds from eucalyptus globulus wood wastes using PEG/phosphate aqueous two-phase systems. Waste Biomass Valori. 2016;8(2):443–452. doi:10.1007/s12649-016-9579-0
35. Yang W, Wang Y, Li X, Yu P. Purification and structural characterization of Chinese yam polysaccharide and its activities. Carbohydr Polym. 2015;117:1021–1027. doi:10.1016/j.carbpol.2014.09.082
36. Pang KS, Cherry WF, Ulm EH. Disposition of enalapril in the perfused rat intestine-liver preparation: absorption, metabolism and first-pass effect. J Pharmacol Exp Ther. 1985;233(3):788–795.
37. Jiang Y, Qi X, Gao K, et al. Relationship between molecular weight, monosaccharide composition and immunobiologic activity of Astragalus polysaccharides. Glycoconj J. 2016;33(5):755–761. doi:10.1007/s10719-016-9669-z
38. Gao J, Zhang T, Jin ZY, et al. Structural characterisation, physicochemical properties and antioxidant activity of polysaccharide from Lilium lancifolium Thunb. Food Chem. 2015;169:430–438. doi:10.1016/j.foodchem.2014.08.016
39. Wu X, Li R, Zhao Y, Liu Y. Separation of polysaccharides from Spirulina platensis by HSCCC with ethanol-ammonium sulfate ATPS and their antioxidant activities. Carbohydr Polym. 2017;173:465–472. doi:10.1016/j.carbpol.2017.06.023
40. Khan BM, Liu Y. High speed counter current chromatography: overview of solvent-system and elution-mode. J Liq Chromatogr Relat Technol. 2018;41(10):629–636. doi:10.1080/10826076.2018.1499528
41. Ren G, Xu L, Lu T, Yin J. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int J Biol Macromol. 2018;115:1202–1210. doi:10.1016/j.ijbiomac.2018.04.132
42. Zhang Z, Guo L, Yan A, Feng L, Wan Y. Fractionation, structure and conformation characterization of polysaccharides from Anoectochilus roxburghii. Carbohydr Polym. 2020;231:115688. doi:10.1016/j.carbpol.2019.115688
43. Zhao Y, Song X, Zhang L, Yu T. High gel chromatography determination of glycyrrhiza polysaccharide molecular weight and molecular weight distribution. J Tianjin Trad Chin Med. 2015;32(1):46–48.
44. Liu Q, Ning J, Ding K. Based on the efficient and gel permeation chromatography tremella polysaccharide quality control study. Chin Herb Med. 2011;42(9):1732–1735.
45. Yang X. The Protective Effect of ARPS on the Alcoholic Liver Injury Mice and ARPS Granule Prepared. Fujian Medical University; 2017.
46. Scholten D, Trebicka J, Liedtke C, Weiskirchen R. The carbon tetrachloride model in mice. Lab Anim. 2015;49(1 Suppl):4–11. doi:10.1177/0023677215571192
47. Yin L, Yu H, Peng J. Molecular mechanism of carbon tetrachloride induced liver damage and the progress of Chinese medicine intervention. J Mod Appl Pharm China. 2015;32(9):1147–1155.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.