Back to Journals » International Journal of General Medicine » Volume 13
Helicobacter pylori Prevalence and Impact: A Histology-Based Report About Children from an Endemic Country
Authors Khdair Ahmad F , Aladily TN , Altamimi M, Ajour M , Alsaber N, Rawashdeh M
Received 18 December 2019
Accepted for publication 7 May 2020
Published 22 May 2020 Volume 2020:13 Pages 207—214
DOI https://doi.org/10.2147/IJGM.S240205
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fareed Khdair Ahmad,1 Tariq N Aladily,2 Motaz Altamimi,3 Maher Ajour,3 Nisreen Alsaber,3 Mohamed Rawashdeh1
1Section of Pediatric Gastroenterology, Hepatology, and Nutrition, Department of Pediatrics, School of Medicine, The University of Jordan, Amman, Jordan; 2Department of Pathology, School of Medicine, The University of Jordan, Amman, Jordan; 3Department of Pediatrics, School of Medicine, The University of Jordan, Amman, Jordan
Correspondence: Fareed Khdair Ahmad Email [email protected]
Background: Helicobacter pylori is spreading worldwide with a high prevalence rate in the developing countries. Our primary goal was to measure the histology-based prevalence of Helicobacter pylori infection in children and to quantify its impact on the gastric inflammation and anemia. Our secondary goal was to study possible predictors for the presence of Helicobacter pylori in this cohort.
Methods: A retrospective chart review was performed for children who underwent Esophago-gastro-duodenoscopy at Jordan university hospital in Jordan from 2008 to 2016. Data collected included epidemiological data, indication for endoscopy, endoscopic findings, and laboratory data. The gastric biopsies were re-examined by a pathologist to check for the presence of Helicobacter pylori, the presence of gastritis, and to grade gastritis according to the updated Sydney criteria.
Results: A total of 98 children (53 girls– 54%) underwent Esophago-gastro-duodenoscopy. The average age was 11.7 years ± 4.7 years. Of them, 53 patients (29 boys– 55%) had Helicobacter pylori identified in the gastric biopsy. The histology-based prevalence rate of Helicobacter pylori was 54%. The most common indication for endoscopy was abdominal pain (53%) followed by vomiting (18%). Nodular gastric mucosa was present in 43% of the Helicobacter pylori-positive group, and in only 11% of the Helicobacter pylori-negative group (P-value < 0.0.5). Moderate to severe chronic gastritis was seen in 59% of the biopsies of Helicobacter pylori-positive group, compared to 31% in the Helicobacter pylori-negative group (p value < 0.05). Presence of anemia was not different between the two groups (p value > 0.05). Presence of endoscopic nodularity, active gastritis by histology, and moderate to severe gastritis by histology were positive predicators for the presence of Helicobacter pylori. (p value < 0.05).
Conclusion: Helicobacter pylori infection in this study cohort of Jordanian children is common, with a histology-based prevalence rate of 54%. Nodularity of the stomach is the most common positive endoscopic feature, and its presence predicts the presence of Helicobacter pylori. Moderate to severe active gastritis is associated with Helicobacter pylori. The presence of Helicobacter pylori does not affect anemia status in this cohort of Jordanian children.
Keywords: Helicobacter pylori, Jordan, children, histology
Introduction
Helicobacter pylori (H. pylori) is spread worldwide, with a high prevalence rate in the developing countries.1 It causes gastritis; gastric and duodenal ulcers; gastric cancer; and mucosa- associated lymphoid tissue (MALT) lymphoma.2–5 It is classified by the World Health Organization as a group 1 carcinogen for gastric adenocarcinoma in adults.6 Unless treated during childhood, H. pylori infection will persist and continue till adulthood causing the above mentioned sequel.2,3 Its prevalence varies among developed and developing countries, ranging (34.7–82%),1,7 with a unique age-specific prevalence pattern in the developing countries manifesting with higher prevalence rates in adults compared to children.1
Several diagnostic tests are used to detect H. pylori infection,8 with the initial diagnosis in children involving endoscopy and histological evaluation.5,9 Endoscopic findings in H. pylori infection in children are variable, with nodular gastric mucosa being a characteristic finding in the high prevalence countries.10,11 Describing histological specimens containing H. pylori is variable,8 with the updated Sydney classification widely used in adults for this purpose.12,13 The classification grades the stomach biopsy in regard to 4 domains: chronicity (based on presence of lymphocytes); activity (based on presence of neutrophils), glandular atrophy; and metaplasia.12,14
H. pylori infection in children has been linked to several extra gastric effects; including iron deficiency anemia (IDA), idiopathic thrombocytopenic purpura (ITP), subnormal growth, short stature, diarrhea, diabetes mellitus, and recently atopy.15–20 The relation of H. pylori to IDA has been widely studied, but the findings are still conflicting.5,9,18,19
Limited data exist about histology-based prevalence (HBP) of H. pylori in Arab children. Studies from Kuwait, Saudi Arabia, Egypt, and Oman showed the prevalence of H. pylori to be 31%, 62%, 65% and 25 %, respectively.21–24 In Jordan, studies estimated the HBP of H. pylori in adults to be 68%–82%,7,25,26 and the presence of H. pylori infection was documented in 50–79% of gastric cancer biopsies in adult Jordanians.27,28 Serology - based studies in asthmatic and healthy Jordanian children estimated H. pylori prevalence to range from 18.1% to 55.5%, respectively.20,29 One study in dyspeptic children from northern Jordan estimated the HBP to be 82%.30
This study aimed to measure, the HBP of H. pylori in symptomatic Jordanian children, and to quantify the impact of H. pylori infection on the gastric inflammation and anemia. Our secondary goal was to study possible predictors for Helicobacter pylori presence in this cohort.
Methods
This was a retrospective chart review study. Children who underwent esophageo-gastro-duodenoscopy (EGD) at the Jordan University Hospital (JUH) between January 2008 and January 2016 were enrolled. Ethical approval was obtained from the institutional review board (IRB) committee at the school of medicine, University of Jordan, Amman, Jordan, and from the IRB committee at JUH.
JUH is a 600-bed tertiary hospital located in Amman city, the capital of Jordan. JUH has about 500,000 yearly patient visits to the outpatient department and about 100,000 yearly visits to the emergency room. Patients come to JUH from all regions of the country but mainly from Amman and central Jordan, which represent the highest population density in the country.
Children aged 1 to 18 years who had EGD done at JUH during the study period were included. Both clinic and hospitalized patients were included in the study. Children who had gastric biopsy obtained at the time of endoscopy were included. Any child who had biopsies and endoscopy done more than once was counted as one unique patient. Children were excluded if their endoscopy or biopsy report were missing from the medical file, or if the gastric biopsy slides were missing. Children known to have the following gastrointestinal diseases were excluded: inflammatory bowel disease IBD; celiac disease; or eosinophilic esophagitis EoE. Children with other nationalities, who had EGD done at JUH during the study period, were excluded from the study.
Data collected about study subjects included age at the time of EGD; gender; residence location; usage of proton pump inhibitors (PPI); indication(s) for endoscopy; endoscopy findings as reported in the endoscopy report; and complete blood count done within 3 months from endoscopy.
Over the study period, EGDs were performed by two pediatric gastroenterologists (MR and FKA) under either general anesthesia or sedation. Biopsies were obtained from the gastric antrum and/or the gastric body, with at least two biopsies were taken from each site. Gastric biopsies were submitted for histology, placed in slides, stained with hematoxylin and eosin stain (Abbey Color, Philadelphia, PA, USA), and were assessed, at the time of endoscopy, by a pathologist. The pathologist organized a report about the findings, and it was kept in the patient’s medical file. The same biopsy slides were examined again at the time of this study by an expert pathologist (TA), who was blinded to the initial pathology report. He looked for the presence of gastritis and H. pylori according to the updated Sydney classification. Results of his new report and old report were compared.
To ensure data privacy, each study subject was assigned a unique study number. This number was linked to the subject’s clinical data. The list containing study subject names with their assigned numbers was kept in a password- protected MS-word file, which was kept on a password - protected computer used only by the primary investigator (FKA). Clinical data was initially recorded on an intake sheet for each de-identified study subject, and then was entered on excel spread sheet. This sheet was password - protected and kept at FKA computer.
Descriptive statistics were used to describe the demographic data. For categorical variables, frequencies and percentages were reported. Comparisons of categorical variables were carried out using Pearson’s chi-square test. Possible predictors of H. pylori presence were assessed using logistic regression analysis for each independent (predicted) variable. An a priori two-tailed level of significance was set at 0.05 levels. Statistical analyses were conducted using statistical Package for the Social Sciences version 20 (SPSS Inc. Chicago, IL, USA).
Results
A total of 228 children had EGD with gastric biopsies taken during the study period. 58 were excluded due to missing biopsy slides; 30 were excluded due to missing clinical data; 18 were excluded due to missing endoscopy or biopsy report; and 12 children were excluded due to prior diagnosis of IBD, celiac disease, or EoE. Three children had EGD done twice, so they were counted as three unique patients instead of six. Another 9 children were excluded because they were non-Jordanian nationals. The total study cohort was 98. Table 1 summarizes the study population characteristics.
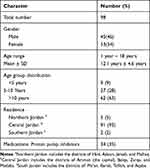 |
Table 1 Demographic Data of the Study Population |
The HBP of H. pylori in this study cohort of 98 children was 54%. Table 2 demonstrates the details of demographic findings. The majority of H. pylori-positive children were boys; older than 10 years of age; and lived in central Jordan. There was a progressive increment of H. pylori prevalence with age in this study cohort (Figure 1). The majority of H. pylori negative children were taking PPI at the time of endoscopy, 32 children out of 45 (%71). No significant difference was found in the prevalence of anemia in regard to the presence or absence of H. pylori infection. This is illustrated in Table 2.
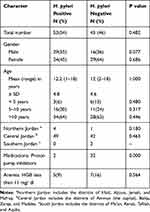 |
Table 2 Demographic Date of H. pylori in Jordanian Children |
 |
Figure 1 Age distribution of H. pylori -positive children. |
Abdominal pain was the most common indication for endoscopy, but it was not statistically different between the group which had H. pylori and the group that did not (p value > 0.05). None of the other indications for endoscopy was significant either. Table 3 lists all indications for endoscopy. Several patients had more than one indication for endoscopy.
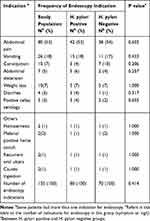 |
Table 3 Frequency of Endoscopy Indications in the Study Population |
The majority of the study cohort had normal endoscopy findings (Table 4). Nodularity of the gastric mucosa was seen in 43% of the H. pylori-positive group and only in 11% of the H. pylori negative group. This was statistically significant, with p-value <0.05. Stomach ulcers were seen in only 3 (3%) children, but only one of them had H. pylori. Ulcers in the other 2 children were due to non-steroidal anti-inflammatory drugs (NSAID) ingestion.
 |
Table 4 Endoscopic Findings in the Study Population |
Based on the updated Sydney classification of gastritis, two thirds of children who had H. pylori had moderate to severe chronic gastritis, whereas two thirds of those who did not have H. pylori had absent to mild chronic gastritis (Table 5). Moderate chronic gastritis was statistically higher in the H. pylori positive group (p value <0.05). The presence of H. pylori was significantly associated with active gastritis (p value <0.05). Glandular atrophy and intestinal metaplasia were rarely seen in this children cohort. There was a 100% concordance between the initial pathology report at the time of endoscopy and the one re-produced during this study.
 |
Table 5 Histology Descriptions Based on Updated Sydney Classification |
Using logistic regression to evaluate for possible predictors for H. pylori (Table 6 ), the presence of nodularity by endoscopy, and the presence of active gastritis by histology were statistically significant variables (p value < 0.05).
 |
Table 6 Variables Predictors for H. pylori Infection |
Discussion
This is the first histology-based study about H. pylori prevalence and impact on Jordanian children. The prevalence rate was 54% among symptomatic children, and it compares to the rates in the literature from other Arab countries.21–24 Table 7 summarizes these histology-based studies. Jordan’s high prevalence rate is similar to those of Egypt and Saudi Arabia and almost double those of Oman and Kuwait.
 |
Table 7 Histology-Based Prevalence of H. pylori in Arab World Countries |
In comparison to Jordanian adult prevalence rate of 82%, the HBP of H. pylori in Jordanian children is not as high.7 The majority of cases in children were above 10 years of age, with a low prevalence of H. pylori among children less than 5 years of age (Table 2). This supports the data about acquisition of H. pylori infection in developing countries during childhood, and higher infection rates in adults.1,23 The high prevalence rate of H. pylori infection in Jordanian children, along with the high rate of H. pylori related gastric cancer in adult Jordanians,28 makes H. pylori eradication an important childhood issue. H. pylori infection is related to the socioeconomic status, sanitation level, living standards and the overall health level in the country.31 This is reflected by the ongoing decline in H. pylori and gastric cancer prevalence rates in the developed countries.32,33
It is likely, though, the prevalence rate of H. pylori in Jordanian children could be higher than what is revealed by our study. This could be attributed to several culprits. The majority of children who did not have H. pylori in this study (70%) were on medications that can decrease the detection rate of H. pylori, like proton pump inhibitors (PPI). Others might have been on antibiotics week(s) before endoscopy. Further, no special stains, like Giemsa, were done to look for H. pylori, and histology was the only criterion used to confirm H. pylori presence. Other tests, like rapid urease test, serology, or tissue culture, were not implemented at the time of endoscopy.
The HBP rate in our study was lower than that conducted by Shatnawi et al in Jordanian children.30 This can be explained in part by the factors mentioned in the preceding paragraph. In addition, Shatnawi et al studied only dyspeptic children who lived in northern Jordan, where as our study looked at dyspeptic and other children, who underwent upper endoscopy from central Jordan. It is possible that the geographical factor contributes to HBP in Jordanian children. Further studies are needed to answer this question.
Despite the known shortcomings of serology testing, the prevalence of H. pylori in this histology -based study is close to the prevalence rate found by a prior serology-based study from Jordan.29 Using enzyme-linked immunosorbent assay (ELISA) for detection of serum IgG and IgA antibodies against H. pylori in 200 children, Bani-Hani et al reported a prevalence of H. pylori in healthy schoolchildren to be 55.5%. Although practice guidelines from the European and North American Society for pediatric gastroenterology, hepatology, and nutrition (ESPGHAN and NASPGHAN) advice against using serology testing for initial diagnosis of H. pylori infection in a clinical setting,5,9 we find in this study that there is a good correlation between our histology-based study and the prior serology-based study. The ESPGHAN/NASPGHAN recommendation is based on the low performance of serology testing in most European and North America countries, which is due to the low disease prevalence there.5,9 Known limitations for serology testing include its inability to differentiate ongoing from old infection and the lag of positive testing behind the clinical condition.8
The majority of H. pylori-infected children in our study lived in central Jordan. This includes the districts of Amman (the capital of Jordan); Balqa, Zarqa; and Madaba. Few children lived in northern and southern Jordan. We believe this is due to the referral bias to our institution, which captures the majority of its patients from central Jordan. More studies at the national level are needed to verify the accurate epidemiology and distribution of H. pylori infection in Jordanian children. Although more boys than girls had H. pylori infection in this study (55% vs 45%), this was not statistically significant. Zamani et al’s meta-analysis showed no gender difference in worldwide H. pylori prevalence,1 whereas Ibrahim et al recently in his meta-analysis showed a slight male predominance.34 More studies are needed to clarify any gender differences.
Although abdominal pain was the most common indication for endoscopy, it was not statistically different between children who had H. pylori infection and those that did not (p-value 0.2). This is likely due to the low prevalence of stomach ulcers in our study (Table 4). The NASPGHAN/ESPGHAN guidelines indicate that H. pylori causes abdominal pain in children only when ulcers are present.5,9 This is also supported by the finding that the prevalence rate of H. pylori in this study of symptomatic children was close to its prevalence in the study of healthy schoolchildren in Jordan, with a rate of 54% and 55.5%, respectively.29
Our study did not find a difference in the prevalence of anemia in the presence or absence of H. pylori infection. This might be due to small sample size and low prevalence of anemia in the whole cohort (13%). Due to the retrospective nature of this study and absent laboratory data, no analysis could have been performed regarding the type of anemia these children had, or to do the correlation between iron stores before and after treatment. Prospective studies can help in finding the answer to this important question.18,19
Our study suffers from a few limitations. The retrospective nature of the study prohibited the evaluation of specific diagnostic methods like Giemsa staining, rapid urease test or tissue culture. It did not allow stopping PPI or antibiotics around the time of endoscopy, and made it difficult to assess the success of H. pylori identification, eradication, or effect on patients’ symptoms or investigations. The study took JUH as the only site for the study, which resulted in the majority of the study population coming from central Jordan. More studies at the country level will provide a better understanding of the disease demography. Moreover, this study was not a mass screening study for all children in Jordan, but rather for the symptomatic ones who were referred to JUH. The result of our study should be interpreted in this context. Prospective screening studies at the country level looking for eradication rates of H. pylori, treatment regimen, and antibiotic sensitivity are needed.
Antibiotics use was not looked at in this study. Antibiotics in Jordan are given sometimes without a prescription in the private sector, and their use or its absence was not recorded routinely in all medical charts. Due to the retrospective nature of the study, it was difficult to track those who had antibiotics from those who did not. Future prospective studies should address this important issue.
Conclusion
In conclusion, H. pylori infection among Jordanian children is common, with a prevalence rate reaching, at least, 54% in symptomatic children from central Jordan. Its prevalence tends to increase with age. Nodularity of the stomach is the most common positive endoscopic finding of its presence. Stomach ulcers were rare in this cohort. Moderate to severe active gastritis are associated with Helicobacter pylori in Jordanian children. The presence of Helicobacter pylori did not affect anemia status in this cohort of Jordanian children. Prospective studies at the country level are needed.
Ethical Approval
Ethical approval was obtained from IRB committee at the school of medicine, University of Jordan, Amman, Jordan, and from the IRB committee at Jordan university hospital.
Acknowledgment
This research was funded by a grant from the University of Jordan – deanship of academic research; grant number 33/2015–2016.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47(7):868–876. doi:10.1111/apt.14561
2. Valle J, Kekki M, Sipponen P, Ihamaki T, Siurala M. Long-term course and consequences of Helicobacter pylori gastritis. Results of a 32-year follow-up study. Scand J Gastroenterol. 1996;31(6):546–550. doi:10.3109/00365529609009126
3. Xia HH, Talley NJ. Natural acquisition and spontaneous elimination of Helicobacter pylori infection: clinical implications. Am J Gastroenterol. 1997;92(10):1780–1787.
4. Cave DR. Transmission and epidemiology of Helicobacter pylori. Am J Med. 1996;100(5a):
5. Koletzko S, Jones NL, Goodman KJ, et al. Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2011;53(2):230–243. doi:10.1097/MPG.0b013e3182227e90
6. Schistosomes, liver flukes and Helicobacter pylori. IARC working group on the evaluation of carcinogenic risks to humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241.
7. Bani-Hani KE, Hammouri SM. Prevalence of Helicobacter pylori in Northern Jordan. Endoscopy based study. Saudi Med J. 2001;22(10):843–847.
8. Guarner J, Kalach N, Elitsur Y, Koletzko S. Helicobacter pylori diagnostic tests in children: review of the literature from 1999 to 2009. Eur J Pediatr. 2010;169(1):15–25. doi:10.1007/s00431-009-1033-x
9. Jones NL, Koletzko S, Goodman K, et al. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (update 2016). J Pediatr Gastroenterol Nutr. 2017;64(6):991–1003. doi:10.1097/MPG.0000000000001594
10. Bahu Mda G, da Silveira TR, Maguilnick I, Ulbrich-Kulczynski J. Endoscopic nodular gastritis: an endoscopic indicator of high-grade bacterial colonization and severe gastritis in children with Helicobacter pylori. J Pediatr Gastr Nutr. 2003;36(2):217–222. doi:10.1097/00005176-200302000-00011
11. Elitsur Y, Raghuverra A, Sadat T, Vaid P. Is gastric nodularity a sign for gastric inflammation associated with Helicobacter pylori infection in children? J Clin Gastroenterol. 2000;30(3):286–288. doi:10.1097/00004836-200004000-00016
12. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated sydney system. International workshop on the histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–1181. doi:10.1097/00000478-199610000-00001
13. Dixon MF, Genta RM, Yardley JH, Correa P. Histological classification of gastritis and Helicobacter pylori infection: an agreement at last? The international workshop on the histopathology of gastritis. Helicobacter. 1997;2(Suppl 1):S17–S24. doi:10.1111/j.1523-5378.1997.06b09.x
14. Sipponen P, Price AB. The sydney system for classification of gastritis 20 years ago. J Gastroenterol Hepatol. 2011;26(Suppl 1):31–34. doi:10.1111/j.1440-1746.2010.06536.x
15. Pacifico L, Osborn JF, Tromba V, Romaggioli S, Bascetta S, Chiesa C. Helicobacter pylori infection and extragastric disorders in children: a critical update. World J Gastroenterol. 2014;20(6):1379–1401. doi:10.3748/wjg.v20.i6.1379
16. Queiroz DM, Rocha AM, Crabtree JE. Unintended consequences of Helicobacter pylori infection in children in developing countries. Gut Microbes. 2013;4(6):494–504. doi:10.4161/gmic.26277
17. Poddar U. Helicobacter pylori: a perspective in low- and middle-income countries. Paediatr Int Child Health. 2019;39(1):13–17. doi:10.1080/20469047.2018.1490100
18. Qu XH, Huang XL, Xiong P, et al. Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J Gastroenterol. 2010;16(7):886–896. doi:10.3748/wjg.v16.i7.886
19. Hudak L, Jaraisy A, Haj S, Muhsen K. An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter. 2017;22:1. doi:10.1111/hel.12330
20. Albataineh E, Alnawaiseh N, Al-Zayadneh E, Al-amer R, Kaplan N, Abu-lobbad M. The relationship between Helicobacter pylori (H. pylori) and atopy and allergic diseases. Jordan J Biol Sci. 2018;11(2):123–128.
21. Radhakrishnan S, Al Nakib B, Kalaoui M, Patric J. Helicobacter pylori-associated gastritis in Kuwait: endoscopy-based study in symptomatic and asymptomatic children. J Pediatr Gastroenterol Nutr. 1993;16(2):126–129. doi:10.1097/00005176-199302000-00005
22. El-Mouzan MI, Abdullah AM, Al-Mofleh IA. Gastritis in Saudi Arab children. Saudi Med J. 2005;26(4):576–579.
23. Al-Sinani S, Sharef SW, Al-Naamani K, Al-Sharji H. Helicobacter pylori infection in Omani children. Helicobacter. 2014;19(4):306–311. doi:10.1111/hel.12134
24. El-Mazary AM, Elfoly MA, Ahmed MF, Abdel-Hamed WM, Hassan ZM. Helicobacter pylori infection in a group of Egyptian children with upper gastro-intestinal bleeding. Gastroenterolo Res. 2013;6(3):95–102. doi:10.4021/gr533e
25. Latif AH, Shami SK, Batchoun R, Murad N, Sartawi O. Helicobacter pylori: a Jordanian study. Postgrad Med J. 1991;67(793):994–998. doi:10.1136/pgmj.67.793.994
26. Shennak MM, Kilani AF. Helicobacter pylori in dyspeptic Jordanian patients. Trop Gastroenterol. 1998;19(1):15–18.
27. Awad HA, Hajeer MH, Abulihya MW, Al-Chalabi MA, Al Khader AA. Epidemiologic characteristics of gastric malignancies among Jordan University Hospital patients. Saudi Med J. 2017;38(9):965–967. doi:10.15537/smj.2017.9.19371
28. Abbasi SY, Taani HE, Saad A, Badheeb A, Addasi A. Advanced gastric cancer in jordan from 2004 to 2008: a study of epidemiology and outcomes. Gastrointest Cancer Res. 2011;4(4):122–127.
29. Bani-Hani KE, Shatnawi NJ, El Qaderi S, Khader YS, Bani-Hani BK. Prevalence and risk factors of Helicobacter pylori infection in healthy schoolchildren. Chin J Dig Dis. 2006;7(1):55–60. doi:10.1111/j.1443-9573.2006.00245.x
30. Shatnawi M, Rawabdeh N, Al-Nahar L, Swaidat S, Jumean S, Malkawi N. Helicobacter pylori infection in dyspeptic children: endoscopic and histological features. J Roy Med Serv. 2015;22(1):52–57. doi:10.12816/0009787
31. Bruce MG, Maaroos HI. Epidemiology of Helicobacter pylori infection. Helicobacter. 2008;13(Suppl 1):1–6. doi:10.1111/j.1523-5378.2008.00631.x
32. Okuda M, Osaki T, Lin Y, et al. Low prevalence and incidence of Helicobacter pylori infection in children: a population-based study in Japan. Helicobacter. 2015;20(2):133–138. doi:10.1111/hel.12184
33. Inoue M. Changing epidemiology of Helicobacter pylori in Japan. Gastric Cancer. 2017;20(Suppl 1):3–7. doi:10.1007/s10120-016-0658-5
34. Ibrahim A, Morais S, Ferro A, Lunet N, Peleteiro B. Sex-differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: systematic review and meta-analysis of 244 studies. Dig Liver Dis. 2017;49(7):742–749. doi:10.1016/j.dld.2017.03.019
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
