Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 16
Heme Metabolism-Related Gene TENT5C is a Prognostic Marker and Investigating Its Immunological Role in Colon Cancer
Authors Han W, Li C, Wang Y, Huo B, Li W , Shi W
Received 3 August 2023
Accepted for publication 30 November 2023
Published 23 December 2023 Volume 2023:16 Pages 1127—1143
DOI https://doi.org/10.2147/PGPM.S433790
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Wei Han,1 Cheng Li,1 Yongheng Wang,1 Binliang Huo,1 Wenhan Li,1 Wen Shi2
1Department of Surgical Oncology, Shaanxi Provincial People’s Hospital, Xi’an, People’s Republic of China; 2Department of Gastroenterology, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China
Correspondence: Wen Shi, Department of Gastroenterology, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710061, People’s Republic of China, Email [email protected]
Background: There is a strong correlation between consuming high amounts of heme and an elevated risk of developing various types of cancer, including colorectal cancer. However, the role of heme metabolism-related genes (HRGs) in colorectal cancer remains unclear. Our study aimed to identify prognostic markers for colorectal cancer patients based on these genes.
Methods: The heme metabolism score was assessed using gene set variation analysis (GSVA). Potential prognostic HRGs were identified from the TCGA-COAD dataset using LASSO and COX regression analyses. The expression level of TENT5C was validated in the GEO database and clinical samples. To explore the association between TENT5C expression and immune cell infiltrations, we performed ESTIMATE and single-sample gene set enrichment analysis (ssGSEA).
Results: The low level of heme metabolism score was associated with a poor prognosis in colorectal cancer patients. TENT5C is a prognostic gene and an independent prognostic biomarker for overall survival. Its expression was confirmed in multiple datasets and clinical samples, showing a positive correlation with immune cells and immune score. GSEA results suggested TENT5C’s significant role in regulating immune and inflammatory responses in colorectal cancer.
Conclusion: TENT5C can be used as a biomarker in colorectal cancer. Additionally, TENT5C is associated with both prognosis and immune infiltration. These findings lay a strong groundwork for future research to delve into the specific role of TENT5C in the development and advancement of colorectal cancer.
Keywords: colon cancer, immune cell infiltrations, prognosis, heme metabolism
Introduction
Colon cancer is a highly malignant tumor that significantly affects the quality of life for millions of individuals.1 Approximately 25% of colon patients are diagnosed with stage IV tumors. Despite some patients being diagnosed early, the cancer can still metastasize.2 Global statistics indicate that there are over 2.2 million new cases of colon cancer annually.3 The current treatment modalities, such as radiotherapy, chemotherapy, surgery, or their combination, have greatly enhanced the survival rate of patients.4 Currently, surgery is the primary approach for treating colon cancer. However, due to the elevated risk of recurrence and metastasis post-surgery, the overall survival rate for patients remains suboptimal.5,6 Biomarkers have been utilized to assist in identifying patients with a heightened risk of tumor progression or recurrence, such as the presence of microsatellite instability, BRAF mutations, or RAS mutations.7,8 Consequently, the investigation of molecular alterations in colon cancer patients has become a central area of research for prognostic biomarkers in colon cancer.9
Heme, an iron-containing porphyrin, acts as a prosthetic group and interacts with a diverse range of proteins. These protein complexes, known as hemoproteins, are essential for various cellular functions, including hemoglobin, myoglobin, and cytochromes, as well as catalase and peroxidase enzymes. Heme is present in all eukaryotic cells and plays a vital role in their survival and behavior.10 Heme also plays a crucial role in various biological functions, including erythroid biogenesis, neurogenesis, pancreatic development, and circadian rhythm.11 Moreover, heme directly controls essential cellular processes such as cell translation, cell transcription, cell death, and cell cycle.12,13 Imbalances in heme levels have been linked to a range of diseases, including coronary heart disease, diabetes, anemia, neurodegenerative diseases, and porphyria, as well as colon, pancreatic, and lung cancers.14–17 Consequently, the regulation of heme levels is highly important. Moreover, epidemiological and experimental research indicated a potential link between the elevated heme levels found in red meat and various diseases, such as cancer, diabetes, and heart disease.12,18,19 Compared to white meat, red meat (including beef, lamb, and pork) contains a significantly higher amount of heme. Numerous studies have established a positive correlation between increased consumption of red meat and a higher risk of developing colorectal cancer.20,21 Heme oxygenase-1 (HO-1) has been identified as a pivotal factor in protecting the gastrointestinal tract’s mucosal defense. The utilization of Kaplan-Meier analysis revealed a notable improvement in the survival rates of colorectal cancer patients who exhibited colonic HO-1 expression.22 Therefore, genes associated with heme metabolism may serve as potential biomarkers for predicting the prognosis of colon cancer. However, there are still limited studies investigating the correlation between heme metabolism-related genes (HRGs) and the outcomes of colon cancer patients.
The progress in gene sequencing technology and the availability of public databases have made it easier to collect gene expression data, thereby speeding up the identification of prognostic biomarkers.23,24 In this study, we utilized the GSVA algorithm to evaluate heme metabolism. Differentially expressed HRGs were identified from the TCGA dataset. We employed LASSO COX regression analysis to identify prognosis-related HRGs. Moreover, TENT5C was discovered as a potential prognostic biomarker with a strong association with immune cell infiltration in colon cancer. Our discoveries lay the groundwork for the clinical prognosis and treatment of colon cancer. Figure 1 shows the flow chart of this study.
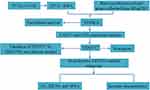 |
Figure 1 Flowchart of the present study. |
Materials and Methods
Data Resources and Processing
RNA-sequencing data and clinicopathological information for colon cancer were obtained from the TCGA database (https://portal.gdc.cancer.gov/). The TCGA cohort included 454 tumor samples and 41 normal samples. TPM format RNA-seq data from TCGA were collected. The expression data were transformed to log2 [TPM+1] and used to generate box plots.25,26 The GSE39582 dataset, which consisted of 566 cancer samples and 19 normal samples, was obtained from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/gds/). HRGs were collected from the molecular signatures database. Additionally, the expression of TENT5C in colon cancer was further examined using the TNMplot.com analysis platform (www.tnmplot.com). In addition, the Cancer Cell Line Encyclopedia (CCLE) database, available at https://portals.broadinstitute.org/ccle, consists of a total of 1457 cancer cell lines with 136,488 distinct data points. Hence, we utilized the CCLE database to investigate the TENT5C expression in colon cancer cell lines.
Gene Set Enrichment Analysis (GSEA)
To identify the most significant pathways among the molecular subgroups, we utilized GSEA with the “ClusterProfiler” package. The “h.all.v7.4.symbols.gmt” subset from the Molecular Signatures Database was downloaded to evaluate relevant pathways and molecular mechanisms. Any p-value below 0.05 was regarded as statistically significant.
Gene Set Variation Analysis (GSVA)
GSVA is an unsupervised method used to evaluate the variation of pathway activity within a population sample.27 In this research, the GSVA algorithm was employed to compute the score for heme metabolism. Subsequently, a Kaplan-Meier survival analysis was conducted to examine the relationship between the heme metabolism score and the overall survival of colon patients.
Identification of Differentially Expressed HRGs
We employed the “limma” package in R to identify differentially expressed HRGs (DEHRGs) between tumor and normal samples, using a threshold of p.adjust < 0.05 and |log fold change (FC)| ≥ 1. To generate the heatmap, we utilized the “pheatmap” package, and for the volcano plot, we utilized the “ggplot2” package.
Functional Enrichment Analysis
We employed the R package “clusterProfiler” to carry out enrichment analysis for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). The “ggplot2” package in R was used to visualize the results. A term was deemed statistically significant if its p-value was less than 0.05.
Screening of Prognostic Genes
The least absolute shrinkage and selection operator (LASSO) analysis based on the DEHRGs was performed using the “glmnet” and “survival” packages. Subsequently, signature genes were further selected through multivariate Cox regression analysis. The “ggplot2” and “survminer” packages were utilized to plot survival curves and assess the relationship between core DEHRGs and overall survival.
The Impact of Genetic Modification and DNA Methylation on Prognosis
We utilized Oncoprinter from cBioportal to assess the genetic alterations, such as gene mutations and copy number alterations, that may influence the expression of TENT5C. Additionally, we investigated the correlation between TENT5C and the survival of patients with colon cancer.28 MethSurv was used to analyze the impact of DNA methylation, a risk factor, on the expression of TENT5C in colon cancer patients. Specifically, the analysis focused on the effect of a single-methylation CpG site on the overall survival of these patients.29
Discovery of Small-Molecule Medications
The Connectivity Map (CMap) was used to identify potential connections between treatments, genes, and diseases by analyzing the impact of disruptions on gene expression.30 We retrieved drug signatures from the CMap database (https://clue.io/). The input data consisted of the top 150 up-regulated genes between normal and colon cancer groups. The analysis yielded scores ranging from −100 to 100. Negative scores for small-molecule drugs indicate the potential to reverse gene expression, indicating therapeutic value. We identified the top ten small-molecule compounds with the lowest CMap scores for further consideration.
Establishment of Nomogram
A nomogram was developed based on the findings of a multivariate analysis to predict the overall survival of patients with colon cancer. The nomogram was created using the “ggplot2” and “survival” R packages, and calibration curves were plotted accordingly.
Identification of TENT5C-Related Subgroups and Enrichment Analysis
We categorized colon cancer patients into low- and high-TENT5C subgroups based on the median TENT5C expression value. Differential gene expression analysis was conducted between these subgroups using the “limma” package in R, with filtering conditions set at p.adjust < 0.05 and |log FC|> 1. To explore the potential biological functions associated with the differentially expressed genes (DEGs), we performed GSEA using the “clusterProfiler” package in R. Enrichment analysis results were also visualized using the “ggplot2” package.
Tumor Microenvironment Analysis
We compared the stromal score, immune score, and ESTIMATE score using the ESTIMATE software in the low-TENT5C and high-TENT5C subgroups. Additionally, the single-sample gene set enrichment analysis (ssGSEA) was used to compare the abundance of immune cells in these subgroups. To analyze and visualize the results, we employed the “ggplot2” package for correlation analysis. We utilized the TISCH database, available at http://tisch.comp-genomics.org, as a valuable resource for investigating the diverse composition of the tumor microenvironment across multiple datasets and cell types.31 This comprehensive collection of single-cell RNA sequencing data allowed us to gain valuable insights into the heterogeneity of the tumor microenvironment in different cell populations.
Validation of Prognostic Gene by Quantitative Reverse Real-Time PCR (qRT-PCR) Analysis
We collected 8 paired tumors and normal colonic tissues from patients with colon cancer who underwent surgery at the Department of Surgical Oncology, Shaanxi Provincial People’s Hospital. All patients provided written informed consent before participating in the study. RNA extraction from the tissue samples was conducted using TRIzol reagent (Invitrogen, CA, USA) following the manufacturer’s protocol. The obtained RNA (2 µg) was then utilized for cDNA synthesis through a cDNA synthesis kit (Takara, Bio, Inc., China). qRT-PCR was performed on a StepOne real-time PCR System (Applied Biosystems, USA). The 2–ΔΔCt method was employed to assess gene expression relative to β-ACTIN expression. The primer sequences employed in the qRT-PCR analysis can be located in Table S1.
Results
The Low Level of Heme Metabolism Score Was Associated with a Worse Poor for Colon Cancer
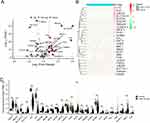
As shown in Figure 2A, the results of GSEA revealed that heme metabolism was significantly enriched in the normal group. Utilizing the GSVA algorithm, the score for the heme metabolism gene set was calculated in each sample. Notably, the colon cancer group displayed a significantly reduced heme metabolism score in comparison to the normal group (Figure 2B). Additionally, the findings from the Kaplan-Meier survival analysis indicated that a lower level of heme metabolism score correlated with a worse prognosis in patients with colon cancer (Figure 2C). The results suggested a potential crucial involvement of the heme metabolism gene set in the progression of colon cancer, thus warranting further exploration and analysis of this specific gene set.
Identification of DEHRGs
Following a comparative analysis of the colon cancer and normal groups, a total of 25 DEHRGs were identified as differentially expressed. Out of these, 14 genes were found to be up-regulated, while 10 genes exhibited down-regulation. To assess the differential expression of DEHRGs between the tumor samples and normal samples, a boxplot and heatmap were utilized to present the expression levels of these genes (Figure 3B and C). Our analysis revealed that the genes SMOX, SLC7A11, TSPAN5, ABCB6, TSPO2, H4C3, ACSL6, KLF1, HBQ1, and TYR exhibited down-regulation in the normal group relative to the colon cancer group, while ABCG2, SLC30A10, CA2, CA1, NR3C1, KAT2B, TENT5C, TNS1, SNCA, TRIM58, ACKR1, ADD2, TAL1, and ALDH1L1 showed up-regulation in the normal group compared to the colon cancer group.
Functional Enrichment Analysis of DEHRGs
To further explore the potential biological behavior of DEHRGs, we performed an enrichment analysis. The results of GO revealed that these DEHRGs were mainly enriched in myeloid cell homeostasis, hemoglobin complex, oxygen carrier activity, etc (Figure 4A). In addition, KEGG enrichment analysis for signaling pathways was significantly associated with bile secretion, ABC transporters, ferroptosis, and nitrogen metabolism (Figure 4B).
 |
Figure 4 Functional annotation for DEHRGs using GO (A) and KEGG (B) enrichment analyses. |
Therapeutic Drugs Prediction
The CMap dataset was used to identify the top 10 drugs/molecules that exhibited negative correlations (Table S2). Amiloride and ipratropium were suggested as drugs suitable for patients with colon cancer. Additional drugs/molecules such as zidovudine, skatole, flupirtine, artemether, dexamethasone, tyrphostin-1, BAY-K8644, and propentofylline have the potential to be used as treatment options for individuals with colon cancer.
Screening of Prognostic Genes in Colon Cancer
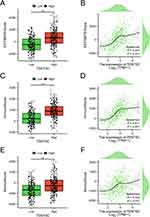
The LASSO regression analysis was conducted using the aforementioned 25 DEHRGs in the current study. Among colon cancer patients, 10 DEHRGs (SMOX, SLC7A11, CA2, ABCB6, TENT5C, H4C3, TRIM58, ACSL6, TYR, and ADD2) with non-zero coefficients were identified through screening (Figure 5A and B). Following this, a multivariate regression analysis was performed on these 10 DEHRGs. The findings revealed that TENT5C and ACSL6 were independent prognostic factors for colon cancer patients (Table S3). Moreover, we performed a comprehensive analysis of TENT5C and ACSL6. As shown in Figure 5C and D, ACSL6 gene expression was significantly upregulated in the colon cancer group compared to the normal group. However, survival analysis showed shorter survival in patients with low ACSL6 gene expression. TENT5C gene expression was significantly downregulated in the colon cancer group compared to the normal group. Moreover, survival analysis showed that patients with low expression of the TENT5C gene had shorter survival (Figure 5E and F). Therefore, TENT5C is an independent prognostic marker for colon cancer patients.
Validation of Prognostic Gene Expression by Multiple Datasets and Clinical Samples
We conducted a thorough validation of TENT5C gene expression using multiple datasets and clinical samples. Our analysis consistently confirmed the significant downregulation of TENT5C in colon cancer. This was evident from the results obtained from the GSE39582 dataset (Figure 6A), the TNMplot.com analysis platform (Figure 6B), and the qRT-PCR analysis (Figure 6C). Furthermore, an examination of the CCLE dataset (https://www.broadinstitute.org/ccle) revealed that the majority of colon cancer cell lines exhibited a decrease in TENT5C expression (Figure S1).
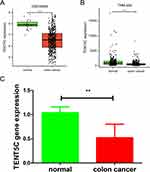 |
Figure 6 Validation of prognostic gene expression. The expression of TENE5C was validated by the GSE39582 dataset (A), TNM plot (B), and clinical samples (C). **p < 0.01, ***p < 0.001. |
DNA Methylation Can Reveal Various Prognoses, but It Does Not Signify Genetic Alterations
We acquired the genetic modification information of TENT5C from the TCGA-COAD cohort through cBioportal. Among the patients, only 1% exhibited genetic alterations in TENT5C (Figure S2A). Interestingly, we observed no significant differences in overall survival rates between patients with and without genetic alterations in TENT5C (Figure S2B). In addition, methylation levels of 5 CpG sites, including cg22424284, cg24498500, cg02854554, cg23098693, and cg11730925 were related to the prognosis of colon patients (Table S4).
Establishment of Nomogram
To predict the 1-, 3-, and 5-year overall survival rates in patients with colon cancer, we developed a nomogram. Figure 7A displays the constructed nomogram, which incorporates TENT5C gene expression. The calibration curve of the nomogram (Figure 7B) demonstrates excellent agreement between the predicted outcomes and the observed results in colon cancer patients. Additionally, the TENT5C gene exhibits a high diagnostic value, as evidenced by the area under the curve (AUC) of 0.918 in Figure 7C. This indicates its potential as a diagnostic marker for colon cancer patients.
Enrichment Analysis of Genes That are Differentially Expressed in Association with TENT5C
As shown in Figure 8A, a comparison between the low- and high-TENT5C subgroups resulted in the identification of 1056 differentially expressed genes (DEGs). Out of these genes, 967 were up-regulated while 89 were down-regulated. The results of GO showed that these DEGs were associated with immune-related pathways, such as lymphocyte differentiation, immune response-regulating signaling pathway, immune receptor activity, cytokine receptor activity, etc (Figure 8B). Further analysis using KEGG revealed a significant enrichment of these DEGs in the cytokine-cytokine receptor interaction, chemokine signaling pathway, neutrophil extracellular trap formation, primary immunodeficiency, etc (Figure 8C). Furthermore, GSEA results revealed that TENT5C-associated DEGs were enriched in Interleukin 10 signaling, IL12-STAT4 pathway, inflammatory response pathway, primary immunodeficiency, TCR pathway, natural killer cell mediated cytotoxicity, etc (Figure 9). In addition, single gene GSEA results showed that immune-related pathways were enriched in TENT5C high-expressed phenotype (Figure S3).
 |
Figure 9 The GSEA results demonstrated alterations in signaling pathways based on DEGs associated with TENT5C. |
Immunocyte Infiltration Analysis
We hypothesized a potential correlation between TENT5C and tumor immune infiltration based on the results of the enrichment analysis. To validate this hypothesis, we performed a comprehensive analysis of immunocyte infiltration using ESTIMATE and ssGSEA. The obtained from ESTIMATE revealed a notable increase in the scores of ESTIMATE, immune, and stromal in the TENT5C-high subgroup as compared to the TENT5C-low subgroup. Furthermore, correlation analyses demonstrated a significant positive association between TENT5C and the ESTIMATE score, immune score, and stromal score (Figure 10).
The ssGSEA results showed that most immune cells were significantly upregulated in the high TENT5C subgroup (Figure 11A). The score of Treg, Th2 cells, Th1 cells, Tgd, TFH, Tem, Tcm, T helper cells, T cells, pDC, NK CD56dim cells, neutrophils, mast cells, macrophages, iDC, eosinophils, DC, cytotoxic cells, CD8 T cells, B cells, and aDC was significantly increased in TENT5C-high subgroup compared to TENT5C-low subgroup (Figure 11B). Correlation analysis showed that the TENT5C gene was positively associated with most immune cells, including T cells, B cells, cytotoxic cells, macrophages, etc (Figure 11C).
By utilizing the TISCH database, we analyzed the expression level of the TENT5C gene in cells associated with the tumor immune microenvironment. Our investigation revealed that TENT5C demonstrated a higher level of infiltration in CD4Tconv, Treg, T prolif, CD8 Tex, and CD8 T cells (Figure 12). These findings suggest that the expression of TENT5C has a role in modulating the infiltration of immune cells, thus impacting the tumor microenvironment.
 |
Figure 12 Single-cell analysis of TENT5C. The heatmap illustrated the expression levels of TENT5C in cells associated with the microenvironment of colon cancer. |
Discussion
In several aspects related to tumor microenvironment reprogramming and metabolic adaptation, inorganic iron has been found to have significant roles.32,33 Similarly, tumor cells may control organic iron, specifically in the form of the iron-containing porphyrin heme, as an ideal molecule to support survival and proliferation, interact with the microenvironment, and regulate energetic metabolism. Due to its participation in various biological processes such as drug metabolism, energy generation, and oxygen transportation, heme, a porphyrin compound containing iron, holds crucial significance for cells.34–36 The significance of dietary heme in cancer development has been emphasized in various carcinoma types. Indeed, the consumption of red and processed meat in large quantities has been linked to a higher occurrence of tumors in the lungs, breast, esophagus, and stomach.36,37 The investigation into heme synthesis in tumors has garnered attention for numerous years. Evidence has demonstrated that inhibiting delta-aminolevulinic acid dehydratase (ALAD) with succinylacetone, an ALAD inhibitor, can decrease tumor cell survival and proliferation by suppressing heme biosynthesis.14,38,39 Targeting heme metabolism holds great promise as it could potentially influence various facets of cancer.40 However, the role of HRGs in colon cancer remains unclear. Therefore, our study was carried out to explore these issues. In the present study, the low level of heme metabolism score was associated with a worse poor in colon cancer. Based on HRGs, TENT5C has been identified as a potential prognostic gene in colon cancer. Analysis of data from the TCGA database and clinical samples revealed a downregulated expression of TENT5C in colon cancer tissues. Furthermore, this decreased expression of TENT5C was associated with an unfavorable prognosis in colon cancer patients.
The alias for TENT5C is FAM46C. Somatic point mutations have been detected in approximately 10% of newly diagnosed multiple myeloma cases, making FAM46C one of the most mutated genes in multiple myeloma.41 The presence of point mutations in FAM46C has been observed in patients with different types of tumors, including hepatocellular carcinoma cells, indicating its potential association with this malignancy.42 A recent study indicates that the PTEN/AKT signaling pathway is influenced by FAM46C, with implications for cell proliferation, the cell cycle, apoptosis, and chemosensitivity in prostate cancer.43 FAM46C demonstrates tumor suppressor characteristics and its impact is, to some extent, mediated by the Wnt/β-catenin pathway in gastric cancer.44 Moreover, in human colorectal cancer, FAM46C is depleted, it has been found to impede cancer cell invasion and suppress MDA MB-435 cancer growth in a xenograft model, thus counteracting the effects of Plk4.45 The results of this study strongly indicate that TENT5C may function as a tumor suppressor, and the reduced expression of TENT5C is closely linked to an unfavorable prognosis among individuals diagnosed with colon cancer. Furthermore, TENT5C has been identified as a reliable prognostic indicator for the survival outcomes of individuals with colon cancer. The effectiveness of TENT5C as a diagnostic tool for colon cancer patients has been confirmed through the use of a nomogram model and ROC analysis. Our signature exhibits a diagnostic performance that is similar to that of the signature associated with lipid metabolism.46
The role of TENT5C in the development and progression of colon cancer is not well understood. To gain further insight into the biological function of TENT5C in colon cancer, we categorized colon cancer patients into two subgroups based on their TENT5C expression levels: high and low. The findings of enrichment analysis revealed that the DEGs between the two subgroups identified were primarily associated with immune and inflammation-related pathways. It is important to note that inflammation and immunity are closely intertwined, with overlapping immune cell populations playing a significant role in both processes. Accumulation of inflammation-triggering mutations and gene abnormalities in the intestinal mucosa of patients with inflammatory bowel disease contributes to the development of colorectal cancer through dysplasia.47 Factors such as interactions with the gut microbiota, biochemical components, the innate immune system, and epithelial cells can play a role in triggering the initial molecular events or influencing the tumor microenvironment, thereby promoting the progression of neoplastic growth.48 Our study revealed a noteworthy elevation in the immune score, stromal score, and ESTIMATE score within the TENT5C-high subgroup. Additionally, in the TENT5C-high subgroup, we found a significant up-regulation in T cells, B cells, macrophages, and neutrophils. Several investigations have revealed a clear correlation between the infiltration of these cells into tumors and the prognostic outlook for individuals diagnosed with colon cancer.49–52 These findings provide additional evidence suggesting a potential correlation between TENT5C expression and a poorer prognosis among colon cancer patients.
Conclusion
In colon cancer, a lower heme metabolism score was found to be linked with a poorer outcome. We have developed a predictive signature for diagnosing colon cancer based on heme metabolism. Additionally, we found that TENT5C serves as an independent prognostic biomarker and is closely associated with immune cell infiltration in colon cancer. This finding lays the groundwork for future research into the role of TENT5C in the development and progression of colon cancer.
Data Sharing Statement
All data can be obtained from the website we provide, and are available from the corresponding author upon request.
Ethics Approval
This study was performed in accordance with the Helsinki Declaration, and approved by the Ethics Committee of Shaanxi Provincial People’s Hospital (approval number: 2023-086).
Funding
This work was supported by the Natural Science Basic Research Program of Shaanxi Province (Grant No.2023-JC-QN-0947, 2021JQ-917) and Shaanxi Provincial People’s Hospital (2021JY-46).
Disclosure
All authors declared that they have no competing interests.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi:10.1136/gutjnl-2015-310912
3. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi:10.1002/cncr.24760
4. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi:10.1016/S0140-6736(13)61649-9
5. Zhai X, Xue Q, Liu Q, Guo Y, Chen Z. Colon cancer recurrence‑associated genes revealed by WGCNA co‑expression network analysis. Mol Med Rep. 2017;16(5):6499–6505. doi:10.3892/mmr.2017.7412
6. Koedam TWA, Bootsma BT, Deijen CL, et al. Oncological outcomes after anastomotic leakage after surgery for colon or rectal cancer: increased risk of local recurrence. Ann Surg. 2022;275(2):e420–e427. doi:10.1097/SLA.0000000000003889
7. Sepulveda AR, Hamilton SR, Allegra CJ, et al. Molecular biomarkers for the evaluation of colorectal cancer. Am J Clin Pathol. 2017;147(3):221–260. doi:10.1093/ajcp/aqw209
8. Koncina E, Haan S, Rauh S, Letellier E. Prognostic and predictive molecular biomarkers for colorectal cancer: updates and challenges. Cancers. 2020;12(2):319. doi:10.3390/cancers12020319
9. Luo B, Lin J, Cai W, Wang M. Identification of the pyroptosis-related gene signature and risk score model for colon adenocarcinoma. Front Genetics. 2021;12:771847. doi:10.3389/fgene.2021.771847
10. Kiening M, Lange N. A recap of heme metabolism towards understanding protoporphyrin ix selectivity in cancer cells. Int J Mol Sci. 2022;23(14):7974. doi:10.3390/ijms23147974
11. Fujiwara T, Harigae H. Biology of heme in mammalian erythroid cells and related disorders. Biomed Res Int. 2015;2015:278536. doi:10.1155/2015/278536
12. Hooda J, Shah A, Zhang L. Heme, an essential nutrient from dietary proteins, critically impacts diverse physiological and pathological processes. Nutrients. 2014;6(3):1080–1102. doi:10.3390/nu6031080
13. Sohoni S, Ghosh P, Wang T, et al. Elevated heme synthesis and uptake underpin intensified oxidative metabolism and tumorigenic functions in non-small cell lung cancer cells. Cancer Res. 2019;79(10):2511–2525. doi:10.1158/0008-5472.CAN-18-2156
14. Hooda J, Cadinu D, Alam MM, et al. Enhanced heme function and mitochondrial respiration promote the progression of lung cancer cells. PLoS One. 2013;8(5):e63402. doi:10.1371/journal.pone.0063402
15. Haines DD, Tosaki A. Heme degradation in pathophysiology of and countermeasures to inflammation-associated disease. Int J Mol Sci. 2020;21(24):9698. doi:10.3390/ijms21249698
16. Ayer A, Zarjou A, Agarwal A, Stocker R. Heme oxygenases in cardiovascular health and disease. Physiol Rev. 2016;96(4):1449–1508. doi:10.1152/physrev.00003.2016
17. Canesin G, Muralidharan AM, Swanson KD, Wegiel B. HO-1 and Heme: g-quadruplex interaction choreograph DNA damage responses and cancer growth. Cells. 2021;10(7):1801. doi:10.3390/cells10071801
18. Cross AJ, Freedman ND, Ren J, et al. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol. 2011;106(3):432–442. doi:10.1038/ajg.2010.415
19. Bastide NM, Pierre FH, Corpet DE. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res. 2011;4(2):177–184. doi:10.1158/1940-6207.CAPR-10-0113
20. Kossenas K, Constantinou C. Epidemiology, molecular mechanisms, and clinical trials: an update on research on the association between red meat consumption and colorectal cancer. Curr Nutr Rep. 2021;10(4):435–467. doi:10.1007/s13668-021-00377-x
21. Sasso A, Latella G. Role of heme iron in the association between red meat consumption and colorectal cancer. Nutr Cancer. 2018;70(8):1173–1183. doi:10.1080/01635581.2018.1521441
22. Becker JC, Fukui H, Imai Y, et al. Colonic expression of heme oxygenase-1 is associated with a better long-term survival in patients with colorectal cancer. Scand J Gastroenterol. 2007;42(7):852–858. doi:10.1080/00365520701192383
23. Fu J, Lin J, Zeng X, Li G, Wei Y, Xian L. GABRP is a promising prognostic biomarker and associated with immune cell infiltration in lung squamous cell carcinoma. Pharm Person Med. 2023;16:357–371. doi:10.2147/PGPM.S403868
24. Wang B, Huang L, Ye S, Zheng Z, Liao S. Identification of novel prognostic biomarkers that are associated with immune microenvironment based on GABA-related molecular subtypes in gastric cancer. Pharm Person Med. 2023;16:665–679. doi:10.2147/PGPM.S411862
25. Shen B, Zhang G, Liu Y, Wang J, Jiang J. Identification and analysis of immune-related gene signature in hepatocellular carcinoma. Genes. 2022;13(10):1834. doi:10.3390/genes13101834
26. Wan R, Bai L, Cai C, et al. Discovery of tumor immune infiltration-related snoRNAs for predicting tumor immune microenvironment status and prognosis in lung adenocarcinoma. Comput Struct Biotechnol J. 2021;19:6386–6399. doi:10.1016/j.csbj.2021.11.032
27. Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinf. 2013;14(1):7. doi:10.1186/1471-2105-14-7
28. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signaling. 2013;6(269):1. doi:10.1126/scisignal.2004088
29. Modhukur V, Iljasenko T, Metsalu T, Lokk K, Laisk-Podar T, Vilo J. MethSurv: a web tool to perform multivariable survival analysis using DNA methylation data. Epigenomics. 2018;10(3):277–288. doi:10.2217/epi-2017-0118
30. Lamb J, Crawford ED, Peck D, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313(5795):1929–1935. doi:10.1126/science.1132939
31. Sun D, Wang J, Han Y, et al. TISCH: a comprehensive web resource enabling interactive single-cell transcriptome visualization of tumor microenvironment. Nucleic Acids Res. 2021;49(D1):D1420–D1430. doi:10.1093/nar/gkaa1020
32. Jung M, Mertens C, Tomat E, Brüne B. Iron as a central player and promising target in cancer progression. Int J Mol Sci. 2019;20(2):273. doi:10.3390/ijms20020273
33. Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM. Iron and cancer. Annu Rev Nutr. 2018;38(1):97–125. doi:10.1146/annurev-nutr-082117-051732
34. Gozzelino R. The pathophysiology of heme in the brain. Curr Alzheimer Res. 2016;13(2):174–184. doi:10.2174/1567205012666150921103304
35. Yuan X, Fleming MD, Hamza I. Heme transport and erythropoiesis. Curr Opin Chem Biol 2013;17(2):204–211. doi:10.1016/j.cbpa.2013.01.010
36. Chiabrando D, Vinchi F, Fiorito V, Mercurio S, Tolosano E. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front Pharmacol. 2014;5:61. doi:10.3389/fphar.2014.00061
37. Chang VC, Cotterchio M, Khoo E. Iron intake, body iron status, and risk of breast cancer: a systematic review and meta-analysis. BMC Cancer. 2019;19(1):543. doi:10.1186/s12885-019-5642-0
38. Ye W, Zhang L. Heme deficiency causes apoptosis but does not increase ROS generation in HeLa cells. Biochem Biophys Res Commun. 2004;319(4):1065–1071. doi:10.1016/j.bbrc.2004.05.089
39. Fukuda Y, Wang Y, Lian S, et al. Upregulated heme biosynthesis, an exploitable vulnerability in MYCN-driven leukemogenesis. JCI Insight. 2017;2(15). doi:10.1172/jci.insight.92409
40. Fiorito V, Chiabrando D, Petrillo S, Bertino F, Tolosano E. The multifaceted role of heme in cancer. Front Oncol. 2019;9:1540. doi:10.3389/fonc.2019.01540
41. Herrero AB, Quwaider D, Corchete LA, Mateos MV, García-Sanz R, Gutiérrez NC. FAM46C controls antibody production by the polyadenylation of immunoglobulin mRNAs and inhibits cell migration in multiple myeloma. J Cell Mol Med. 2020;24(7):4171–4182. doi:10.1111/jcmm.15078
42. Zhang QY, Yue XQ, Jiang YP, Han T, Xin HL. FAM46C is critical for the anti-proliferation and pro-apoptotic effects of norcantharidin in hepatocellular carcinoma cells. Sci Rep. 2017;7(1):396. doi:10.1038/s41598-017-00313-6
43. Ma L, He H, Jiang K, et al. FAM46C inhibits cell proliferation and cell cycle progression and promotes apoptosis through PTEN/AKT signaling pathway and is associated with chemosensitivity in prostate cancer. Aging. 2020;12(7):6352–6369. doi:10.18632/aging.103030
44. Shi J, Zhu Q, Wu J, Zhu P. FAM46C suppresses gastric cancer by inhibition of Wnt/beta-catenin. Front Biosci. 2020;25(3):549–563. doi:10.2741/4820
45. Kazazian K, Haffani Y, Ng D, et al. FAM46C/TENT5C functions as a tumor suppressor through inhibition of Plk4 activity. Commun Biol. 2020;3(1):448. doi:10.1038/s42003-020-01161-3
46. Huang Y, Zhou J, Zhong H, Xie N, Zhang FR, Zhang Z. Identification of a novel lipid metabolism-related gene signature for predicting colorectal cancer survival. Front Genetics. 2022;13:989327. doi:10.3389/fgene.2022.989327
47. Hirano T, Hirayama D, Wagatsuma K, Yamakawa T, Yokoyama Y, Nakase H. Immunological mechanisms in inflammation-associated colon carcinogenesis. Int J Mol Sci. 2020;21(9):3062. doi:10.3390/ijms21093062
48. Yamauchi M, Lochhead P, Morikawa T, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61(6):794–797. doi:10.1136/gutjnl-2012-302014
49. Couzin J. Cancer. T cells a boon for colon cancer prognosis. Science. 2006;313(5795):1868–1869. doi:10.1126/science.313.5795.1868b
50. Xia J, Xie Z, Niu G, et al. Single-cell landscape and clinical outcomes of infiltrating B cells in colorectal cancer. Immunology. 2023;168(1):135–151. doi:10.1111/imm.13568
51. Feng Q, Chang W, Mao Y, et al. Tumor-associated macrophages as prognostic and predictive biomarkers for postoperative adjuvant chemotherapy in patients with stage II colon cancer. Clin Cancer Res. 2019;25(13):3896–3907. doi:10.1158/1078-0432.CCR-18-2076
52. Zhou G, Peng K, Song Y, et al. CD177+ neutrophils suppress epithelial cell tumourigenesis in colitis-associated cancer and predict good prognosis in colorectal cancer. Carcinogenesis. 2018;39(2):272–282. doi:10.1093/carcin/bgx142
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.