Back to Journals » Cancer Management and Research » Volume 12
Heat Shock Cognate Protein 70 Enhanced Integrin β1 Mediated Invasion in Cancer Cells
Authors Sun G, Cao Y, Guo J, Li M, Dai Y
Received 23 October 2019
Accepted for publication 11 December 2019
Published 11 February 2020 Volume 2020:12 Pages 981—991
DOI https://doi.org/10.2147/CMAR.S235791
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Guan Sun, 1,* Ying Cao, 2,* Jun Guo, 1 Min Li, 3,* Yuyu Dai 4
1Department of Neurosurgery, Yancheng City No.1 People’s Hospital, The Fourth Affiliated Hospital of Nantong University, Yancheng, People’s Republic of China; 2Department of Ear-Nose-Throat, The Second People’s Hospital of Huai’an, Huai’an Affiliated Hospital of Xuzhou Medical University, Huai’an, People’s Republic of China; 3Department of Neurosurgery, Jiangning Hospital Affiliated with Nanjing Medical University, Nanjing, People’s Republic of China; 4Department of Neurosurgery, Yancheng Third People’s Hospital, Yancheng, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuyu Dai
Department of Neurosurgery, Yancheng Third People’s Hospital, Yancheng 224001, People’s Republic of China
Email [email protected]
Min Li
Department of Neurosurgery, Jiangning Hospital Affiliated with Nanjing Medical University, Nanjing 211100, People’s Republic of China
Email [email protected]
Purpose: Glioblastoma is one of the most common malignant cancers worldwide. In our previous work, we have shown that heat shock cognate protein 70 (Hsc70) functions as a positive growth regulator in glioma. We investigated the role of Hsc70 in integrin β 1 mediated invasion of glioma cells.
Methods: In order to investigate whether the down-regulation of Hsc70 would affect the expression of integrin β 1 subunit, HeLa cells were transiently transfected with Hsc70-AS or pcDNA3.0 vectors and the down-regulation of Hsc70 was confirmed by Western blotting. Human brain glioma U87 cells were stably transfected with Hsc70-AS or pcDNA3.0 vectors to further elucidate the relationship between Hsc70 and integrin β 1 in human glioma cells. Cellular localization of integrin β 1 was detected using immunofluorescence confocal microscopy analysis.
Results: Here we reported that down-regulation of the expression of Hsc70 in U87 cells by transfection with antisense cDNA specifically increased the expression of cell surface integrin β 1 without changing its mRNA. Meanwhile, the integrin β 1 125-kD mature form increased while 105-kD precursor form decreased when Hsc70 was down-regulated. Mechanically, the U87 cells transfected with antisense cDNA of Hsc70 decreased the Golgi localization of integrin β 1, strengthened its interaction with integrin α 5 subunit, and enhanced the adhesion ability to fibronectin (FN) and the phosphorylation level of focal adhesion kinase (FAK).
Conclusion: Overall, these results suggested that the down-regulation of Hsc70 expression could promote the expression of cell surface integrin β 1 and subsequently inhibit glioma invasion phenotype.
Keywords: Hsc70, integrin β 1, glioma, focal adhesion kinase, invasion
Introduction
Glioblastoma is the most common and malignant primary central nervous system tumor, which is characterized by a high degree of invasion.1 Therapy approaches including irradiation and surgery, with chemotherapy constitute an important strategy to glioblastoma. However, most patients with glioblastoma still have a poor prognosis, largely due to a high rate of post-surgical recurrence and metastasis.2 In cancer clinical practice, invasion and metastasis is an important prognostic factor of glioblastoma, and elucidation of the mechanism of invasion and metastasis at the molecular level is desirable.3
Cancer invasion and metastasis is a complex process and is influenced by many biological factors.4 Integrins are transmembrane receptors that facilitate cell-extracellular matrix (ECM) adhesion. The presence of integrins allows rapid and flexible responses to events at the cell surface.5 Integrins are obligate heterodimers, which consist of α- and β-subunits, and 24 α-subunits and nine β-subunits assemble into at least 24 different integrins. Among these, 12 integrins contain the β1 subunit.6 In recent years, changes in carbohydrates on integrin β1 have been reported to regulate the activity of integrin, resulting in altered cell adhesion and metastasis.7 β-1,4-Galactosyltransferase (β-1,4-GalT) V is a constitutively expressed enzyme that can effectively express enzyme galactosylating the GlcNAcβ1-6Man group of the highly branched N-glycans which are characteristic of tumor cells.8 Overexpression of β-1,4-GalT has been reported to increase lactosamine glycans on integrin β1, resulting in enhanced migration and invasion of cancer cells.9 The invasion of SHG44 human glioma cells has been shown to be suppressed by transfection with antisense β-1,4-GalT V cDNA, indicating that the expression of the β-1,4-GalT V is associated with the tumorigenic and invasive potentials of glioma cancer cells.10
Quite interestingly, in our previous studies, Hsc70 was found to interact with β-1,4-GalT V and played an important role in tumor metastasis and invasion. However, the effects of Hsc70 on the glycosylation and integrin β1 mediated invasion of human glioma cells have not been reported. Hsc70 is a constitutively expressed molecular chaperone which belongs to the heat shock protein 70 (HSP70) family. Studies have displayed that Hsc70 is an important factor in kinds of tumors.11 It has been found that the expression of Hsc70 is higher in some cancer cell lines than in normal cell lines, including esophageal cancer, lung adenocarcinoma, colon cancer cell, renal cell carcinoma, leukemia, lymphoma and so on.12 The increased expression of Hsc70 is often closely related to tumor grade and tumor metastasis.13 These findings implied that Hsc70 may enhance malignant properties and invasion phenotype of glioma cells via modifying glycosylation and signaling of integrin β1.
In this study, we investigated the role of Hsc70 in integrin β1 mediated invasion of HeLa and glioma cells. We found that down-regulation of Hsc70 expression could promote the expression of cell surface integrin β1 and subsequently inhibit glioma invasion phenotype.
Materials and Methods
Materials
Anti-mouse IgG, anti-rabbit IgG, fibronectin, DMEM, Golgi specific dye NBD were purchased from Sigma (St. Louis, MO, USA). Rhodamine or FITC-conjugated goat anti-mouse secondary antibody were purchased from Molecular Probe (Eugene, OR, USA). Monoclonal antibody against integrin β1 and Endoglycosidase H (Endo H) were obtained from BD Pharmingen (SanJose, CA, USA). Trizol reagent was purchased from Tiangen Biotech (Beijing, China). PrimeScript RT Master Mix was purchased from Takara (Shiga, Japan). The pcDNA3.0 vector and Transfection Reagent Sofast were purchased from Invitrogen (Carlsbad, CA, USA). The plasmid antisense cDNA of Hsc70 (Hsc70-AS) was purchased from Santa Cruz (Santa Cruz, CA, USA). Monoclonal antibody against β-actin, FAK, integrin α5 were purchased from Santa Cruz (Santa Cruz, CA, USA).
Cell Culture and Transfection
Human glioma cell line U87 and cervical cell line HeLa were purchased from Shanghai Institutes for Biological Sciences (SIBS) of the Chinese Academy of Sciences (CAS) (Shanghai, China). Cells were cultured in DMEM/F-12 media supplemented with 10% fetal bovine serum and PenStrep [penicillin (100 U/mL) and streptomycin (100 μg/mL)] (Gibco, Grand Island, NY, USA) in a humidified atmosphere in 5% CO2 at 37°C. The U87 cells stably transfected with Hsc70-As and pcDNA3.0 vector plasmids were named as Hsc70-AS/U87 and Vector/U87 cells, respectively. Transient transfections were performed with SofastTM reagent according to the manufacturer’s instruction.
Subcellular Localization of Integrin β1 and Golgi Co-Localization Study
U87 cells in 60-mm plates were fixed with 4% paraformaldehyde for 30 min at 4°C, permeabilized with 0.1% TritonX100 in PBS for 30 min at 4°C, and washed three times with cold PBS. After blocked with 1% BSA, cells were stained with anti-integrin β1 mouse monoclonal antibody, followed by incubation with rhodamine-conjugated anti-mouse IgG and incubated at 4°C for 0.5 h. Then, cells were stained with 100-nm Golgi specific dye NBD for 30 min at for 0.5 h. After washed with PBS, cells were viewed using a Zeiss LSM780 CLSM (Carl Zeiss, Gottingen, Germany).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted using the Trizol reagent (Tiangen Biotech, Beijing, China) according to the manufacturer’s instructions. RNA was reverse transcripted into cDNA by PrimeScript RT Master Mix (Takara, Shiga, Japan) and Q-PCR was subsequently performed using SYBR Premix Ex Taq II (Takara, Shiga, Japan) according to the manufacturer’s protocol. The primers used were: for integrin β1 forward: 5ʹ-AACTTGATCCCTAAGTCAGCAGTAG-3ʹ, reverse: 5ʹ-ATCAGCAGTAATGCAAGGCC-3ʹ; for integrin α5 forward: 5ʹ-ACCAAGGCCCCAGCTCCATTAG-3ʹ, reverse: 5ʹ-GCCTCACACTGCAGGCTAAATG-3ʹ; forβ-actin forward, 5ʹ-GCGCGGCTACAGCTTCAC-3ʹ; reverse, 5ʹ-GGGGCCGGACTCGTCATA-3ʹ. Relative band intensities were determined using Image J software (NIH, Maryland, USA).
Detection of Integrin on Cell Surface with Fluorescence-Activated Cell Sorter (FACS)
Cells were grown to subconfluence and detached with 2 mM EDTA in PBS. Cells (106) were washed, resuspended in 100 μL of FACS buffer (PBS containing 1% BSA and 0.01% sodium azide), and then incubated with antibody against α5 or β1 integrin (1:100) at 4°C for 30 min, followed by washing three times with FACS buffer. Cells were then labeled with FITC-conjugated secondary antibodies or FITC-streptavidin (10 μL) at 4°C for 30 min. Analyses were then performed on FACScan (BD Biosciences, San Jose, CA, USA).
Immunoprecipitation
Immunoprecipitation of focal adhesion kinase (FAK) experiment was performed according to the manufacturer’s instructions. The cells were harvested and lysed by IP lysis buffer (250 mmol/L HEPES, 50 mmol/L NaCl, 1 mmol/L EDTA, 0.1 mmol/L neocuproine, 1% NP-40, Protease Inhibitor Cocktail). Antibodies specific to Integrin α5 or FAK (Santa Cruz, CA, USA) were added to supernatants followed by an incubation. Immune complexes were then precipitated with protein A agarose beads. Bound proteins were eluted by boiling with loading buffer and analyzed by Western blotting with anti-Integrin β or PY-20 antibodies.
Cell Adhesion Assays
Ninety-six-well microtiter plates were coated with 0.1 mL of human plasma fibronectin in PBS, incubated at 37 oC for 1 h, and blocked by 1% BSA at 37 oC for 30 min after washing. Cells, 3×104, suspended in 100 μL of serum-free DMEM were added to each coated well and incubated at 37 oC for 30 min. Wells were gently washed three times with 100 μL of ice-cold PBS to remove unbound cells, followed by fixation of adherent cells using 3.5% formaldehyde for 15 min. Cells were then stained with a 0.5% crystal violet solution. After the wells were washed twice with PBS, the absorbance of each well at 595 nm was measured using an automated microtiter plate spectrophotometer.
Endoglycosidase Digestion
Cells were harvested and lysates in lysis buffer. One milligrams per milliliter of diluted cell lysates proteins, determined using BCA protein assay procedure, were added with Endo H (30 milliunits/mL) and incubated at 37°C for 24 h, followed by addition of acetone and incubated at 4°C for 1 h. The lysates were centrifuged at 12,000 g for 10 min at 4°C. The precipitate was dissolved in lysis buffer and then boiled in 2× SDS sample buffer for 10 min. Then, the samples were subjected to immunoblotting analysis with an anti-integrin β1 antibody.
Wound-Healing Assay
Cells, 1.2×106, were seeded on six-well plates coated with fibronectin with DMEM containing 10% FBS and grown to confluence. The cells were scratched with a sterile 1000-μL pipette tip to create artificial wounds. At 0, 24 and 48 h after wounding, phase-contrast images of the wound-healing process were photographed digitally using an inverted Olympus IX50 microscope with a 10× objective lens. Eight images per treatment were analyzed to determine averaging parameters of positioning of the migrating cells at the wound edges by digitally drawing lines using the Image J software (NIH, Maryland, USA).
Western Blot Analysis
Protein samples (20 μg/lane) were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membrane was blocked with 5% bovine serum albumin and 0.1% Tween 20 in Tris-buffered saline for 1 h at room temperature. The membranes were incubated with antibodies against overnight at 4°C and then incubated with peroxidase-conjugated secondary antibodies for 2 h at room temperature. The bands were visualized with an enhanced chemiluminescence detection kit (BeyoECL plus, Beyotime, China), and the band densities were analyzed using Image J software. All fold changes in band densities were normalized to that of the control group. Each experiment was carried out in three biological replicates and average fold changes are reported.
Statistical Analysis
Quantitative data were presented as means ± SEM. Student’s t test was used to determine differences between two groups. And one-way ANOVA was adopted to compare differences among multiple groups. A value of P<0.05 was considered statistically significant. Data were analyzed using SPSS software (SPSS version 17.0) (SPSS, Chicago, IL, USA).
Results
Down-Regulation of Hsc70 Promoted the Expression of Integrin β1 in HeLa Cell Surface
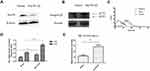
In order to investigate whether the down-regulation of Hsc70 would affect the expression of integrin β1 subunit, human cervical carcinoma HeLa cells were transiently transfected with Hsc70-AS or pcDNA3.0 vectors and the down-regulation of Hsc70 was confirmed by Western blotting (Figure 1A). Then, an equal amount of cellular protein from these transfected cells was subjected to immunoblot analysis with anti-integrin β1 and anti-β-actin antibodies. The down-regulation of Hsc70 significantly promoted the expression of both integrin β1 125-kD mature form and 105-kD precursor form compared with the vector (Figure 1B and C). Furthermore, increased expression of integrin β1 on the cell surface of HeLa cells transiently transfected with Hsc70-AS was also verified by FACS. As shown in Figure 1D, the fluorescence intensity of cells transfected with Hsc70-AS was about 2.1-fold higher than that of control cells. Interestingly, the mRNA level of integrin β1 was not affected (data not shown). The above results provided us the initial evidence that Hsc70 could affect the expression of integrin β1.
Down-Regulation of Hsc70 in U87 Cells Promoted the Expression of Integrin β1 Specifically
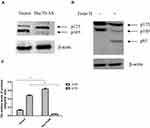
In order to further elucidate the relationship between Hsc70 and integrin β1 in human glioma cells, human brain glioma U87 cells were stably transfected with Hsc70-AS or pcDNA3.0 vectors, which were called Hsc70-AS/U87 and Vector/U87 cells, respectively, and the down-regulation of Hsc70 was confirmed by Western blotting (Figure 2A). Consistent with the results in HeLa cells, down-regulation of Hsc70 promoted the expression of integrin β1 proteins on Hsc70-AS/U87 cell surface, while its mRNA level was not affected (Figure 2B and C). Integrins are obligate heterodimers, which consist of α- and β-subunits, in order to investigate whether Hsc70 affected integrin β1 specifically, the expression of the most common integrin α5 subunit was investigated.14 As shown in Figure 2D and E, the results showed that both the mRNA level and protein expression on Hsc70-AS/U87 cell surface were not affected, suggesting the specificity of Hsc70 to integrin β1 subunit.
Down-Regulating the Expression of Hsc70 Increased P125 and Decreased P105 of Integrin β1
Integrin β1 is a transmembrane glycoprotein, after synthesized as an 85-kDa polypeptide, it undergoes glycosylation in the endoplasmic reticulum (ER), and then in the Golgi apparatus.15 The incompletely glycosylated form of integrin β1 has a mass of 105 kDa and is referred to as p105. The completely glycosylated form of integrin β1 has a mass of 125 kDa (p125). The mature p125 is transferred to the cell membrane and exerts its biological effects.16 The above results have demonstrated the stable expression of integrin β1 at the transcriptional level. We hypothesized that the increased expression of integrin β1 on Hsc70-AS/U87 cell surface might be attributed to the transportation of the mature form of integrin β1. Western blot was performed to investigate the protein levels of integrin β1 in Hsc70-AS/U87 and Vector/U87 cells (Figure 3A and C). There was no obvious difference in total integrin β1 proteins. However, the ratio of mature form p125 to precursor p105 was much higher in Hsc70-AS/U87 cells than that in Vector/U87 cells. Endoglycosidase H (Endo H) cleaves N-linked glycoproteins to remove the chitobiose core of mannose and some hybrid oligosaccharides. Endo H digestion completely converted p105 to a band of 85 kDa (the size of the core protein), whereas p125, which is glycosylated by medial- and trans-Golgi enzymes, was Endo H resistant.17 As shown in Figure 3B, lysates of U87 cells transiently transfected with Hsc70-AS were digested with Endo H before Western blot analyses. The 85-kDa band of integrin β1 was seen after Endo H digestion.
Integrin β1 Was Localized Near the Cell Surface in Hsc70-AS/U87 Cells
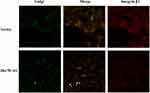
The above results indicated that down-regulation of Hsc70 in glioma cells might prompt the processing of integrin β1 in Golgi and its transportation to the cell surface. Thus, cellular localization of integrin β1 was detected using immunofluorescence confocal microscopy analysis. Vector/U87 cells or Hsc70-AS/U87 cells were reacted with anti-integrin β1 mouse monoclonal antibody followed by incubation with rhodamine-conjugated goat anti-mouse IgG (red) and C6-NBD (Golgi specific dye, green). As shown in Figure 4, integrin β1 is in red and the Golgi marker is in green. The yellow image is a red/green merge to show colocalization. In Vector/U87 cell, the colocalization was much higher than that in Hsc70-AS/U87 cells. In Hsc70-AS/U87 cells, the integrin β1 was localized near the cell surface (Lower panel, white arrow), suggesting down-regulation of Hsc70 could promote the transportation of integrin β1 from Golgi to the cell surface.
The Interaction Between β1 and α5 Subunits Was Strengthened in Hsc70-AS/U87 Cells
Integrin β1 interacts with many alpha integrins to exert biological functions.18 It has been reported that the expression of β-1,4-GalT regulated glycosylation and function of α5β1 integrin.19 Thus, whether down-regulation of Hsc70 in glioma cells could affect the function of integrin β1 through regulating the interaction between integrins α5 and β1 subunits was investigated. As shown in Figure 5A, the interaction between integrins α5 and β1 subunits was strengthened in Hsc70-AS/U87 cells demonstrated by immunoprecipitation assay. FAK is a key mediator for cell migration and invasion.20 We further investigated the expression of FAK and phosphorylated FAK in Hsc70-AS/U87 cells by immunoprecipitation and the results showed that the phosphorylation of FAK was increased, suggesting the activation of integrin-FAK signaling axis (Figure 5B).
Cell Adhesion Was Increased in Hsc70-AS/U87 Cells
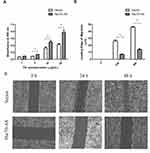
Fibronectin (FN) is a ~440-kDa glycoprotein of the extracellular matrix that binds to integrins, which plays a major role in cell adhesion, growth, migration, and differentiation.21 As shown in Figure 6A, in FN-coated plates, the adhesive ability of Hsc70-AS/U87 cells was increased compared with Vector/U87 cells. Furthermore, a wound-healing experiment was performed to evaluate the migration ability of Hsc70-AS/U87 cells. As shown in Figure 6B and C, the result was consistent with that in cell adhesion assay in Figure 6A, the migration ability of Hsc70-AS/U87 cells was significantly attenuated.
Discussion
Glioblastoma is one of the most common malignant tumors in the human central nervous system. Until now, the pathogenesis of glioblastoma has not been studied clearly. The significance of β-1,4-GalT V in invasion and metastasis of cancer cells has been verified.22,23 Research has indicated the increased galactosylation in astrocytomas, which was caused by alterations of expression of β-1,4-GalT V, and the malignant degree of astrocytoma is correlated with the expression of β-1,4-GalT V.24 In our previous study, we have shown that β-1,4-GalT V is over-expressed in glioma tissues and cells, and is positive correlated with the grade of gliomas. Interestingly, Hsc70 was found to interact with β-1,4-GalT V and played an important role in tumor metastasis and invasion in our previous studies. However, the detailed mechanism elucidating the function of Hsc70 in the invasion of glioma cells has not been uncovered. In this manuscript, we found that the down-regulation of Hsc70 significantly promoted the expression of integrin β1 125-kDa mature form, indicating the involvement of integrin β1 in Hsc70-mediated invasion of glioma cells.
Integrins are transmembrane receptors that facilitate cell extracellular matrix (ECM) adhesion. Changes in the expression of cell surface integrins are important in the genesis and development of cancer, impacting on various aspects of cancer metastasis and invasion.25 Glycosylation of integrin β1 in the Golgi complex has been related to its function in multiple cell processes, e.g., invasion, matrix adhesion, and migration.26 The mature integrin β1 subunit pairs with at least 12 different α-subunits to form transmembrane adhesion receptors for ECM proteins, e.g., collagen, FN, and laminin. The function of β1 depends on its accurate glycosylation catalyzed by enzymes located in morphologically and biochemically distinct cisternae of the Golgi system. Mature integrin β1 is transported to the cell surface, where it mechanically links plasma membrane adhesion complexes to the actin cytoskeleton for bidirectional transmembrane as well as intracellular signaling.27 Many factors can affect the maturation of integrin β1 and its transport to the cell surface. In this study, we found that down-regulating the expression of Hsc70 in U87 cells specifically up-regulated the expression of cell surface integrin β1 without the change of its mRNA. Meanwhile, the integrin β1 125-kDa mature form increased and 105-kDa precursor form decreased when Hsc70 was down-regulated. Furthermore, the U87 cells transfected with antisense cDNA of Hsc70 decreased the Golgi localization of integrin β1 and increased the transportation of integrin β1 from Golgi to the cell surface. From these results, we can speculate the possibility that the enhancement of cell surface integrin β1 in Hsc70-AS/U87 cells is not caused by increment of mRNA transcription of integrin β1, but may be caused by the accelerated transformation of the precursor form of 105 kDa to mature form of 125 kDa.
FAK, also known as protein tyrosine kinase 2, is a focal adhesion-associated protein kinase involved in cellular adhesion and spreading processes. This cytosolic kinase has been implicated in diverse cellular roles including cell locomotion, mitogen response and cell survival.28 It has been shown that when FAK was blocked, breast cancer cells became less metastatic due to decreased mobility.29 FAK is phosphorylated in response to integrin engagement and recruited to focal adhesions. In Hsc70-AS/U87 cells, down-regulation of Hsc70 increased the function of integrin β1 through regulating the interaction between integrins α5 and β1 subunits, increasing the phosphorylation of FAK and activating integrin-FAK signaling pathway.
Conclusion
In our previous studies, the expression of Hsc70 was found to increase in the process of human cancer cells, and the overexpression of Hsc70 promoted the growth and invasion of human glioma cells. The Hsc70-AS/U87 cells in this study demonstrate the suppression of tumor development and migration, suggesting that Hsc70 could represent a novel target in glioma therapy.
Acknowledgments
This work was supported by the China Natural Science Foundation (81672499, 81000963), Jiangsu Province’s Natural Science Foundation (BK20141256, BK20161318), the Youth Medical Talent Project of the Jiangsu Province (QNRC2016470), Jiangsu Postdoctoral Research Funding Program (2019K061) and Top Talent Project of “six one projects” for High-level Health talents in Jiangsu Province (YY-206).
Disclosure
The authors have no conflicts of interest in this work.
References
1. Xie Q, Mittal S, Berens ME. Targeting adaptive glioblastoma: an overview of proliferation and invasion. Neuro Oncol. 2104;16:1575–1584. doi:10.1093/neuonc/nou147
2. Aref D, Croul S. Medulloblastoma: recurrence and metastasis. CNS Oncol. 2013;2:377–385. doi:10.2217/cns.13.30
3. Mahajan-Thakur S, Bien-Möller S, Marx S, et al. Sphingosine 1-phosphate (S1P) signaling in glioblastoma multiforme-a systematic review. Int J Mol Sci. 2017;18:2448. doi:10.3390/ijms18112448
4. Aladowicz E, Ferro L, Vitali GC, et al. Molecular networks in melanoma invasion and metastasis. Future Oncol. 2013;9:713–726. doi:10.2217/fon.13.9
5. Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18:533–548. doi:10.1038/s41568-018-0038-z
6. Pan L, Zhao Y, Yuan Z, et al. Research advances on structure and biological functions of integrins. Springerplus. 2016;5:1094. doi:10.1186/s40064-016-2502-0
7. Glavey SV, Huynh D, Reagan MR, et al. The cancer glycome: carbohydrates as mediators of metastasis. Blood Rev. 2015;29:269–279. doi:10.1016/j.blre.2015.01.003
8. Ishii T, Miyauchi K, Nitta Y, et al. Mechanism for decreased gene expression of beta4-galactosyltransferase 5 upon differentiation of 3T3-L1 mouse preadipocytes to adipocytes. Biol Pharm Bull. 2018;41:1463–1470. doi:10.1248/bpb.b18-00360
9. Chang HH, Chen CH, Chou CH, et al. Beta-1,4-Galactosyltransferase III enhances invasive phenotypes via beta1-integrin and predicts poor prognosis in neuroblastoma. Clin Cancer Res. 2013;19:1705–1716. doi:10.1158/1078-0432.CCR-12-2367
10. Chen X, Jiang J, Yang J, et al. Down-regulation of the expression of beta1,4-galactosyltransferase V promotes integrin beta1 maturation. Biochem Biophys Res Commun. 2006;343:910–916. doi:10.1016/j.bbrc.2006.03.046
11. Liu T1, Daniels CK, Cao S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol Ther. 2012;136:354–374. doi:10.1016/j.pharmthera.2012.08.014
12. Liao Y, Tang L. The critical roles of HSC70 in physiological and pathological processes. Curr Pharm Des. 2014;20:101–107. doi:10.2174/13816128113199990585
13. Vila-Carriles WH, Zhou Z-H, Bubien JK, et al. Participation of the chaperone Hsc70 in the trafficking and functional expression of ASIC2 in glioma cells. J Biol Chem. 2007;282:34381–34391. doi:10.1074/jbc.M705354200
14. Mierke CT. Phagocytized beads reduce the alpha5beta1 integrin facilitated invasiveness of cancer cells by regulating cellular stiffness. Cell Biochem Biophys. 2013;66:599–622. doi:10.1007/s12013-012-9506-3
15. Naydenov NG, Feygin A, Wang L, et al. N-ethylmaleimide-sensitive factor attachment protein alpha (alphaSNAP) Regulates matrix adhesion and integrin processing in human epithelial cells. J Biol Chem. 2014;289:2424–2439. doi:10.1074/jbc.M113.498691
16. Gens JS, Reuzeau C, Doolittle KW, et al. Covisualization by computation optical-sectioning microscopy of integrin and associated proteins at the cell membrane of living onion protoplasts. Protoplasma. 1996;194:215–230. doi:10.1007/BF01882029
17. Bednarczyk JL, Szabo MC, McIntyre BW, et al. Post-translational processing of the leukocyte integrin alpha 4 beta 1. J Biol Chem. 1992;267:25274–25281.
18. Borza CM, Chen X, Mathew S, et al. Integrin {alpha}1{beta}1 promotes caveolin-1 dephosphorylation by activating T cell protein-tyrosine phosphatase. J Biol Chem. 2010;285:40114–40124. doi:10.1074/jbc.M110.156729
19. Yang H, Hu L, Chen J, et al. Lipopolysaccharide induced upregulation of beta-1,4-galactosyltransferase-I in Schwann cell. Inflammation. 2009;32:279–286. doi:10.1007/s10753-009-9131-5
20. Kong DB, Chen F, Sima N, et al. Focal adhesion kinases crucially regulate TGFbeta-induced migration and invasion of bladder cancer cells via Src kinase and E-cadherin. Onco Targets Ther. 2017;10:1783–1792. doi:10.2147/OTT.S122463
21. Wang JP, Hielscher A. Fibronectin: how its aberrant expression in tumors may improve therapeutic targeting. J Cancer. 2017;8:674–682. doi:10.7150/jca.16901
22. Xu S, Zhang S, Chen C, et al. Over-expression of beta-1, 4-galactosyltransferase V increases the growth of astrocytoma cell line. J Exp Clin Cancer Res. 2002;21:409–414.
23. Cui C, Chen X1, Liu Y, et al. Beta1,4-Galactosyltransferase V activates Notch1 signaling in glioma stem-like cells and promotes their transdifferentiation into endothelial cells. J Biol Chem. 2018;293:2219–2230. doi:10.1074/jbc.RA117.000682
24. Xu S, Zhu X, Zhang S, et al. Over-expression of beta-1,4-galactosyltransferase I, II, and V in human astrocytoma. J Cancer Res Clin Oncol. 2001;127:502–506. doi:10.1007/s004320100246
25. Xu H, Yuan Y, Wu W, et al. Hypoxia stimulates invasion and migration of human cervical cancer cell lines HeLa/SiHa through the Rab11 trafficking of integrin alphavbeta3/FAK/PI3K pathway-mediated Rac1 activation. J Biosci. 2017;42:491–499. doi:10.1007/s12038-017-9699-0
26. Kim LT, Ishihara S, Lee CC, et al. Altered glycosylation and cell surface expression of beta 1 integrin receptors during keratinocyte activation. J Cell Sci. 1992;103 (Pt 3):743–753.
27. Berthier R, Jacquier-Sarlin M, Schweitzer A, et al. Adhesion of mature polyploid megakaryocytes to fibronectin is mediated by β1 integrins and leads to cell damage. Exp Cell Res. 1998;242:315–327. doi:10.1006/excr.1998.4119
28. Vitillo L, Kimber SJ. Integrin and FAK regulation of human pluripotent stem cells. Curr Stem Cell Rep. 2017;3:358–365. doi:10.1007/s40778-017-0100-x
29. Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185:357–370. doi:10.1083/jcb.200809110
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.