Back to Journals » International Journal of General Medicine » Volume 14
Heat Shock Protein Beta 1 is a Prognostic Biomarker and Correlated with Immune Infiltrates in Hepatocellular Carcinoma
Authors Long S, Peng F, Song B, Wang L, Chen J, Shang B
Received 22 July 2021
Accepted for publication 27 August 2021
Published 10 September 2021 Volume 2021:14 Pages 5483—5492
DOI https://doi.org/10.2147/IJGM.S330608
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Shengyi Long,1,* Fang Peng,1,* Baohui Song,1,* Liang Wang,2 Jun Chen,2 Bingbing Shang1
1The Second Hospital of Dalian Medical University, Dalian, Liaoning Province, People’s Republic of China; 2Laboratory Animal Center, Dalian Medical University, Dalian, Liaoning Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Chen Email [email protected]
Bingbing Shang Email [email protected]
Background: Hepatocellular carcinoma (HCC) is one of the most serious malignancies. The main features of HCC are vascular invasion and drug resistance. Ferroptosis is a novel cell program that is involved in several diseases, such as cancer. Heat shock protein beta 1 (HSPB1) is a major component of heat shock proteins. A recent study showed that HSPB1 could be a new therapeutic target for colorectal cancer with 5-fluorouracil-acquired resistance. However, the functional role of HSPB1 in HCC remains unclear.
Aim: The aim of this study is to clarify HSPB1 expression in HCC and its potential therapeutic and prognostic value.
Methods: We collected data on HSPB1 expression levels in HCC and normal liver tissues from The Cancer Genome Atlas and Gene Expression Omnibus databases. We then validated it using immunohistochemistry (IHC). Receiver operating characteristic and Kaplan–Meier survival curves were used to investigate the role of HSPB1 in the prognosis analysis of HCC. Further, we used the online Search Tool for the Retrieval of Interacting Genes/Proteins website, Gene Ontology, and Kyoto Encyclopedia of Genes and Genomes to conduct enrichment analysis and identify the predictive signaling pathways. Meanwhile, we used the TIMER and GSVA package of R (v3.6.3) to analyze the association between HSPB1 and immunocyte infiltration.
Results: Compared to normal tissues, there was differential expression of HSPB1 in pan-cancers. HSPB1 expression was higher in HCC tissues than in normal tissues (p< 0.05). There was an evident significant difference between HSPB1 mRNA levels and histologic grade, vascular invasion, and alpha-fetoprotein level (all p values< 0.05). Univariate analysis indicated that HCC patients with high HSPB1 levels had shorter overall survival rates than those with low HSPB1 levels (p< 0.05). MAPK14, HSPA8, MAPKAPK3, MAPKAPK5, and MAPKAPK2 are essential proteins that interact with HSPB1. There was a significant correlation between HSPB1 expression levels and immune cell infiltration, including CD4+ T cells (r=0.203, p< 0.05).
Conclusion: High HSPB1 expression is closely associated with a worse prognosis in HCC patients, and HSPB1 may be a target of immunotherapy in HCC.
Keywords: hepatocellular carcinoma, ferroptosis, HSPB1, immune cells infiltration, bioinformatics analysis
Introduction
As one of the most serious malignant tumors, primary liver cancer was the third most frequent cause of cancer-associated deaths worldwide in 2020.1 Hepatocellular carcinoma (HCC) comprises 75–85% of primary liver cancer cases. The risk factors for HCC include chronic hepatitis B virus (HBV) infection, aflatoxin-contaminated foods, heavy alcohol intake, and diabetes. The major risk factors for HCC in China are chronic HBV infection and aflatoxin exposure.2 Because the onset of HCC in the early stage is insidious, approximately half of the patients with HCC are diagnosed in the late stage. The prognosis of HCC is poor. The 5-year survival rate of HCC is approximately 3–5%.3,4 Therapeutic approaches for HCC include operation, chemotherapy, and radiotherapy. Among these, surgical resection can only be performed in early-stage HCC patients. Application of immunotherapy in malignant tumors has been used to treat liver cancer.5 However, current immune checkpoint inhibitors for liver cancer therapy are limited.6 Hence, there is an urgent need to seek more effective treatments, especially for late-stage patients.
Ferroptosis is different from apoptosis and other programed cell death. It is iron-ion-mediated lipid accumulation.7,8 Studies have shown that ferroptosis has been employed as a new approach to treat many diseases, including HCC.9–12
As a type of molecular chaperone, heat shock proteins (HSPs) play vital roles in regulating primary cellular activities in many physiological and pathological processes, such as heat shock and hypoxia. Heat shock protein beta 1 (HSPB1) is a class of small HSPs, also referred to as human HSP27 or mouse HSP25. One study showed that HSPB1 could serve as a novel anticancer therapy via ferroptosis.13,14 In order to seek new therapeutic target, we aim to clarify HSPB1 expression in HCC and its potential therapeutic and prognostic value.
Materials and Methods
RNA-Sequencing Data and Bioinformatics Analysis
We extracted the data of 424 samples, including 50 para-carcinoma tissues and 374 tumor tissues, from The Cancer Genome Atlas (TCGA). Transcripts per kilobase million were used for further assays. Data from HCC patients were collected from TCGA database. All participants have been informed why the research is being conducted, whether or not anonymity is assured, and how the data they are collecting is being stored. Ethics approval from ethics committee of Second Hospital of Dalian Medical University (approval number 2021-059) have been obtained prior to conducting study. After approval by the Medical Ethics Committee, consent was obtained by the study participants prior to study commencement. And pathological tissues were obtained from the Second Hospital of Dalian Medical University. Written informed consents were obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki. In addition, the gene expression profiles of GSE60502 and GSE62232 datasets were collected from the Gene Expression Omnibus (GEO) database to validate the HSPB1 expression level. We used IHC to validate HSPB1 gene expression in HCC and normal liver tissues (antibody A16332, 1:200, ABclonal).
Multiple Models on Prognosis Analysis
First, receiver operating characteristic (ROC) and Kaplan-Meier survival curves were used to analyze the diagnostic and prognostic values of HSPB1 in HCC patients. Second, univariate, and multivariate regression analyses were performed to illustrate the association between HSPB1 expression and overall survival (OS) rates of HCC patients. When p<0.05, the Cox regression analysis was statistically significant.
Protein-Protein Interaction (PPI) Network Construction and Functional Enrichment Analysis
We constructed a PPI network of HSPB1 using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database to explore HSPB1-related proteins. We then analyzed the prediction pathway of HSPB1 and its associated proteins via Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG).
Correlation Analysis of Immune Cell Infiltration
We used Tumor Immune Estimation Resource (TIMER) and single sample Gene Set Enrichment Analysis (ssGSEA) to evaluate the relationships between HSPB1 and tumor purity, as well as several immunocytes, including B cells, neutrophils, macrophages, CD4+T cells, and CD8+T cells. Spearman correlation analysis was used to study the correlation between HSPB1 and immune cell infiltration.
Statistical Analyses
Statistical significance was set at p<0.05. We calculated the data using R software (v.3.6.3). Both the Chi-square test and Fisher’s exact test were used in the clinical information analysis. Furthermore, the Wilcoxon rank sum test was used.
Results
Clinical Characteristics
The clinical histories of 374 patients were obtained from TCGA, including age, sex, race, TNM stage, pathologic stage, adjacent hepatic tissue inflammation, vascular invasion, and alpha-fetoprotein (AFP) level. The details are presented in Table 1. Clinical information was calculated using data filtering.
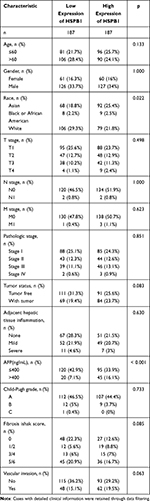 |
Table 1 Clinical Characteristics of the LIHC Patients Based on TCGA |
HSPB1 Expression in HCC Was Elevated
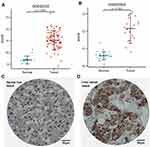
Differential expression of HSPB1 was analyzed in pan-cancer cells. We observed that the mRNA level of HSPB1 increased in adrenocortical carcinoma, breast cancer, cervical squamous carcinoma, cholangiocarcinoma, lymphoid neoplasm diffuses large B-cell lymphoma, glioblastoma multiforme, kidney chromophobe, kidney renal clear cell carcinoma, kidney renal papillary cell carcinoma, HCC, lung adenocarcinoma, lung squamous cell carcinoma, ovarian serous cystadenocarcinoma, pancreatic adenocarcinoma, stomach adenocarcinoma, testicular germ cell tumors, or thymoma. Compared to normal tissue, HSPB1 expression level was higher in HCC tissues (p<0.05). The correlation analysis revealed that there was a statistical difference between HSPB1 and histologic grade (p<0.05), vascular invasion (p<0.05), and AFP level (p<0.05). There was no statistically significant difference in age (p=0.36) and T stage (p=0.32) (Figure 1). HSPB1 expression was validated in GSE60502 and GSE62232 (p<0.001). Further analysis showed homology with IHC results (Figure 2).
Correlation Analysis of Prognosis
The area under the ROC curve was 0.944. This result suggested that HSPB1 was able to distinguish between normal and tumor tissues. The Kaplan-Meier survival curve shows that high HSPB1 levels are associated with poor prognosis (Figure 3). Figure 4 shows that high HSPB1 levels (p=0.035) are associated with high T stage, tumor status, and pathologic stage in OS events in the univariate Cox model (p<0.01). In the multivariate Cox model, only tumor status was an independent factor related to the OS of HCC patients (p=0.005; Figure 4).
PPI Networks and Enrichment Analysis
In this study, we constructed a network of HSPB1 and its related genes using the STRING tool. HSPA4-related genes included MAPKAPK2, DAXX, MAPK14, MAPKAPK5, CYCS, HSPA8, CDC5L, HSPA1A, MAPKAPK3, and ESR1. Their scores were greater than 0.9. The details are shown in Figure 5. In combination with enrichment analysis via GO/KEGG, we found that HSPB1, MAPK14, MAPKAPK3, and MAPKAPK2 are enriched in the vascular endothelial growth factor (VEGF) signaling pathway (Figure 6).
Correlation Analysis of Immune Cell Infiltration
Liver cancer is considered an immunogenic tumor, which is closely associated with viral infection and the inflammatory environment. Data from TIMER suggests statistical significance between HSPB1 and B cells, CD4+ T cells, macrophages, and dendritic cells (both p< 0.05), whereas there is no statistically significant difference between B cells and neutrophils (p>0.05; Figure 7A). The correlation between HSPB1 and other immunocytes was determined using the GSVA package (Figure 7B).
Discussion
High HSPB1 expression is necessary to maintain the malignant characteristics of tumor cells, including uncontrollable invasion, resistance to chemotherapy and radiation, persistent angiogenesis, and metastasis. HSPB1 is considered a promising potential target for cancer therapy,15–19 as HSPB1-induced chemoradiotherapy resistance properties in malignancies have been confirmed.20 However, there have been few studies on HSPB1 expression in HCC. Therefore, it is important to clarify HSPB1 expression in HCC and its potential therapeutic and prognostic value. First, we investigated the differential expression of HSPB1 in pan-cancer and HCC in this study. We discovered that the level of HSPB1 was higher in HCC tissues than in normal liver tissue. HSPB1 mRNA levels were markedly distinct based on histologic grade, vascular invasion, and AFP level. Next, we verified the correlation using the GEO database and IHC. We performed ROC and Kaplan–Meier survival analyses to confirm the association between HSPB1 and survival rate in HCC patients. We further evaluated HSPB1 expression and other clinicopathological factors associated with OS in HCC via univariate and multivariate regression analyses. All results indicate that high HSPB1 expression is associated with worse prognosis, and HSPB1 could be used as a good predictor of prognosis in HCC.
In this study, the PPI network was used to identify the co-regulatory proteins of HSPB1. We found that HSPB1-related genes (Figure 6), including MAPK14, MAPKAPK3, and MAPKAPK2, were enriched in the VEGF signaling pathway. As a type of chaperone, HSPB1 can serve as an inhibitor signaling pathway, which is related to cell death. Thus, when HSPB1 is elevated in cancer cells, it could indirectly stimulate tumor cell growth.21 Several studies have demonstrated that HSPB1 released from tumor cells can bind to its receptors, stimulating VEGF transcription and exerting pro-angiogenic effects.22 Extracellular HSPB1 promotes angiogenesis via direct interaction with VEGF.23 It has been reported that HSPB1 expression also favors invasion and metastasis24,25 triggered by hepatocyte growth factors.24
Furthermore, HSPB1 could negatively regulate ferroptosis by reducing the production of reactive oxygen species (ROS) mediated by iron.13 Although iron is an essential element in cell survival, excessive iron accumulation inevitably leads to cell death.26–28 Ferroptosis is confirmed to be caused by the iron-dependent accumulation of ROS, which provides a novel mechanism for the prevention of tumor development.29 Recent studies on HSPB1,13,30 which could serve as an important regulator in the ferroptotic process of tumor cell death, have provided promising insights into the mechanism of ferroptosis, as well as a theoretical foundation for combinational therapy targeting both HSPB1 and ferroptosis.
In addition, liver cancer is closely associated with viral infection and the inflammatory environment.31,32 Studies have shown that ferroptosis participates in tumor immunoregulation.9,33 Ferroptosis plays a vital role in anti-tumor immunotherapy. However, there are few studies on the association between HSPB1 and immunocytes in HCC. In this study, the correlation between HSPB1 and immunocyte infiltration was calculated using TIMER and ssGSEA. Our results suggest that there is a significant difference between HSPB1 and B cells, CD4+T cells, macrophages, and dendritic cells. However, there were some limitations in this study, such as, the number of normal samples from TCGA database was relatively small, and the hypothesis was not validated using animal models. Certainly, these factors need to be validated in further studies. Therefore, we will generate cell and animal models in the future to further prove our conclusions through experiments.
Conclusion
In conclusion, our investigations demonstrated the distinct prognostic roles of HSPB1 expression levels in HCC patients, and there was a significant correlation between HSPB1 expression levels and immune cell infiltration. Thus, our results suggest that HSPB1 expression may have a distinct prognostic value in HCC patients, and it might be a potential target for immunotherapy in HCC.
Data Sharing Statement
The datasets TCGA for this study can be found in the https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga. And the datasets GEO can be found in the https://www.ncbi.nlm.nih.gov/geo/.
Contribution to the Field Statement
HSPB1-induced chemoradiotherapy resistance properties in malignancies have been confirmed. However, there have been few studies on HSPB1 expression in HCC. In this study, we discovered that the level of HSPB1 was higher in HCC tissues than in normal liver tissue. We demonstrated the distinct prognostic roles of HSPB1 expression levels in HCC patients, and there was a significant correlation between HSPB1 expression levels and immune cell infiltration. Our results suggest that HSPB1 expression may have a distinct prognostic value in HCC patients, and it might be a potential target for immunotherapy in HCC.
Research Ethics and Consent
All participants have been informed why the research is being conducted, whether or not anonymity is assured, and how the data they are collecting is being stored. Ethics approval from ethics committee of Second Hospital of Dalian Medical University have been obtained prior to conducting study (approval number 2021-059). The consent was obtained by the study participants prior to study commencement.
Acknowledgments
We would like to acknowledge the contributions of colleagues, institutions, or agencies that aided the efforts of the authors.
Author Contributions
Jun Chen and Bingbing Shang conceived the study and wrote the manuscript; Baohui Song, Fang Peng, Liang Wang and Shengyi Long conducted the experiments and contributed to the analysis of data. Fang Peng, Shengyi Long collected clinical sample data. Baohui Song, Peng Fang, Jun Chen, Bingbing Shang revised the manuscript. All authors read and approved the final manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Asada Y, Tsuruta M, Okabayashi K, et al. Inhibition of heat-shock protein 27 reduces 5-fluorouracil-acquired resistance in human colon cancer cells. Anticancer Res. 2021;41(3):1283–1290. doi:10.21873/anticanres.14885
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Schmidt S, Follmann M, Malek N, et al. Critical appraisal of clinical practice guidelines for diagnosis and treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26(12):1779–1786. doi:10.1111/j.1440-1746.2011.06891.x
4. Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clin Mol Hepatol. 2016;22(1):7–17. doi:10.3350/cmh.2016.22.1.7
5. Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525–543.
6. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6
7. Stockwell BR, Jiang X, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30(6):478–490. doi:10.1016/j.tcb.2020.02.009
8. Chen X, Li J, Kang R, et al. Ferroptosis: machinery and regulation. Autophagy. 2020:1–28. doi:10.1080/15548627.2020.1810918
9. Du X, Zhang Y. Integrated analysis of immunity- and ferroptosis-related biomarker signatures to improve the prognosis prediction of hepatocellular carcinoma. Front Genet. 2020;11:614888. doi:10.3389/fgene.2020.614888
10. Mahoney-Sánchez L, Bouchaoui H, Ayton S, et al. Ferroptosis and its potential role in the physiopathology of parkinson’s disease. Prog Neurobiol. 2021;196:101890. doi:10.1016/j.pneurobio.2020.101890
11. Guo J, Xu B, Han Q, et al. Ferroptosis: a novel anti-tumor action for cisplatin. Cancer Res Treat. 2018;50(2):445–460. doi:10.4143/crt.2016.572
12. Liu Y, Guo F, Guo W, et al. Ferroptosis-related genes are potential prognostic molecular markers for patients with colorectal cancer. Clin Exp Med. 2021;21(3):467–477. doi:10.1007/s10238-021-00697-w
13. Sun X, Ou Z, Xie M, et al. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34(45):5617–5625. doi:10.1038/onc.2015.32
14. Xie Y, Hou W, Song X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369–379. doi:10.1038/cdd.2015.158
15. Lu H, Sun C, Zhou T, et al. HSP27 knockdown increases cytoplasmic p21 and cisplatin sensitivity in ovarian carcinoma cells. Oncol Res. 2016;23(3):119–128. doi:10.3727/096504015X14496932933656
16. Hsu H-S, Lin J-H, Huang W-C, et al. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer. 2011;117(7):1516–1528. doi:10.1002/cncr.25599
17. Stope MB, Weiss M, Preuss M, et al. Immediate and transient phosphorylation of the heat shock protein 27 initiates chemoresistance in prostate cancer cells. Oncol Rep. 2014;32(6):2380–2386. doi:10.3892/or.2014.3492
18. Jin HO, Hong SE, Kim JY, et al. Induction of HSP27 and HSP70 by constitutive overexpression of Redd1 confers resistance of lung cancer cells to ionizing radiation. Oncol Rep. 2019;41(5):3119–3126.
19. Fei Z, Lijuan Y, Jing Z, et al. Molecular characteristics associated with ferroptosis in hepatocellular carcinoma progression. Hum Cell. 2021;34(1):177–186. doi:10.1007/s13577-020-00431-w
20. Choi C, Kam H, Kim KY, Park SI, Lee YS. Targeting heat shock protein 27 in cancer: a druggable target for cancer treatment? Cancers. 2019;11(8):1195. doi:10.3390/cancers11081195
21. Calderwood SK, Gong J. Heat shock proteins promote cancer: it’s a protection racket. Trends Biochem Sci. 2016;41(4):311–323. doi:10.1016/j.tibs.2016.01.003
22. Choi S-H, Lee H-J, Jin YB, et al. MMP9 processing of HSPB1 regulates tumor progression. PLoS One. 2014;9(1):e85509. doi:10.1371/journal.pone.0085509
23. Thuringer D, Jego G, Wettstein G, et al. Extracellular HSP27 mediates angiogenesis through toll-like receptor 3. FASEB J. 2013;27(10):4169–4183. doi:10.1096/fj.12-226977
24. Pavan S, Musiani D, Torchiaro E, et al. HSP27 is required for invasion and metastasis triggered by hepatocyte growth factor. Int J Cancer. 2014;134(6):1289–1299. doi:10.1002/ijc.28464
25. Cordonnier T, Bishop JL, Shiota M, et al. Hsp27 regulates EGF/β-catenin mediated epithelial to mesenchymal transition in prostate cancer. Int J Cancer. 2015;136(6):E496–E507. doi:10.1002/ijc.29122
26. Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9–17. doi:10.1038/nchembio.1416
27. Salnikow K. Role of iron in cancer. Semin Cancer Biol. 2021. doi:10.1016/j.semcancer.2021.04.001
28. Liu J, Kang R, Tang D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2021. doi:10.1111/febs.16059
29. Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi:10.1016/j.cell.2017.09.021
30. Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26(3):165–176. doi:10.1016/j.tcb.2015.10.014
31. Saran U, Humar B, Kolly P, et al. Hepatocellular carcinoma and lifestyles. J Hepatol. 2016;64(1):203–214. doi:10.1016/j.jhep.2015.08.028
32. An L, Zeng HM, Zheng RS, et al. [Liver cancer epidemiology in China, 2015]. Zhonghua Zhong Liu Za Zhi. 2019;41(10):721–727. Chinese.
33. Ndiaye H, Liu J, Hall A, et al. Immunohistochemical staining reveals differential expression of ACSL3 and ACSL4 in hepatocellular carcinoma and hepatic gastrointestinal metastases. Biosci Rep. 2020;40(4):BSR20200219. doi:10.1042/BSR20200219
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.