Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 16
Hepatic Artery Infusion Chemotherapy Sequential Hepatic Artery Embolization Combined with Operation in the Treatment of Recurrent Massive Hepatocellular Carcinoma Achieved Pathological Complete Response: A Case Report
Authors Chen J, Liao X , Wu Y, Ou S , Qin W, Yang C , Tan Y , Lao Q, Peng M, Peng T , Ye X
Received 19 July 2023
Accepted for publication 6 October 2023
Published 1 November 2023 Volume 2023:16 Pages 949—958
DOI https://doi.org/10.2147/PGPM.S426791
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Junjie Chen,1,* Xiwen Liao,1,2 Yining Wu,1,* Shenjian Ou,1 Wei Qin,1 Chengkun Yang,1,2 Yufeng Tan,1 Quan Lao,1 Minhao Peng,1 Tao Peng,1,2 Xinping Ye1
1Department of Hepatobiliary Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, 530021, People’s Republic of China; 2Guangxi Key Laboratory of Enhanced Recovery After Surgery for Gastrointestinal Cancer, Nanning, 530021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinping Ye; Tao Peng, Tel +86-771-5356528, Fax +86-771-5350031, Email [email protected]; [email protected]
Abstract: Hepatocellular carcinoma (HCC) recurrence, which encompasses both true recurrence resulting from cancer spread and de novo tumors developing within the same cancer-prone liver, presents a complication in approximately 70% of cases within a 5-year timeframe. The efficacy of neoadjuvant therapy for recurrence after hepatectomy for hepatocellular carcinoma is still unclear. We report a case of recurrent massive advanced hepatocellular carcinoma with pathological complete remission was treated by continuous hepatic arterial infusion chemotherapy (HAIC) and sequential transcatheter arterial embolization (TAE) combined with secondary operation. One month after resection, the patient recurred (massive type 141mm× 76mm). After 4 times of HAIC sequential TAE conversion therapy, the tumor shrank significantly (70mm× 29mm), alpha-fetoprotein(AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) levels decreased significantly, residual liver volume[left half liver accounted for 39.85% of standard liver volume(left half liver + right anterior lobe) accounted for 80.17% of standard liver volume] and Indocyanine green 15-minute retention(ICG R15 8.0%) complies with surgical requirement.The second operation was performed, and the tumor was completely resected after hepatic blood flow occlusion Requirement. The postoperative pathological results showed complete remission (PCR) of the tumor, and no recurrence was found during the follow-up of 16 months. In this case, HAIC sequential TAE conversion therapy has good short-term effect on patients with postoperative recurrence of hepatocellular carcinoma, tumor burden is significantly reduced, the second surgery pathology achieves complete remission, safety and tolerance, it is worthy of study and promotion.
Keywords: hepatocellular carcinoma, recurrence after resection, two-stage hepatectomy, complete remission, sequential therapy
Background
Hepatocellular carcinoma (HCC) is a common malignant tumor with a high morbidity and mortality in the world.1 According to epidemiological investigation, Guangxi is a high incidence area of HCC in China.2,3 The occurrence of HCC was significantly associated with high risk factors such as hepatitis B virus (HBV) and hepatitis C virus (HCV) infection,4–6 Alcohol,7 metabolic and endocrine diseases,8 and high exposure to aflatoxin B1 (AFB1) exposure.9–12 Radical surgery is still the most common and effective treatment for HCC, but the main problem is the 5-year recurrence rate is around 50% to 70% after surgery.13 There are many treatment options available for HCC recurrence after surgery, including systemic chemotherapy, hepatic arterial infusion chemotherapy, transcatheter arterial chemoembolization, Radiofrequency Ablation and surgery.14–29 The treatment choice hinges on the extent of tumor thrombosis, the severity of underlying cirrhosis, and the patient’s performance status, all of which can impact the prognosis. Nevertheless, there are currently no guidelines stipulating the preferred non-surgical treatment based on the available evidence.
Currently, research into neoadjuvant therapy for hepatocellular carcinoma (HCC) is still in its nascent stages.30 In a study conducted at Kaohsiung Veterans General Hospital, Mok et al observed limited benefits associated with hepatectomy when compared to multimodal therapies involving transcatheter arterial embolization (TAE) or HAIC for patients with giant HCC.31 Nonetheless, massive HCC is often associated with compromised liver reserve function, a heightened risk of intrahepatic metastasis, and vascular invasion, rendering it unsuitable for surgical resection. Consequently, transcatheter arterial embolization/chemoembolization (TAE/TACE) has been acknowledged as a palliative treatment option for unresectable large HCC.32 However, prior research has indicated that TACE may be less effective in managing extensive HCC, with TACE-related mortality rates ranging from 6.5% to 20%.31 Hepatic Arterial Infusion Chemotherapy serves as an alternative palliative treatment option for inoperable advanced HCC. It involves the direct injection of a high concentration of chemotherapeutic agents into the liver through the hepatic artery, aiming to maximize the anti-tumor effect at the tumor site. Furthermore, this approach is expected to result in fewer systemic side effects owing to the liver’s first-pass effect. Nevertheless, its efficacy is often limited, necessitating the combination with systemic therapy to enhance its effectiveness. The combination of HAIC with Transcatheter Arterial Embolization (TAE) can optimize the local concentration and duration of chemotherapeutic drugs through arterial infusion chemotherapy, effectively enhancing the cytotoxic impact of chemotherapy. In this context, we present a case of a patient with recurrent massive hepatocellular carcinoma after hepatectomy who underwent successful HAIC followed by TAE, subsequently leading to resection and achieving a pathological complete response (PCR).
Case Presentation
Our study received approval from the ethics committee of The First Affiliated Hospital of Guangxi Medical University (Ethics No:2023-E572-01). Written informed consent was obtained from the patient for participation in this study.
The patient, a 47-year-old male, was admitted to our hospital complaining of “right upper abdominal pain for ten days”. Initial diagnosis indicated a hepatic space-occupying lesion, and the patient reported a history of hepatitis B without any other significant medical history. Following comprehensive examinations, the diagnosis of HCC was confirmed.
On March 11, 2021, the patient underwent robot-assisted hepatectomy, cholecystectomy, and diaphragm repair. The postoperative diagnosis identified S6 segment HCC, with a Child-Pugh Score of 5 points, BCLC-A (Barcelona Clinical Liver Cancer), and CNLC I b (Chinese Liver Cancer) staging.
One month later, multiple lesions were observed in the S5 and S6 segments of the liver, with the largest measuring 14.7 cm×7.6 cm. Serum alpha-fetoprotein (AFP) levels were measured at 14.02 ng/mL, and abnormal prothrombin (PIVKA-II) levels were 38.77 mAU/mL, indicating postoperative recurrence of HCC. Subsequently, HAIC sequential TAE treatments were administered in June, July, September, and October 2021. Following these treatments, serum AFP levels decreased to 2.48 ng/mL, and PIVKA-II levels decreased to 7.06 ng/mL. Upper-abdominal enhanced computed tomography (CT) scans revealed a reduction in the tumor size to 7.0 cm×2.9 cm. The remaining liver volume was assessed to be suitable for surgical resection. On December 6, 2021, a surgical resection was performed, and postoperative pathology confirmed a complete response to treatment.
Treatment Process
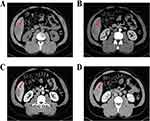
The first operation took place on March 8, 2021, when the patient was admitted due to “right upper abdominal pain persisting for 10 days”. A CT scan was conducted in the outpatient department of our hospital, which raised the suspicion of S6 segment liver cancer (Figure 1), The patient’s AFP level was measured at 381.74 ng/mL, and the PIVKA-II level was 9,997.07 ng/mL. A preliminary diagnosis of HCC was made (S6 Child -A 5 points, CNLC stage I b) based on these findings. Preoperative assessment was completed, and on March 11, 2021, a robot-assisted hepatectomy, cholecystectomy, and diaphragm repair were performed under general anesthesia in our hospital’s operating room. Postoperative pathology indicated that the shape of the mass in S6 was consistent with moderately differentiated hepatocellular carcinoma, with invasive tumor growth and no evidence of microvascular invasion (MVI) (grade = M0). The tumor was located adjacent to the surgical margin, with a margin distance of less than 1mm. The GS grade was G1S1, and the Ishak score showed 3 points for inflammation and 2 points for fibrosis (Figure 2). Immunohistochemistry was not performed due to the patient’s discharge. Postoperative diagnosis: 1. HCC (S 6, Child-A grade 5 points, BCLC-A, CNLC-I b); 2. Gallstones; 3. Clonorchiasis?
The treatment for postoperative recurrence commenced on June 10, 2021, following an outpatient reexamination of the upper abdomen using enhanced CT. The CT scan revealed multiple space-occupying lesions in segments S5/6/7, tumor thrombus formation in the right branch of the portal vein, and possible involvement of the hepatic flexure of the colon and right peritoneum. Additionally, there was enlargement of hepatic hilar and paraaortic lymph nodes, with metastasis not being ruled out. Laboratory examination indicated an AFP level of 77.5 ng/mL, PIVKA-II level of 910.35 AU/L, and a hepatitis B virus DNA level of 5.71 × 10^3.
Following an intra-group evaluation, it was determined that the patient had experienced a recurrent massive HCC post-operation and was not suitable for a secondary operation. The treatment plan involved primary percutaneous HAIC, with the second stage of treatment awaiting surgical consideration.
Relevant examinations were completed, and HAIC (FOLFOX 48h protocol) in combination with TAE was administered in June, July, September, and October 2021, respectively. The sequential treatment regimen comprised oxaliplatin at 85mg/m2 intra-arterial infusion for 2 hours, calcium folinate at 400mg/m2 intra-arterial infusion for 1 hour, or oxaliplatin at 130mg/m2 intra-arterial infusion for 3 hours, calcium folinate at 200mg/m2 intra-arterial infusion for 2 hours, followed by combined 5-fluorouracil at 400mg/m2 intra-arterial infusion and 5-fluorouracil at 2400mg/m2 intra-arterial infusion over 46 hours. After the infusion, transcatheter arterial tumor embolization was performed, with a treatment cycle lasting for 4 days and repeated every 3–4 weeks.
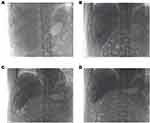
Serum AFP and PIVKA-II levels were monitored during each treatment cycle, and CT enhancement of the upper abdomen was performed every 8 weeks until meeting the surgical indication criteria (sufficient remaining liver volume after radical resection and no evidence of distant metastasis). The Lipiodol deposition area of the tumor after the fourth conversion treatment was significantly reduced (> 75%) compared to the first conversion treatment, The change is shown in the Figure 3. On October 12, during the reexamination with enhanced CT of the upper abdomen, it was observed that the liver segments S5/6/7 had partially fused into a mass, and the tumor diameter had decreased from 141mm to 70mm. According to the RECIST v1.1 standard, the tumor exhibited a reduction of 50.3%, thereby achieving a partial response. See Figure 4. AFP and PIVKA-II levels exhibited a continuous decrease throughout the course of treatment, and they returned to normal after the first two HAIC sequential TAE conversion treatments, as shown in Figure 5.
 |
Figure 5 Dynamic changes of tumor marker levels during treatment. (A) The reduction in levels of alpha-fetoprotein and (B) des-gamma-carboxyprothrombin reflects a reduction in tumor burden. |
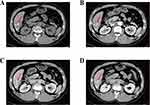
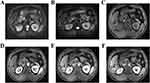
On December 8, 2021, After the preoperative CT and MR imaging evaluation (see Figures 6 and 7), the second hepatectomy was performed. Intraoperatively, it was determined that the tumor was located in liver segments S5/6/7, with multiple tumors present. The larger tumor, after fusion, measured approximately 7.0cm × 2.3cm. Notably, the tumor appeared to be adhering to the diaphragm muscle, which exhibited hardness and thickening. There was suspicion that the tumor had invaded the diaphragm muscle. Consequently, a portion of the diaphragm was excised during the surgery, and the excised tissue was sent for frozen pathology examination, which indicated the absence of cancer. No abnormalities were detected in the remaining tissue. Given the patient’s history of HCC recurrence after the initial resection and the high risk of recurrence and metastasis, a right hepatectomy was initially planned based on preoperative assessment. However, during the surgery, the residual liver volume following a right hepatectomy was estimated to be approximately 39%. To make a final determination, an intraoperative indocyanine green (ICG) measurement was conducted. The liver function of the remaining left liver was assessed after temporarily blocking the right hepatic pedicle. The retention rate of ICG at 15 minutes was found to be 37.7%. Consequently, it was decided to proceed with the surgical resection plan, which involved partial resection of liver segment S5 and complete resection of segments S6 and S7. The Pringle maneuver was employed during the liver surgery to minimize blood loss. For short periods of use, it is very effective in reducing intraoperative blood loss.
The closest distance from the tumor to the surgical margin was measured to be 2cm (as demonstrated in Figure 8) when the specimen was examined in vitro. Postoperative pathology analysis revealed that all specimens and masses obtained after the radical operation for HCC following interventional therapy were sectioned and examined. Under the microscope, multiple necrotic nodules were observed within the tumor. Additionally, the surrounding area showed signs of fibroplasia, lymphocyte infiltration, and a foam cell reaction. Notably, no residual tumor cells were detected under the microscope, indicating a complete response to treatment. Furthermore, the evaluation of microvascular invasion (MVI) indicated a grade of M0, signifying the absence of microvascular invasion. Upon palpation, no evidence of cancer was detected at the surgical margin. The surrounding liver tissue displayed chronic inflammatory changes, with a G2S1 classification. The Ishak score for inflammation was 4 points, and for fibrosis, it was 2 points. The results of special staining for Ag (silver) and PAS (Periodic Acid-Schiff) supported the aforementioned diagnosis. Pathological results showed complete response (PCR) as shown in Figure 9. Considering the high risk of recurrence and metastasis following the second operation, HAIC combined with TAE consolidation therapy was administered once more, one month after the surgery. The patients were subsequently monitored for a period of 18 months. AFP levels remained negative, and there was no evidence of recurrence or metastasis upon reexamination through an enhanced CT scan of the upper abdomen.
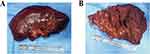 |
Figure 8 The second hepatectomy specimen. (A and B) show part of liver in segment V and all of segment VI and VII. |
Discussion
Hepatocellular carcinoma is a leading cause of cancer-related mortality globally, ranking as the third most prevalent cancer in men and the seventh most common in women.33,34 The recurrence and metastasis rates of hepatocellular carcinoma remain elevated even five years after hepatectomy, posing a significant clinical challenge and closely linked to poor patient prognosis. It is widely acknowledged that conversion therapy following a relapse is pivotal in enhancing both the quality of life and the survival duration of patients. In recent years, minimally invasive interventional therapies have expanded the treatment options for cases of massive HCC where immediate surgical intervention is not feasible. Only a limited number of patients can undergo neoadjuvant conversion therapy to stimulate compensatory proliferation in the remaining healthy liver tissue, thereby increasing liver reserve capacity and potentially enabling subsequent curative surgery. Despite the absence of robust high-level evidence to firmly establish the role of neoadjuvant therapy in HCC, the exploration of novel strategies is essential given the high rates of metastasis and postoperative recurrence associated with HCC. It’s worth noting that TACE stands as the standard treatment for unresectable HCC.35–37but the long-term efficacy of TACE is not satisfactory for patients with tumors>10 cm in diameter, with a disease control rate (DCR) of <50% and a median overall survival (OS) of 6.5–9.1 months.38,39 In recent years, hepatic arterial infusion chemotherapy with the FOLFOX regimen has seen rapid advancements, with its therapeutic efficacy gaining recognition. In comparison to TACE, HAIC is administered over multiple days, leading to a significantly prolonged local retention time of chemotherapy drugs and an enhanced drug concentration within the effective range. According to Gourd40 and other clinical data analysis, the OS of patients in FOLFOX-HAIC group was significantly longer than that of patients in TACE group (23.10 months vs 16.07 months, P<0.01), the incidence of treatment-related serious adverse events was also lower (19% vs 30%, P=0.03), and the proportion of patients in FOLFOX-HAIC group receiving radical surgical resection after conversion therapy was also significantly higher (23.8% vs 11.5%, P=0.004). The results of the study further confirmed the value of FOLFOX-HAIC as a means of HCC transformation therapy. Although FOLFOX-HAIC has obvious advantages over conventional methods as a translational therapy, its overall transformation success rate for unresectable HCC is still less than 30%. Although Dr. W Guo’s retrospective study41 from 2015 to 2016 has already explored the efficacy of TAE+HAIC, the contribution of this case report lies in the modification of the sequential treatment steps by adopting a method of first perfusion and then embolization, using iodized oil as the embolic agent. Our rationale is as follows: 1. Embolizing first may potentially occlude tumor peripheral vessels, affecting the subsequent penetration of chemotherapy drugs into the tumor, thereby impacting drug efficacy; 2. Embolization after perfusion chemotherapy can, on one hand, block the vessels, reducing the possibility of chemotherapy drug reflux, lowering drug concentration, and minimizing chemotherapy drug side effects. On the other hand, iodized oil is phagocytosed by tumor macrophages, aiding in imaging CT assessment of treatment efficacy, enabling the evaluation of tumor boundaries, and diagnosing subfocus, among other benefits. It is these characteristics that make this combination therapy more effective, achieving complete tumor pathologic remission, warranting further research with larger sample sizes and animal experiments for validation. If the results are indeed significant, it is worth considering further clinical application. Performing iodized oil embolization therapy after continuous arterial infusion chemotherapy in patients leverages the advantages of arterial infusion chemotherapy to the fullest extent, extending the onset time of high-concentration chemotherapy drugs as much as possible, providing a better environment for chemotherapy drugs to target tumor cells. Monitoring the levels of AFP and PIVKA-II during the course of salvage therapy is helpful in evaluating the efficacy of the HCC system. The assessment of systemic treatment efficacy requires a comprehensive consideration of tumor markers and imaging. The trend of tumor markers may not always align with imaging system assessments throughout the process, suggesting that a certain period may be needed to achieve a response to transition therapy.
In summary, the customization and integration of our existing treatment strategies into a potent tool for effectively eradicating tumors while safeguarding patients is an ongoing subject that demands continuous research and exploration. This case demonstrates that the combination of hepatic arterial infusion chemotherapy (HAIC) followed by transcatheter arterial embolization (TAE) as preoperative adjuvant therapy for recurrent massive hepatocellular carcinoma is both safe and feasible, showcasing effectiveness and potential as a novel approach in liver cancer treatment. However, further validation of the efficacy of this treatment approach requires a substantial number of randomized controlled trials in the future.
Abbreviations
HAIC, hepatic arterial infusion chemotherapy; TAE, transcatheter arterial embolization; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; PCR, pathological complete remission; HCC, Hepatocellular carcinoma; HBV, hepatitis B virus; AFB1, aflatoxin B1; CT, computed tomography; FOLFOX, fluorouracil combined with folic acid and oxaliplatin; OS, overall survival; Ag, silver; PAS, Periodic Acid-Schiff.
Ethics Approval and Consent to Participate
This study involving patient was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. The patient provided his written informed consent to participate in this study.
Consent for Publication
Written informed consent was obtained from the patient to publish this case report.
Acknowledgments
The authors would like to thank Professor Xinping Ye and Professor Minhao Peng, Department of Hepatobiliary Surgery, Guangxi Medical University, for their contributions in interpreting the significance of the results of this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that taking part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported in part by the Natural Science Foundation of Guangxi Province of China (No. 2020GXNSFAA159127).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Liu DM, Song TQ. Changes in and challenges regarding the surgical treatment of hepatocellular carcinoma in China. Biosci Trends. 2021;15(3):142–147. doi:10.5582/bst.2021.01083
2. Wang XY, Huang JM, Lu XM, et al. Changing risk factors for hepatocellular carcinoma in hyperendemic regions in the era of universal hepatitis B vaccination. Cancer Epidemiol. 2020;67:101775. doi:10.1016/j.canep.2020.101775
3. Yuan JM, Govindarajan S, Henderson BE, Yu MC. Low prevalence of hepatitis C infection in hepatocellular carcinoma (HCC) cases and population controls in Guangxi, a hyperendemic region for HCC in the People’s Republic of China. Br J Cancer. 1996;74(3):491–493. doi:10.1038/bjc.1996.389
4. McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19(2):223–238. doi:10.1016/j.cld.2015.01.001
5. Wong MCS, Huang JLW, George J, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16(1):57–73. doi:10.1038/s41575-018-0055-0
6. Ng J, Wu J. Hepatitis B- and hepatitis C-related hepatocellular carcinomas in the United States: similarities and differences. Hepat Mon. 2012;12(10 HCC):e7635. doi:10.5812/hepatmon.7635
7. Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112(3):580–593. doi:10.1038/bjc.2014.579
8. Jinjuvadia R, Patel S, Liangpunsakul S. The association between metabolic syndrome and hepatocellular carcinoma systemic review and meta-analysis. J Clin Gastroenterol. 2014;48(2):172–177. doi:10.1097/MCG.0b013e3182a030c4
9. Han CY, Yu TD, Qin W, et al. Genome-wide association study of the TP53 R249S mutation in hepatocellular carcinoma with aflatoxin B1 exposure and infection with hepatitis B virus. J Gastrointest Oncol. 2020;11(6):1333–+. doi:10.21037/jgo-20-510
10. Zhu Q, Ma YR, Liang JB, et al. AHR mediates the Aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct Tar. 2021;6:1.
11. Zhou R, Liu MZ, Liang XL, Su M, Li R. Clinical features of aflatoxin B1-exposed patients with liver cancer and the molecular mechanism of aflatoxin B1 on liver cancer cells. Environ Toxicol Phar. 2019;2019:71.
12. Long XD, Ma Y, Zhou YF, Ma AM, Fu GH. Polymorphism in xeroderma pigmentosum complementation group C Codon 939 and aflatoxin B1-related hepatocellular carcinoma in the guangxi population. Hepatology. 2010;52(4):1301–1309.
13. Sugawara Y, Hibi T. Surgical treatment of hepatocellular carcinoma. Biosci Trends. 2021;15(3):138–141.
14. Liu JG, Zhang JJ, Wang YJ, Shu GM, Lou C, Du Z. HAIC versus TACE for patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Medicine. 2022;101:51.
15. Mei JH, Yu HY, Qin LH, Jia ZZ. FOLFOX-HAIC for unresectable large hepatocellular carcinoma: the effectiveness has yet to be determined. J Clin Oncol. 2022;40(16):1841–+. doi:10.1200/JCO.21.02533
16. Yao W, Wei R, Jia J, et al. Development and validation of prognostic nomograms for large hepatocellular carcinoma after HAIC. Ther Adv Med Oncol. 2023;2023:15.
17. Sidaway P. FOLFOX-HAIC active in large HCC. Nat Rev Clin Oncol. 2022;19(1):5. doi:10.1038/s41571-021-00577-y
18. Comito T, Loi M, Franzese C, et al. Stereotactic radiotherapy after incomplete transarterial (Chemo-) Embolization (TAE\TACE) versus Exclusive TAE or TACE for Treatment of Inoperable HCC: a Phase III Trial (NCT02323360). Curr Oncol. 2022;29(11):8802–8813. doi:10.3390/curroncol29110692
19. Luo L, He Y, Zhu G, et al. Hepatectomy after conversion therapy for initially unresectable HCC: what is the difference? J Hepatocell Carcinoma. 2022;9:1353–1368. doi:10.2147/JHC.S388965
20. Kudo M. A novel treatment strategy for patients with intermediate-stage HCC who are not suitable for TACE: upfront Systemic therapy followed by curative conversion. Liver Cancer. 2021;10(6):539–544. doi:10.1159/000519749
21. Terzi E, Piccinnu M, Piscaglia F, et al. Survival of patients with hepatocellular Carcinoma (Hcc) within the bologna liver oncology group: comparison with international guidelines. Digest Liver Dis. 2015;47:E33–E. doi:10.1016/j.dld.2015.01.074
22. Ozdemir BH. Tumor microenvironment: necroptosis switches the subtype of liver cancer while necrosis promotes tumor recurrence and progression. Exp Clin Transplant. 2023;21(4):291–298. doi:10.6002/ect.2021.0457
23. Wu ZQ, Fan J, Qiu SJ, Zhou J, Tang ZY. The value of postoperative hepatic regional chemotherapy in prevention of recurrence after radical resection of primary liver cancer. World J Gastroentero. 2000;6(1):131–133. doi:10.3748/wjg.v6.i1.131
24. Qin S, Zhang X, Guo W, et al. Prognostic nomogram for advanced hepatocellular carcinoma treated with FOLFOX 4. Asian Pac J Cancer Prev. 2017;18(5):1225–1232. doi:10.22034/APJCP.2017.18.5.1225
25. Li S, Mei J, Wang Q, et al. Postoperative adjuvant transarterial infusion chemotherapy with FOLFOX could improve outcomes of hepatocellular carcinoma patients with microvascular invasion: a preliminary report of a phase III, randomized controlled clinical trial. Ann Surg Oncol. 2020;27(13):5183–5190. doi:10.1245/s10434-020-08601-8
26. Hsu SJ, Xu X, Chen MP, et al. Hepatic arterial infusion chemotherapy with modified FOLFOX as an alternative treatment option in advanced hepatocellular carcinoma patients with failed or unsuitability for transarterial chemoembolization. Acad Radiol. 2021;28(1):S157–S66. doi:10.1016/j.acra.2021.01.024
27. Li S, Mei J, Wang Q, et al. Correction to: postoperative adjuvant transarterial infusion chemotherapy with FOLFOX could improve outcomes of hepatocellular carcinoma patients with microvascular invasion: a preliminary report of a phase III, randomized controlled clinical trial. Ann Surg Oncol. 2021;28(Suppl S3):874. doi:10.1245/s10434-021-09813-2
28. Huang J, Huang W, Zhan M, et al. Drug-eluting bead transarterial chemoembolization combined with FOLFOX-based hepatic arterial infusion chemotherapy for large or huge hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:1445–1458. doi:10.2147/JHC.S339379
29. Li S, Mei J, Wang Q, et al. Transarterial infusion chemotherapy with FOLFOX for advanced hepatocellular carcinoma: a multi-center propensity score matched analysis of real-world practice. Hepatobiliary Surg Nutr. 2021;10(5):631–645. doi:10.21037/hbsn.2020.03.14
30. Shinkawa H, Tanaka S, Takemura S, et al. Nomograms predicting extra- and early intrahepatic recurrence after hepatic resection of hepatocellular carcinoma. Surgery. 2021;169(4):922–928. doi:10.1016/j.surg.2020.10.012
31. Mok KT, Wang BW, Lo GH, et al. Multimodality management of hepatocellular carcinoma larger than 10 cm. J Am Coll Surgeons. 2003;197(5):730–738. doi:10.1016/j.jamcollsurg.2003.07.013
32. He Q, Yang J, Jin Y. Development and validation of tace refractoriness-related diagnostic and prognostic scores and characterization of tumor microenvironment infiltration in hepatocellular carcinoma. Front Immunol. 2022;13:869993. doi:10.3389/fimmu.2022.869993
33. Fitzmaurice C, Dicker D, Pain A, et al. The global burden of cancer 2013 global burden of disease cancer collaboration. JAMA Oncol. 2015;1(4):505–527. doi:10.1001/jamaoncol.2015.0735
34. Akinyemiju T, Abera S, Ahmed M, et al.; Global Burden of Disease Liver Cancer C. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3(12):1683–1691. doi:10.1001/jamaoncol.2017.3055
35. Liver EAS, Liver EAS, Canc EORT. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(6):1430.
36. Verslype C, Rosmorduc O, Rougier P, Grp EGW. Hepatocellular carcinoma: ESMO-ESDO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:41–48. doi:10.1093/annonc/mds225
37. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi:10.1002/hep.24199
38. Xue TC, Le F, Chen RX, et al. Transarterial chemoembolization for huge hepatocellular carcinoma with diameter over ten centimeters: a large cohort study. Med Oncol. 2015;32(3). doi:10.1007/s12032-015-0504-3
39. Huang YH, Wu JC, Chen SC, et al. Survival benefit of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma larger than 10 cm in diameter. Aliment Pharm Ther. 2006;23(1):129–135. doi:10.1111/j.1365-2036.2006.02704.x
40. Gourd K, Lai C, Reeves C. ESMO Virtual Congress 2020. Lancet Oncol. 2020;21(11):1403–1404. doi:10.1016/S1470-2045(20)30585-4
41. Guo W, Gao J, Zhuang W, Wu Z, Li B, Chen S. Efficacy and safety of hepatic arterial infusion chemotherapy combined with transarterial embolization for unresectable hepatocellular carcinoma: a propensity score‐matching cohort study. JGH Open. 2019;4(3):477–483. doi:10.1002/jgh3.12285
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.