Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Hepatic Safety of Febuxostat and Allopurinol for Gout Patients: A Systematic Review of Randomized Controlled Trial
Authors Dewi C, Puspita F, Puspitasari IM , Zakiyah N
Received 6 June 2023
Accepted for publication 22 August 2023
Published 18 September 2023 Volume 2023:19 Pages 731—743
DOI https://doi.org/10.2147/TCRM.S424598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Christiyanti Dewi,1 Falerina Puspita,1 Irma Melyani Puspitasari,1,2 Neily Zakiyah1,2
1Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, Indonesia; 2Center of Excellence for Pharmaceutical Care Innovation, Universitas Padjadjaran, Bandung, Indonesia
Correspondence: Neily Zakiyah, Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, 45363, Indonesia, Tel +62 22 84288888, Ext. 3510, Email [email protected]
Purpose: This study aims to systematically review the hepatic safety of febuxostat and allopurinol in adult gout patients.
Methods: We searched for information using the following databases: PubMed, Cochrane Library, and Scopus. The inclusion criteria were to review all randomized controlled trials (RCT) that compared allopurinol and febuxostat for adult gout patients that had an assessment of liver function outcomes. Non-English studies on case reports, case series, reviews, and abstracts only were excluded. We extracted information from the studies to answer the research question, ie, study design, publication year, population, sample size, patient characterization, duration, Jadad score, and liver function outcomes.
Results: We screened 512 publications from the databases and identified 11 studies that met the inclusion criteria. Ten out of 11 included studies were double-blind RCTs. In the majority of the included studies, no statistically significant differences were observed in terms of hepatic safety data between febuxostat and allopurinol. However, in studies where allopurinol titration was used, it posed a challenge to maintain blinding. Notably, consistent adverse events related to liver function findings were observed across all reviewed RCTs. These abnormal liver function test results sometimes led to study withdrawal based on the investigators’ assessment. Nevertheless, the investigators classified most liver function test elevations as mild to moderate in severity.
Conclusion: Our analysis concluded that adult gout patients enrolled in the included RCTs exhibited similar hepatic safety profiles for both febuxostat and allopurinol treatment. Liver function abnormalities were identified in all RCTs included in this systematic review. Consequently, it is important for the product labeling information of both allopurinol and febuxostat to present and describe the current safety data to guide healthcare practitioners when prescribing these medications to patients. Pharmacovigilance and post-marketing pharmacoepidemiology data are essential in establishing the comprehensive safety profile.
Keywords: febuxostat, allopurinol, gout, hepatic safety
Introduction
Both febuxostat and allopurinol have been extensively studied in clinical trials and have shown efficacy and safety as urate-lowering therapies for gout patients.1–4 Gout is a metabolic disease characterized by elevated serum uric acid levels and the deposit of monosodium urate crystal, resulting of inflammation and pain.5–8 It primarily affects males over 40 years of age, with a higher risk observed in individuals with cardiovascular disease, diabetes, obesity, renal disease, hyperlipidemia, and post-menopausal women.5,7,9–11 Optimal control of serum uric acid levels can reduce the clinical and financial burden linked to gout.12,13
Allopurinol is a xanthine oxidase inhibitor that is typically initiated at a starting dose of 100 mg/day and can be gradually increased to 900 mg/ day for patients with good renal function.14,15 The effective daily dose of allopurinol is generally 300 mg.2,5,16,17 Allopurinol is metabolized by xanthine oxidase to an active metabolite called oxypurinol, which inhibits xanthine oxidase. Due to the renal excretion of oxypurinol, patients with impaired renal function may experience prolonged excretion and elevated level of uric acids, which have been associated with the development of allopurinol hypersensitivity syndrome.15,18–20
On the other hand, febuxostat is a non-purine selective inhibitor of Xanthine Oxidase (NP-SIXO) and belongs to the class of 2-arylthiazole derivatives. Febuxostat selectively inhibits the Xanthine Oxidase enzyme to reduce serum uric acid levels. Unlike allopurinol, febuxostat does not significantly affect other enzymes involved in purine or pyrimidine metabolism at therapeutic doses. It is primarily metabolized in the liver through glucuronide formation and oxidation.21,22
Based on both efficacy and safety data for both drugs in several countries, including the United States, United Kingdom, Spain, and Korea, have designated allopurinol and febuxostat as first-line medications for gout patients.6,23–27
While there have been numerous clinical trials demonstrating the superior efficacy of febuxostat compared to allopurinol,1–4 there is currently a lack of systematic analysis data regarding the hepatic safety of febuxostat compared to allopurinol in gout patients. This study aims to assess the hepatic safety of febuxostat and allopurinol in adult gout patients through a systematic review.
Materials and Methods
Systematic Search Strategy
For this systematic review, multiple databases, including PubMed, The Cochrane Library, and Scopus, were utilized to gather relevant data until May 15th, 2023. The search was conducted to discover articles that provide information on the hepatic safety data of febuxostat and allopurinol in adult gout patients. The electronic search terminologies used in this study include “febuxostat”, “allopurinol”, “randomized controlled trial”, “gout”, “hyperuricemia”, and “adverse event”. The search was limited to the English language. The detail of the search terms in the databases is provided in Supplementary Table 1 . References from identified articles were also reviewed to determine relevant publications. Furthermore, The Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) checklist was used for this systematic review. The objectives and methods of this review were registered in the International Prospective Register of Systematic Reviews (PROSPERO) 2023, number CRD42023423942. The details are provided in Supplementary Tables 2–4.
Eligibility and Study Selection
The eligibility criteria comprised published randomized controlled trials (RCTs) involving adult patients (≥18 years - <65 years) with gout. Specifically, the studies that compared allopurinol and febuxostat reported liver function safety results as a means of identifying hepatic safety outcomes. We excluded case reports, case series, article reviews, conference proceedings, non-peer-reviewed papers, protocols, abstracts-only publications, and studies with non-RCT design. The collected studies included assessments on liver function outcomes outweigh upper limits normal (ULN) such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), or total bilirubin. The assessment of the effects in this study will focus on hepatotoxicity and liver function outcomes. These outcomes will be evaluated based on the number of events reported as a percentage, pertaining to specific criteria to at least one of the following outcomes ie, elevation of AST ≥ 1.5 × ULN, elevation of ALT ≥ 1.5 × ULN, elevation of ALT, AST or both ≥ 1.5 × ULN, elevation of ALP ≥ 2 × ULN, Hy’s case (ALT ≥ 3 × ULN and total bilirubin ≥ 1.5 × ULN), liver-related treatment, discontinuations, and liver-related hospitalization.
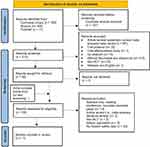
To ensure the accuracy of the selection process, two reviewers independently screened the articles during both initial title-and-abstract and full-text screening. Any duplicates and irrelevant articles were excluded. The eligibility of the remaining studies was assessed independently by the two reviewers, and any disagreements were resolved through consensus. The full-text data extraction and quality assessment were performed for all included studies. The full-text articles were thoroughly reviewed, and relevant information regarding study characteristics and safety outcomes was extracted. Figure 1 depicts the PRISMA flow diagram illustrating the study selection process.
Data Collection and Quality Assessment
The records were manually managed using an Excel spreadsheet to organize the information obtained from the included studies. Information from the included studies was extracted ie, study design, author, publication year, population (indication), intervention (type, dose, duration, and frequency), sample size, characteristic of patients (gender, age), study duration, type of safety outcomes (ie, liver function test abnormality, liver-related treatment discontinuation, hospitalization due to hepatic cause or acute liver failure, or death).
Jadad score was used to appraise the quality of included studies to evaluate the validity of the eligible RCTs. The Jadad score assesses the methodological quality of the included studies and comprises three components: randomization, blinding, and extent of loss to follow-up.28 Two reviewers independently assessed the methodological quality of the studies using the Jadad score, and any disagreements were resolved through consensus.
Results
Systematic Search
In this systematic review, a total of 619 electronic citations were initially identified from PubMed, the Cochrane Library, and Scopus. After removing 107 duplicate records, 512 records were screened based on their title and abstract up to May 6th, 2023. We further excluded 181 records that were article reviews, systematic reviews, meta-analyses, and case reviews, as well as 20 trial protocols, 1 cost-effectiveness study, 15 records without abstracts, 215 studies that did not involve febuxostat and allopurinol, 23 non-RCTs, and 1 non-English abstract.
Following the title and abstract screening, we sought the full text of 56 studies; however, one study could not be retrieved. During the full-text screening, we identified one additional article through snowballing, resulting in a total of 56 studies for final eligibility review. In total, 11 studies met the inclusion and exclusion criteria and were included in this systematic review. The remaining studies were excluded for various reasons, including 14 records with abstracts only or conference proceedings, non-peer-reviewed papers, 2 article reviews (meta-analysis, literature review), 5 non/poor RCTs, 3 studies focusing solely on the elderly population (≥65 years), and 20 trials lacking hepatic safety data.
The Main Characteristics of Included Studies
Table 1 presents the final inclusion of 11 studies.1,2,16–18,29–34 These studies were conducted in different countries, including 3 RCTs from United States,2,16,34 2 from the United States/ Canada,1,16 4 from China,29,30,32,33 1 from Japan,18 and 1 from multi countries (11 European countries and Brazil).31 The most recent study was conducted in 202233 while the oldest dates back to 2005.1
 |
Table 1 Characteristics of Included Studies |
The baseline demographic of the included studies mainly comprised male patients ≥ 18 years old, reflecting the higher prevalence of gout in males over 40 years old. The total number of patients included in the RCTs comparing febuxostat and allopurinol was 7810 patients.1,2,16–18,29–34 The studies evaluated various daily doses of febuxostat, ranging from low doses of 10/20 mg,18,33 40 mg,2,29,30,32 80 mg,1,2,16,17,29,30,32,34 120 mg1,16,17,31 and 240 mg17 per day, compared to different doses of allopurinol (50/100/200/300/600 mg per day).1,2,16,17,30,34 The duration of the studies varied, ranging from 7–9 days31 to up to 3 years.16 The gout study population was mainly referred to the American College of Rheumatology, American Rheumatism Association, or American Rheumatology Association categorization.1,2,16,17,30
Study Design
The majority of studies included in this systematic review were conducted with double-blind randomized study design (9 of 11).1,2,17,18,29–32,34 Two studies were performed as open-label or not explicitly described as double-blind.16,33 However, it should be noted that in studies involving the titration of allopurinol, blinding may be compromised.1,2,16,17,32
Hepatic Safety Data
Table 2 presents the hepatic safety data from all the studies included in this analysis.1,2,16–18,29–33 The hepatic safety data described in the studies were defined as abnormal findings on liver function tests, investigator-defined liver function abnormalities leading to discontinuation/ withdrawal, ALT increased, AST increased, both ALT and AST increased, AST/ ALT and total bilirubin increased, AST/ ALT and alkaline phosphatase elevation, hepatobiliary disorders, liver-related treatment-emergent Adverse Event (TEAE) or Treatment-emergent signs and symptoms (TESS), cholestasis, hepatotoxicity, and increased levels of gamma-glutamyltransferase. In addition, there were no reported data in the full-paper studies regarding Hy’s cases, liver-related hospitalization, and death.
 |
Table 2 Reported Liver Function Result and Other Hepatic Safety Evidence |
Main Findings
We observed consistent adverse events in liver function for both febuxostat and allopurinol. These abnormalities, as identified through liver function tests, sometimes resulted in study withdrawal, according to investigators’ assessment. However, the majority of elevations in liver function parameters were classified as mild to moderate in severity. Many of the included studies did not show statistically significant differences in hepatic safety data between febuxostat and allopurinol.2,16,18,27,29,31–34 However, the study by Schumacher et al in 2008 reported a significant increase in ALT (≥ 1.5 times the ULN) and AST (≥ 1.5 times the ULN) with febuxostat 80 mg compared to placebo. These events were classified as mild to moderate in severity, except for one case that was not transient. This particular subject, who received febuxostat 80 mg, had pre-existing hepatitis C and entered the study with elevated hepatic enzymes that persisted and led to premature discontinuation.17
Another study by Becker et al, 2005 showed significant differences in abnormal liver function test results, which were the most frequent adverse event leading to subject withdrawal. The comparison between 120 mg febuxostat (2.79%) and the allopurinol group (0.4%) demonstrated statistical significance (P=0.04). However, most adverse events were assessed as mild to moderate in severity and reversible after discontinuation of febuxostat.1
In another study by Spina et al in 2015 which assessed febuxostat versus allopurinol in patients with hematologic malignancies at intermediate to high risk of tumor lysis syndrome (TLS), the hepatobiliary disorders were experienced in the allopurinol group (0.6% for both overall hepatobiliary disorders, cholestasis, and hepatotoxicity preferred terms) and none were reported in the febuxostat 120 mg group.31
Discussion
To date, there is currently a lack of systematic analysis data regarding hepatic safety of febuxostat compared to allopurinol of gout patients. In this systematic review, we aimed to evaluate the hepatic safety profile of febuxostat compared to allopurinol in published RCTs. We identified a total of 11 eligible studies conducted between 2005 to 2022.1,2,16–18,29–34 The findings of this review indicate that most of the hepatic safety parameters assessed in adult patients did not show statistically significant differences between allopurinol and febuxostat (9 out of 11 studies).2,16,18,27,29,31–34 These results suggest that the hepatic safety data of febuxostat is comparable to that of allopurinol. This finding aligns with a meta-analysis conducted by Guo et al, in 2020, which assessed the risk of liver damage associated with both allopurinol and febuxostat. The meta-analysis included geriatric patients and found no statistically significant differences in the risk of liver damage between allopurinol (RR: 1.42; 95% CI: 0.89, 2.40) and febuxostat (RR: 1.47; 95% CI: 0.96, 2.40).35 Aside of that, indirectly comparison of allopurinol and febuxostat in a network meta-analysis (NMA) of six trials (1269 patients) did not result in a higher risk of impaired liver function of urate-lowering therapies.36
Regular monitoring of liver function tests is recommended in the management of chronic gout with urate-lowering therapy to identify self-limited liver test abnormalities and resolve them by discontinuing the medication.6,24,36 The hepatotoxicity mechanism of febuxostat is believed to be associated with its metabolism in the liver, primarily through glucuronidation and to a lesser extent via Cytochrome (CYP) 450 system.37 Based on the historical cohort data in Japan, liver disease was a common clinical comorbidities and demographic characteristic of hyperuricemia (31.4%) or gout (15.5%) patients.38 There is a limited review that discusses the hepatic safety of those two drugs as a comparison.
Allopurinol-induced hepatotoxicity is believed to be related to immunoallergic reactions, such as severe allopurinol hypersensitivity skin reaction, which are closely associated with the HLA B*58:01 allele. These reactions are commonly seen in cases of allopurinol liver injury accompanied by drug reaction with eosinophilia and systematic symptoms (DRESS syndrome) or Stevens Johnson syndrome/ toxic epidermal necrolysis (SJS/TEN).19,20,39–43 An included study showed hepatobiliary disorders were experienced in allopurinol groups only for cholestasis (0.6%) and hepatotoxicity (0.6%) and assessed as severe in intensity. These liver abnormalities did not appear in the febuxostat groups.31 Concerning this finding, we believed that all treatment-related adverse events found in the respective clinical trials should be reflected in the product labeling information, although for old-mature products like allopurinol.
The product labeling information of both allopurinol and febuxostat should provide accurate and up-to-date safety data to guide healthcare practitioners in prescribing these medications to patients. According to the U.S National Library of Medicine, transient and minor liver test abnormalities are associated with allopurinol therapy in 2–6% of patients,42 which is categorized as common in the latest product labeling information or summary product characterization (SmPC) of allopurinol.44 Similarly, the SmPC for febuxostat mentions the common occurrence of liver function abnormalities,45 which is consistent with the average of hepatotoxic adverse reactions (3.5%) reported in the US National Library of Medicine data.37
Nowadays, the shared decision-making (SDM) between patients and healthcare practitioners increases patients’ knowledge, and awareness and allows active interactions role in decision-making across diseases, especially gout. United States rheumatologists reported that they engaged in SDM for three of the five medication decisions with gout patients and need more support for patients in making choices for gout treatment.46 In fact, it was literate that the comprehensive patient information leaflet of both allopurinol and febuxostat would be valuable to support gout SDM decision.
The strength of our study was that we focused on intervention studies with minimal variability or bias, comparing allopurinol and febuxostat head-to-head and describing liver function outcomes. We also described the treatment duration in the eligible studies; however, the long-term safety studies of more than 3 years of monitoring were still lacking. In studies where allopurinol titration was used, it posed a challenge to maintain blinding. Meanwhile, febuxostat undergoes hepatic metabolism, and its dose adjustment and effect are less affected by the patient’s renal function to maintain blinding. The number of included studies was too few to describe the gout adult heterogeneous population treated with allopurinol and febuxostat. In the included studies, we also found that the hepatic safety result was not completely described and compared in the different ethnic groups, age, gender, and other patient demographic data.
Due to the limited number of patients involved in clinical trials of febuxostat and allopurinol, continuous monitoring of pharmacovigilance databases is crucial. An accurate description of the hepatic safety profile of allopurinol and febuxostat will assist healthcare practitioners in determining appropriate liver monitoring for patients receiving these medications for the management of gout.
Although this systematic review focused on the adult gout population in the setting of trials, we consistently observed liver function-related adverse events in all the reviewed RCTs. It is recommended further to examine available pharmacovigilance databases for hepatic safety data, encompassing a more diverse patient population and real-world data. The roles of pharmacovigilance and post-marketing pharmacoepidemiology data are crucial in establishing the comprehensive safety profiles of both febuxostat and allopurinol. Conducting future pharmacoepidemiology studies to explore the hepatic safety data of allopurinol and febuxostat is of utmost importance, given their frequent use by patients to treat this chronic disease. Such studies would be highly beneficial for ensuring patient safety and understanding any potential risks associated with these medications.
Conclusion
Based on our analysis, the hepatic safety data of adult gout patients enrolled in the included RCTs indicate that febuxostat and allopurinol have comparable safety profiles. Liver function abnormalities were observed in all studies. Regular monitoring of liver function tests is recommended in the management of chronic gout with urate-lowering therapy to identify self-limited liver test abnormalities. In addition, it is important to incorporate this information for the product labeling information and patient information leaflet of both allopurinol and febuxostat to provide accurate and current safety data as a reference for healthcare practitioners in prescribing these medications to patients indicating the roles of pharmacovigilance and post-marketing surveillance data in establishing the ultimate safety profile for both febuxostat and allopurinol.
Acknowledgments
The authors acknowledge the participating researchers who provided the raw data and published journals analyzed in this study.
Funding
This study is supported by a grant from Universitas Padjadjaran.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Becker MA, Schumacher HR, Wortmann RL, et al. Febuxostat Compared with Allopurinol in Patients with Hyperuricemia and Gout. Vol 23; 2005. Available from: www.nejm.org.
2. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63. doi:10.1186/ar2978
3. Sapankaew T, Thadanipon K, Ruenroengbun N, et al. Efficacy and safety of urate-lowering agents in asymptomatic hyperuricemia: systematic review and network meta-analysis of randomized controlled trials. BMC Nephrol. 2022;23(1). doi:10.1186/s12882-022-02850-3
4. Suzuki S, Yoshihisa A, Yokokawa T, et al. Comparison between febuxostat and allopurinol uric acid-lowering therapy in patients with chronic heart failure and hyperuricemia: a multicenter randomized controlled trial. J Int Med Res. 2021;49(12):030006052110627. doi:10.1177/03000605211062770
5. Hainer BL, Matheson E, Wilkes RT Diagnosis, Treatment, and Prevention of Gout. Vol 90; 2014. Available from: www.aafp.org/afp.
6. Neilson J, Bonnon A, Dickson A. Gout: diagnosis and management NICE guideline; 2022. Available from: www.nice.org.uk/guidance/ng219.
7. Singh JA, Gaffo A. Gout epidemiology and comorbidities. Semin Arthritis Rheum. 2020;50(3):S11–S16. doi:10.1016/j.semarthrit.2020.04.008
8. Singh JA. Quality of life and quality of care for patients with gout. Curr Rheumatol Rep. 2009; 11 (2) :154–160.
9. Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–662. doi:10.1038/nrrheum.2015.91
10. Li Q, Li X, Wang J, et al. Diagnosis and treatment for hyperuricemia and gout: a systematic review of clinical practice guidelines and consensus statements. BMJ Open. 2019;9(8):e026677. doi:10.1136/bmjopen-2018-026677
11. Pillinger MH, Mandell BF. Therapeutic approaches in the treatment of gout. Semin Arthritis Rheum. 2020;50(3):S24–S30. doi:10.1016/j.semarthrit.2020.04.010
12. Flores NM, Nuevo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1–6. doi:10.1080/13696998.2018.1532904
13. Jeon YK, Pearce F, Thong BYH, Aziz MIA, Aziz MIA. Cost-effectiveness of sequential urate lowering therapies for the management of gout in Singapore. J Med Econ. 2020;23(8):838–847. doi:10.1080/13696998.2020.1757456
14. Kannangara DRW, Graham GG, Wright DFB, et al. Individualising the dose of allopurinol in patients with gout. Br J Clin Pharmacol. 2017;83(9):2015–2026. doi:10.1111/bcp.13307
15. Proudman C, Lester SE, Gonzalez-Chica DA, Gill TK, Dalbeth N, Hill CL. Gout flares, and allopurinol use: a population-based study. Arthritis Res Ther. 2019;21(1). doi:10.1186/s13075-019-1918-7
16. Becker MA, Schumacher HR, MacDonald PA, Lloyd E, Lademacher C. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J Rheumatol. 2009;36(6):1273–1282. doi:10.3899/jrheum.080814
17. Schumacher HR, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, Phase III, randomized, double-blind, parallel-group trial. Arthritis Care Res. 2008;59(11):1540–1548. doi:10.1002/art.24209
18. Kamatani N, Fujimori S, Hada T, et al. An allopurinol-controlled, randomized, double-dummy, double-blind, parallel between-group, comparative study of febuxostat (TMX-67), a non-purine-selective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan: Phase 3 clinical study. J Clin Rheumatol. 2011;17(4 Suppl 2). doi:10.1097/rhu.0b013e31821d36cc
19. Iqbal U, Siddiqui HU, Anwar H, Chaudhary A, Quadri AA. Allopurinol-induced granulomatous hepatitis: a case report and review of literature. J Investig Med High Impact Case Rep. 2017;5(3). doi:10.1177/2324709617728302
20. Ng WL, Lim KS, Hariraj V, et al. Incidence of allopurinol-induced severe cutaneous adverse drug reaction in Malaysia. Br J Clin Pharmacol. 2022;88(8):3782–3788. doi:10.1111/bcp.15327
21. Gaffo AL, Saag KG Core evidence febuxostat: the evidence for its use in the treatment of hyperuricemia and gout; 2009. Available from: www.dovepress.com.
22. Chen C, Chung W, Lin Y, Chang C, Chang S. Risk of Severe Adverse Reactions Related to Allopurinol and Febuxostat in Asians. Value Health. 2018;21:S106. doi:10.1016/j.jval.2018.07.801
23. Malaysia, Ministry of Health Clinical Practice GuidelinesManagement of Gout Malaysian Society of Rheumatology, Academy of Medicine Malaysia Malaysia Health Technology Assessment Section, Medical Development Division 2021.
24. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020;72(6):744–760. doi:10.1002/acr.24180
25. Jeong H, Choi E, Suh A, Yoo M, Kim B. Risk of cardiovascular disease associated with febuxostat versus allopurinol use in patients with gout: a retrospective cohort study in Korea. Rheumatol Int. 2022;43(2):265–281. doi:10.1007/s00296-022-05222-0
26. Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American college of rheumatology guidelines for management of gout. part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64(10):1431–1446. doi:10.1002/acr.21772
27. Zhang S, Xu T, Shi Q, et al. Cardiovascular safety of febuxostat and allopurinol in hyperuricemic patients with or without gout: a network meta-analysis. Front Med. 2021:8. doi:10.3389/fmed.2021.698437
28. Stephen H, Halpern M, Douglas J. Appendix: Jadad Scale for Reporting Randomized Controlled Trials: Evidence-Based Obstetric Anesthesia. Blackwell Publishing Ltd; 2007:237–238.
29. Huang X, Du H, Gu J, et al. An allopurinol-controlled, multicenter, randomized, double-blind, parallel between-group, comparative study of febuxostat in Chinese patients with gout and hyperuricemia. Int J Rheum Dis. 2014;17(6):679–686. doi:10.1111/1756-185X.12266
30. Xu S, Liu X, Ming J, et al. A phase 3, multicenter, randomized, allopurinol-controlled study assessing the safety and efficacy of oral febuxostat in Chinese gout patients with hyperuricemia. Int J Rheum Dis. 2015;18(6):669–678. doi:10.1111/1756-185X.12648
31. Spina M, Nagy Z, Ribera JM, et al. FLORENCE: a randomized, double-blind, Phase III pivotal study of febuxostat versus allopurinol for the prevention of tumor lysis syndrome (TLS) in patients with hematologic malignancies at intermediate to high TLS risk. Ann Oncol. 2015;26(10):2155–2161. doi:10.1093/annonc/mdv317
32. Zhang F, Liu Z, Jiang L, et al. A randomized double-blind, non-inferiority study of febuxostat versus allopurinol in hyperuricemic Chinese subjects with or without gout. Rheumatol Ther. 2019;6(4):543–557. doi:10.6084/m9.figshare.9771893
33. Yang N, Cao B. Low-dose febuxostat exhibits a superior renal-protective effect and non-inferior safety profile compared to allopurinol in chronic kidney disease patients complicated with hyperuricemia: a double-centre, randomized, controlled study. J Clin Pharm Ther. 2022;47(12):2214–2222. doi:10.1111/jcpt.13794
34. Goldfarb DS, MacDonald PA, Gunawardhana L, et al. Randomized controlled trial of febuxostat versus allopurinol or placebo in individuals with higher urinary acid excretion and calcium stones. Clin J Am Soc Nephrol. 2013;8(11):1960–1967. doi:10.2215/CJN.01760213
35. Zhang S, Xie Q, Xie S, et al. The association between urate-lowering therapies and treatment-related adverse events, liver damage, and major adverse cardiovascular events (MACE): a network meta-analysis of randomized trials. Pharmacotherapy. 2021;41(9):781–791. doi:10.1002/phar.2609
36. Tien YY, Shih MC, Tien CP, Huang HK, Tu YK. To treat or not to treat? Effect of urate-lowering therapy on renal function, blood pressure and safety in patients with asymptomatic hyperuricemia: a systematic review and network meta-analysis. J Am Board Fam Med. 2022;35(1):140–151. doi:10.3122/jabfm.2022.01.210273
37. U.S National Library of Medicine. Febuxostat national center for biotechnology information; 2018. Available from: https://www/ncbi.nml.nih.gov/books/.
38. Akari S, Nakamura T, Furusawa K, Miyazaki Y, Kario K. The reality of treatment for hyperuricemia and gout in Japan: a historical cohort study using health insurance claims data. J Clin Hypertens. 2022;24(8):1068–1075. doi:10.1111/jch.14539
39. Cardoso CS, Vieira AM, Oliveira AP. DRESS syndrome: a case report and literature review. BMJ Case Rep. 2011;2011:bcr0220113898. doi:10.1136/bcr.02.2011.3898
40. Zeng M, Zhang M, Liu F, Yan W, Kong Q, Sang H. Drug eruptions induced by allopurinol associated with HLA-B*5801. Indian J Dermatol Venereol Leprol. 2015;81(1):43–45. doi:10.4103/0378-6323.148566
41. Villanueva-Paz M, Niu H, Segovia-Zafra A, et al. Critical review of gaps in the diagnosis and management of drug-induced liver injury associated with severe cutaneous adverse reactions. J Clin Med. 2021;10(22):5317. doi:10.3390/jcm10225317
42. U.S. National Library of Medicine. Allopurinol. National Center for Biotechnology Information; 2020. Available from: https://www/ncbi.nlm.nih.gov/books/.
43. Markel A. Allopurinol-induced DRESS syndrome. IMAJ. 2005;7(10):656.
44. Electronic Medicine Compendium, Allopurinol 300mg Tablets_SMPC_13SEP2018. https://www.medicines.org.uk/emc/product/9468/smpc/print.
45. Electronic Medicine Compendium, Febuxostat 80 mg film-coated tablets_SMPC_24APR2023. https://www.medicines.org.uk/emc/product/10521/smpc/print 2018.
46. Singh JA, Richards JS, Chang E, Toupin-April K, Barton JL. Shared decision-making in gout treatment: a national study of rheumatology provider opinion and practice. Clin Rheumatol. 2021;40(2):693–700. doi:10.1007/s10067-020-05421-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.