Back to Journals » Journal of Pain Research » Volume 10
Heat pain detection threshold is associated with the area of secondary hyperalgesia following brief thermal sensitization: a study of healthy male volunteers
Authors Hansen MS , Wetterslev J , Pipper CB, Asghar MS, Dahl JB
Received 31 August 2016
Accepted for publication 7 November 2016
Published 27 January 2017 Volume 2017:10 Pages 265—274
DOI https://doi.org/10.2147/JPR.S121189
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Morten Sejer Hansen,1 Jørn Wetterslev,2 Christian Bressen Pipper,3 Mohammad Sohail Asghar,1 Jørgen Berg Dahl4
1Department of Anesthesiology, 4231, Centre of Head and Orthopedics, Rigshospitalet, 2Copenhagen Trial Unit, Centre for Clinical Intervention Research, Department 7812, 3Section of Biostatistics, Faculty of Health, Copenhagen University, Copenhagen, 4Department of Anesthesiology, Department Z, Bispebjerg Hospital, Copenhagen, Denmark
Introduction: The area of secondary hyperalgesia following brief thermal sensitization (BTS) of the skin and heat pain detection thresholds (HPDT) may both have predictive abilities in regards to pain sensitivity and clinical pain states. The association between HPDT and secondary hyperalgesia, however, remains unsettled, and the dissimilarities in physiologic properties suggest that they may represent 2 distinctively different pain entities. The aim of this study was to investigate the association between HPDT and BTS-induced secondary hyperalgesia.
Methods: A sample of 121 healthy male participants was included and tested on 2 separate study days with BTS (45°C, 3 minutes), HPDT, and pain during thermal stimulation (45°C, 1 minute). Areas of secondary hyperalgesia were quantified after monofilament pinprick stimulation. The pain catastrophizing scale (PCS) and hospital anxiety and depression scale (HADS) were also applied.
Results: A significant association between HPDT and the size of the area of secondary hyperalgesia (p<0.0001) was found. The expected change in area of secondary hyperalgesia due to a 1-degree increase in HPDT was estimated to be −27.38 cm2, 95% confidence interval (CI) of −37.77 to −16.98 cm2, with an R2 of 0.19. Likewise, a significant association between HADS-depression subscore and area of secondary hyperalgesia (p=0.046) was found, with an estimated expected change in secondary hyperalgesia to a 1-point increase in HADS-depression subscore of 11 cm2, 95% CI (0.19–21.82), and with R2 of 0.03. We found no significant associations between secondary hyperalgesia area and PCS score or pain during thermal stimulation.
Conclusion: HPDT and the area of secondary hyperalgesia after BTS are significantly associated; however, with an R2 of only 19%, HPDT only offers a modest explanation of the inter-participant variation in the size of the secondary hyperalgesia area elicited by BTS.
Keywords: pain, central nervous system sensitization, hyperalgesia, pain threshold, healthy volunteers, catastrophization, secondary hyperalgesia, central sensitization
Corrigendum for this paper has been published
Introduction
Clinical pain models may bridge the gap between animal and human research and may be applied in the investigation of pain sensitivity. Sufficient prediction of pain sensitivity, for example prior to surgery, may improve our ability to prevent severe acute and chronic pain following surgery,1 as well improve the inclusion procedure in pharmaceutical drug trials by allowing initial grouping of participants in high- and low-pain responders.2,3
Current evidence suggest that the development of secondary hyperalgesia to punctate mechanical stimuli after a cutaneous heat injury in healthy volunteers is mediated by heat- and mechanosensitive type-I and/or mechanosensitive (heat-insensitive) A-fiber nociceptors, and is due to changes in the central nervous system, that is, central sensitization.4–9 Central sensitization encompasses a functional change in neuron properties and nociceptive pathways, with increased membrane excitability and synaptic efficacy, and decreased synaptic inhibition resulting in increased and sometimes pathological responses to mechanical and noxious stimulation.4,9 The transcription-dependent long-lasting phase of central sensitization is assumed to play a key role in several pathological pain conditions, for example, osteoarthritis and fibromyalgia,4,9–11 and investigation of secondary hyperalgesia following a standardized burn injury may therefore provide insight into central sensitization.
Studies in healthy volunteers have indicated that the size of the area of secondary hyperalgesia following standardized cutaneous sensitization procedures has a large inter-individual to intra-individual variance,12,13 is modifiable by certain analgesics,14–18 and may be predictive of individual pain responses.4,9,19,20 The area of secondary hyperalgesia following the cutaneous heat pain model of brief thermal sensitization (BTS)13,14,16,18,21,22 quantified by monofilament stimulation12–14,16–18,21–28 has been demonstrated to be a reproducible phenomenon that may be used in phenotype characterization of healthy volunteers.12,13
Heat pain detection threshold (HPDT) has been applied in several studies,17,18,22,25,29–33 and the acute first pain elicited by the rapid heating of the skin is believed to be transmitted in A-fiber type-II mechano- and heat-sensitive nociceptors (in hairy skin), and mechano- and heat-sensitive C fibers.5 HPDT has been proven to be reproducible,34 and evidence suggests that HPDT may have a predictive value when investigating postoperative pain.35,36
However, the dissimilarities in physiologic properties between secondary hyperalgesia to mechanical pinprick stimulation and HPDT suggest that they may represent 2 distinctively different pain entities.
As a first step to explore secondary hyperalgesia following BTS and its potential predictive abilities, we aim to investigate the association between HPDT and secondary hyperalgesia induced by BTS. We hypothesized that HPDT and areas of secondary hyperalgesia were two predominantly independent entities, and that the area of secondary hyperalgesia was poorly explained by HPDT.
Methods
The study was approved by the local Danish Committee on Health Research Ethics for the Capital Region (Identifier: H-8-2014-012) and the Danish Data Protection Agency (Identifier: 30–1436); the study is also reported on the international database clinicaltrials.gov (Identifier: NCT02527395).
The design and methods of this prospective study is based upon a previous study done by Hansen et al;13 moreover, an extensive description of the design and methods of this study has been published in a preceding methods paper, which is publicly available for review.37
Study participants
Healthy male participants aged >18 and <35 years who could understand and speak the Danish language were included in the study. Written informed consent was obtained from all participants prior to inclusion, and all participants received EUR 20 (USD 27) per hour for their participation in the study. Participants were recruited by advertisement in the medical student magazine at Copenhagen University and online at www.forsøgspersoner.dk. Exclusion criteria were failure to cooperate with the tests, a weekly intake of >21 units of alcohol, consummation of >3 units of alcohol 24 hours before study day, substance abuse, intake of analgesics within 3 days before study day, intake of antihistamines 48 hours before study day, intake of prescription medicine and/or antidepressant medicine within 30 days before study day, neurological illnesses, chronic pain conditions, psychiatric diagnoses, tattoos on the extremities, eczema, wounds or sunburns at the sites of testing, and a body mass index (BMI) of >30 kg/m2 and <18 kg/m2.
Setting
The study was conducted in a quiet secluded room (temperature of 22°C–25°C), where only the investigator and the participant were present. The participants were placed in a supine position, on their back, throughout the assessments. The study was conducted during the time from 8 AM to 6 PM at the Department of Anesthesiology, 4231, Rigshospitalet, Copenhagen, Denmark in the period from October 1, 2015 to December 2, 2015.
Design
The study consisted of 1 screening/information day and 2 separate study days. To avoid a possible carry-over effect of the applied tests, the screening day and the 2 study days were separated with a minimum of 7 days.13 Height, weight, arterial blood pressure, and pulse frequency of all participants were measured; moreover, data on age, right/left-handedness, and parental ethnicity were collected. On the 2 separate study days, the study participants were tested with 3 types of pain models: BTS, HPDT, and pain during 1-minute thermal stimulation (p-TS) in a predefined sequence (see pain models and randomization and allocation concealment). On the information day, the participants were provided with the psychological tests, pain catastrophizing scale (PCS)38–40 and hospital anxiety and depression scale (HADS)41–43 (see psychological testing), which they completed at home and returned on the first study day in sealed opaque envelopes to ensure blinding. Opening of the envelopes was deferred until all participants had completed the study. All other assessments and tests were performed by the same investigator throughout the study (MSH).
Pain models
All pain testing was conducted with a computer-controlled thermode (MSA Thermotester™), size 2.5×5 cm
Brief thermal sensitization (BTS)
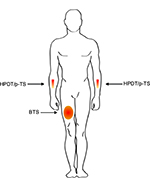
BTS was induced by placing the computer-controlled thermode on the skin of the participant, centrally on the anterior part of the right thigh in the midline between the anterior superior iliac spine and the base of patella (Figure 1). The starting temperature of the thermode was 32°C, and with an increase of 1°C/second, the thermode was heated to 45°C. After 3 minutes, the assessment of secondary hyperalgesia (see below) was conducted while the thermode at 45°C was still positioned on the skin of the participant.14,16,18,21,22 The assessment of secondary hyperalgesia took ~1–2 minutes, resulting in a maximum duration of heat stimulation of 5 minutes.
Assessment of secondary hyperalgesia
The area of secondary hyperalgesia was quantified after pinprick stimulation with a 19G monofilament (von Frey hair) in 4 linear paths arranged in 90° around the center of the thermode. Stimulation began well outside the area of secondary hyperalgesia, minimum 15 cm from the edge of the thermode, and advanced in steps of 5 mm/second toward the thermode. When the participant stated a clear change in sensation (intense burning, pricking, and tenderness), the spot was marked with a felt pen, and the longitudinal and transverse axes were measured with a pliable measuring tape for rectangular area calculation.12–14,16–18,21–28
Heat pain detection threshold (HPDT)
HPDT was evaluated by placing the thermode on the skin of the participant on the anterior part of the dominant lower arm (Figure 1). The start temperature of the thermode was 32°C and the temperature was then increased by 1°C/second. When the participant perceived the heat as painful he pressed a button, the temperature was registered, and the thermode returned to a temperature of 32°C. If a temperature of 52°C was reached, the thermode would automatically return to 32°C and 52°C would be registered as the threshold. The HPDT was estimated as an average of 4 separate stimulations with an interval of 6–10 seconds.13,14,16–18,22–25
Pain during thermal stimulation (p-TS)
The thermode was placed on the participant’s skin centrally on the anterior lower non-dominant arm (Figure 1). The start temperature of the thermode was 32°C, and with an increase of 1°C/second, the thermode was heated to 45°C and remained 45°C for 1 minute. During the 1 minute heating of the skin the participant evaluated the pain using the electronic visual analog scale (VAS; Somedic USB-VAS), with an index of 0–100 mm, where 0 mm represented “no pain”, and 100 mm represented “worst pain imaginable”. The software provided with the electronic VAS automatically calculated an area under the curve (AUC) and a maximum VAS score for the time period. The participant was not able to see the computer screen during the assessment.17,22–25
Psychological testing
Pain Catastrophizing Scale (PCS)
PCS is 13-point questionnaire on a 5-point Likert scale with values from 0 to 4. The highest achievable score is 52, and the PCS can be subdivided into 3 sections that evaluate 1) rumination, 2) magnification, and 3) helplessness.38–40
Hospital Anxiety and Depression Scale (HADS)
HADS is a 14-point questionnaire on a 4-point Likert scale with values ranging from 0 to 3. The highest achievable score is 53, and the HADS can be subdivided into 2 sections that evaluate 1) anxiety and 2) depression.41–43
Randomization and allocation concealment
The sequence of BTS and HPDT was randomized so that on 1 study day the sequence was: 1) BTS, 2) HPDT, and 3) p-TS, and on the other study day the sequence was: 1) HPDT, 2) BTS, and 3) p-TS. The randomization was performed with a computer-generated random allocation sequence, conducted by the Copenhagen Trial Unit, and stored in sealed opaque envelopes to secure adequate allocation concealment.
Test results and assessments for each study day were entered in a standardized case report form and placed in an opaque sealed envelope to ensure that the investigator was unable to see previous test results. Completed psychological tests were kept in sealed opaque envelopes and the blinding was first broken after all study participants had completed the study.
Outcome measures
Primary analysis
The association between HPDT and area of secondary hyperalgesia induced by BTS.
Secondary analyses
The association between area of secondary hyperalgesia induced by BTS and
- VAS-AUC following p-TS
- Max VAS-score following p-TS
- PCS-score
- HADS-score
- PCS and HADS subscales (PCS-rumination, PCS-magnification, PCS-helplessness, HADS-anxiety, and HADS-depression)
Sample size
A simulation-based sample size calculation was performed with data from our previous study;13 and with an α of 0.05 and β of 0.01, we estimated that a number of 120 participants were needed in order to provide an empirical power of 99.9% (for further description see the published protocol37). All simulation-based calculations were made using the open-source statistical programming environment R.44
Statistical analysis
Individual levels of areas of secondary hyperalgesia, HPDT, VAS-AUC, and VAS-max were obtained as estimated best linear unbiased predictors (EBLUPS). The association between area of secondary hyperalgesia and HPDT was evaluated by multiple linear regression adjusting for individual body surface area. Models were validated graphically by means of residuals and QQ plots. Normality of residuals was assessed by the Kolmogorov–Smirnov test. The ability of HPDT to predict the size of the area of secondary hyperalgesia was quantified by R2 and illustrated with prediction limits.
In a secondary analysis, we additionally included VAS-AUC, Max VAS-score following p-TS, PCS-score, and HADS-score as predictors in a multiple linear regression on area of secondary hyperalgesia. The importance of these predictors was assessed by backward elimination with a 5% cut-off level.
p-Values corresponded to F tests and were evaluated at a 5% significance level.
Additionally, 3 post hoc sensitivity analyses were performed to assess the robustness of the findings. In the first sensitivity analysis, further adjustment by age, weight, BMI, and mean arterial pressure (MAP) was performed. In the second sensitivity analysis, only right-handed participants were included; and finally, in the third sensitivity analysis, only ethnic Scandinavians were included.
Body surface area was calculated using the Mosteller formula.45 Distributions of variables are summarized by medians and interquartile ranges. All analyses were made using the open-source statistical programming environment R.44
Results
A sample of 131 healthy male volunteers was assessed for eligibility, and a total of 121 were included in the study (Figure 2). All 121 study participants completed the study, and data from all the participants were analyzed for the primary and secondary outcome measures. Of the 121 participants, 12 had one or more parents with non-Scandinavian ethnicity and 16 were left-handed. The median interval between the 2 study days was 7 days (interquartile range [IQR], 7–8). Relevant data on the included participants’ characteristics are presented in Table 1. The median size of the area of secondary hyperalgesia was 447.78 cm2 (IQR, 346.19–528.99) and the median HPDT was 45.57°C (IQR, 43.79–46.61). Results from the p-TS, PCS, and HADS are presented in Tables 2 and 3. No adverse or serious adverse events were reported.
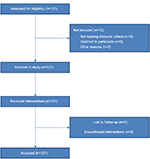  | Figure 2 Flowchart of included study participants. |
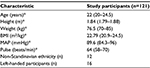  | Table 1 Characteristics of included participants Note: *Data are reported as median and interquartile range. Abbreviations: BMI, body mass index; MAP, mean arterial pressure. |
  | Table 3 Psychological test scores, total, and subscores Abbreviations: HADS, hospital anxiety and depression scale; IQR, interquartile range; PCS, pain catastrophizing scale. |
Primary analysis
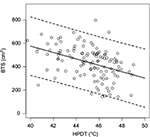
We found a significant association between HPDT and the size of the area of secondary hyperalgesia (p<0.0001). We estimated the expected change in area of secondary hyperalgesia due to a 1-degree increase in HPDT to −27.38 cm2 with a 95% confidence interval (CI) of −37.77 to −16.98 cm2. The R2 was calculated to 0.19, and the prediction limits at a given HPDT of 46°C and body surface of 1.99 m2 were estimated to 167.42–656.07 cm2 (Figure 3).
Secondary analyses
We found a significant association between HADS-depression score and area of secondary hyperalgesia (p=0.046). The estimated expected change in secondary hyperalgesia area to a 1-point increase in HADS-depression was 11 cm2 (95% CI, 0.19–21.82; R2, 0.03). No significant associations were found in any of the other secondary outcome measures.
Post hoc analyses
The 3 post hoc sensitivity analyses did not demonstrate noticeably different results when compared to our primary analysis.
Discussion
In the present study, we aimed to investigate the association between HPDT and secondary hyperalgesia elicited by BTS. We demonstrated a significant association between HPDT and secondary hyperalgesia, where increasing levels of HDPT were associated with decreasing sizes of secondary hyperalgesia areas. In addition to the highly significant association, we found an R2 of 19%, illustrating that HPDT only offers a modest explanation of the inter-participant variation in secondary hyperalgesia following BTS. The estimated prediction interval for areas of secondary hyperalgesia at an HPDT of 46°C and a body surface of 1.99 m2 were estimated to 167.42–656.07 cm2 (Figure 3), indicating that although we find a highly significant result, HPDT and areas of secondary hyperalgesia are only modestly associated. Likewise, we also found a significant association between increasing HADS-depression subscore and increasing size of secondary hyperalgesia area; however, R2 was estimated to 3%, and in this study, HADS-depression subscore only offered a very modest explanation for the variation in the area of secondary hyperalgesia.
We have applied an experimental pain model (BTS) with a high reliability (intraobserver intra-participant correlation of 0.85).13 Moreover, our post hoc sensitivity analyses did not demonstrate noticeable differences compared to our primary analysis, illustrating the robustness of our results. In our study, BMI, age, MAP, left-handedness, and ethnicity did not have any influence on the association between HPDT and secondary hyperalgesia to mechanical punctate stimuli. The high number of included participants provides an empirical power of 99.9%, which practically eliminates the risk of type-II errors and once again illustrates the robustness of our results.
The results in this study confirm the results from our previous study where a significant association was demonstrated with an R2 of 20%.13 Likewise, in our current study we find a high inter-participant difference in areas of secondary hyperalgesia ranging from 135 to 788 cm2 (Table 2), as well as high inter-participant differences in HPDT ranging from 38.7°C to 51.02°C (Table 2).
The weak association between HPDT and secondary hyperalgesia area is noteworthy because it has been suggested that both parameters may to some extent be important in categorizing pain sensitivity; however, evidence on the predictive value of these parameters is contradicting with diverse results both for20,46–48 and against31,36 HPDT and secondary hyperalgesia areas as predictors of pain sensitivity. The physiologic properties in the neural mediation of HPDT and secondary hyperalgesia may, in part, account for the weak association. HPDT is primarily mediated by A-fiber type-II mechano- and heat-sensitive nociceptors, and mechano- and heat-sensitive C fibers, and secondary hyperalgesia to punctate mechanical stimuli is mediated by heat- and mechano-sensitive type-I and mechanosensitive (heat-insensitive) A-fiber nociceptors.5–7 In a recent study, results even suggested that secondary hyperalgesia was mediated only by heat-insensitive mechanosensitive A-fiber nociceptors.8 Finally, it is believed that the development of secondary hyperalgesia is caused by central sensitization due to changes in the central nervous system,4,7,9 which leads to the suggestion that HPDT and secondary hyperalgesia to mechanical punctate stimuli may be 2 distinctively different pain entities. A biological explanation, although speculative, may be that HPDT represent an acute warning system against nociceptive stimuli, whereas secondary hyperalgesia represents a somewhat later occurrence of sensitization of central neurons, which may serve other purposes in the nociceptive process.
Studies have demonstrated that patients suffering from persistent pain due to rheumatoid arthritis or fibromyalgia display larger areas of secondary hyperalgesia when compared with healthy individuals.49,50 Likewise, clinical studies have indicated that increasing sizes of secondary hyperalgesia areas surrounding surgical wounds are associated with an increased risk of developing chronic pain following surgery.20,51 These findings indicate that a large area of secondary hyperalgesia is found in persons with high levels of central sensitization. Thus, the investigation of secondary hyperalgesia areas may provide insight in individual levels of central sensitization. With Woolf’s description of a central sensitization syndrome,4 where pain hypersensitivity syndromes may share common contributions of central sensitization, the investigation of secondary hyperalgesia may provide insight into already known pain hypersensitivity syndromes, and may also contribute to the phenotyping of pain sensitivity in healthy persons. A recent brain magnetic resonance imaging study of healthy volunteers indicated that participants with differences in areas of secondary hyperalgesia exhibited structural and functional differences when comparing healthy participants with a large vs small area of secondary hyperalgesia,52 suggesting differences in sensory discrimination, pain suppression, and avoidance behavior. However, the practical applicability of secondary hyperalgesia areas is not yet fully understood or described, and thorough investigations of central sensitization, as well as factors influencing individual propensity to develop central sensitization may have a role in the future of analgesic therapy and pain research.
Contrary to our previous study,13 we found a significant association between increasing HADS-depression subscore and increasing size of secondary hyperalgesia area. However, R2 was estimated to 3%, and in this study, HADS-depression subscore only offered a very modest explanation for the variation in secondary hyperalgesia areas. Several clinical studies have demonstrated significant associations between postoperative pain and personality traits, such as depression, anxiety, and pain catastrophizing.53–56 Moreover, in a study by Salomons et al,57 it was demonstrated that pain-focused cognitive training reduced the area of secondary hyperalgesia in healthy volunteers. However, in a recently published review it was concluded that the influence of psychological variables on experimental pain responses is still largely unclear.58 Our very strict inclusion criteria, that specifically excluded women, chronic pain patients, and persons with prior psychological history, may have resulted in a sampling bias that reduced the inter-individual variance of secondary hyperalgesia areas, PCS (IQR, 7–17), and HADS score (IQR, 3–8.5), and could be responsible for the weak association between HADS, HPDT, and secondary hyperalgesia. A sufficient investigation of psychological variables and pain should attempt to conduct consecutive inclusion of patients prior to, for example, surgery or restricted inclusion of volunteers with high psychiatric vulnerability.
Our study has some limitations. 1) As emphasized before, we applied very strict inclusion criteria, and consequently, included a very homogenous population; inclusion of, for example, females and chronic pain patients could potentially have increased the inter-individual variance and resulted in a higher R2. However, individual characteristics, such as sex,25,59–62 obesity,63 and menstrual hormone cycle,64 may potentially influence pain thresholds and sensitivity and to accommodate for all these variables, hereby minimizing the unknown factors of variation, and to focus only on the association between HPDT and secondary hyperalgesia areas, we chose to apply very strict inclusion criteria. Additionally, BTS has only been validated in healthy male volunteers,13 and consequently, the results of this study only apply to young and healthy, male volunteers.
2) We did not evaluate dietary intake, stress and hormone levels, genetics, brain anatomy, or skin receptor density of the included participants. Studies have suggested that diets high on tryptophan,65,66 high stress levels of serum cortisol and testosterone,67 and even certain genetic markers68–72 may influence the pain sensitivity. An inter-participant differentiable diet and hormone level, as well as differences in stress levels, genetics, and brain anatomy could be explanatory factors of the high inter-individual variance of HPDT and areas of secondary hyperalgesia.
Finally, 3 patients reported HPDTs well outside the interquartile ranges, 2 patients <40°C and 1 patient >50°C, which may indicate that they misunderstood the procedure.
In our study, HPDT only offered a modest explanation of the inter-individual size of the area of secondary hyperalgesia; and the inter-individual differences in secondary hyperalgesia observed in numerous studies remain largely unexplained. Studies investigating postoperative pain and secondary hyperalgesia before and after surgery could provide insight on the predictive value of secondary hyperalgesia areas, and finally, as secondary hyperalgesia is believed to occur as a result of central neuronal plasticity,4,9 future research should attempt to investigate variables in the central nervous system in both patients and healthy participants, with modalities such as structural and functional magnetic resonance imaging, electroencephalography, and magnetoencephalography.
In conclusion, our study demonstrated a statistically significant association between HPDT and the size of the area of secondary hyperalgesia. However, with an R2 of only 19%, HPDT offers only a modest explanation of the inter-participant variation in the size of the secondary hyperalgesia area elicited by BTS.
Acknowledgments
This work was supported by grants from the Augustinus foundation (grant number: 14-3907), Toyota Fonden – Denmark (grant number: OH/BG-8610), and the Aase and Ejnar Danielsen’s foundation (grant number: 10-001341). The funders had no role in the conception or design of the study, the collection, analysis or interpretation of data, the writing of the report, or on the decision to publish the results.
The authors would like to thank illustrator Sarah Egbert Eiersholt for her valuable contribution to the artwork in Figure 1.
Disclosure
The authors report no conflicts of interest in this work.
References
Werner MU, Mjobo HN, Nielsen PR, Rudin A. Prediction of postoperative pain: a systematic review of predictive experimental pain studies. Anesthesiology. 2010;112(6):1494–1502. | ||
Arendt-Nielsen L, Hoeck HC. Optimizing the early phase development of new analgesics by human pain biomarkers. Expert Rev Neurother. 2011;11(11):1631–1651. | ||
Chizh BA, Priestley T, Rowbotham M, Schaffler K. Predicting therapeutic efficacy – experimental pain in human subjects. Brain Res Rev. 2009;60(1):243–254. | ||
Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(Suppl 3):S2–S15. | ||
Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120(11):3760–3772. | ||
Treede RD. Chapter 1 Pain and hyperalgesia: definitions and theories. Handb Clin Neurol. 2006;81:3–10. | ||
Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain. 1999;122 (Pt 12):2245–2257. | ||
van den Broeke EN, Lenoir C, Mouraux A. Secondary hyperalgesia is mediated by heat-insensitive A-fibre nociceptors. J Physiol. 2016;594(22):6767–6776. | ||
Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. | ||
Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10(6):556–572. | ||
Edwards RR. Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology. 2005;65(3):437–443. | ||
Werner MU, Petersen KL, Rowbotham MC, Dahl JB. Healthy volunteers can be phenotyped using cutaneous sensitization pain models. PLoS One. 2013;8(5):e62733. | ||
Hansen MS, Wetterslev J, Pipper CB, Ostervig R, Asghar MS, Dahl JB. The area of secondary hyperalgesia following heat stimulation in healthy male volunteers: inter- and intra-individual variance and reproducibility. PLoS One. 2016;11(5):e0155284. | ||
Frymoyer AR, Rowbotham MC, Petersen KL. Placebo-controlled comparison of a morphine/dextromethorphan combination with morphine on experimental pain and hyperalgesia in healthy volunteers. J Pain. 2007;8(1):19–25. | ||
Mathiesen O, Imbimbo BP, Hilsted KL, Fabbri L, Dahl JB. CHF3381, a N-methyl-D-aspartate receptor antagonist and monoamine oxidase-A inhibitor, attenuates secondary hyperalgesia in a human pain model. J Pain. 2006;7(8):565–574. | ||
Petersen KL, Iyengar S, Chappell AS, et al. Safety, tolerability, pharmacokinetics, and effects on human experimental pain of the selective ionotropic glutamate receptor 5 (iGluR5) antagonist LY545694 in healthy volunteers. Pain. 2014;155(5):929–936. | ||
Petersen KL, Jones B, Segredo V, Dahl JB, Rowbotham MC. Effect of remifentanil on pain and secondary hyperalgesia associated with the heat–capsaicin sensitization model in healthy volunteers. Anesthesiology. 2001;94(1):15–20. | ||
Petersen KL, Meadoff T, Press S, Peters MM, LeComte MD, Rowbotham MC. Changes in morphine analgesia and side effects during daily subcutaneous administration in healthy volunteers. Pain. 2008;137(2):395–404. | ||
You DS, Creech SK, Meagher MW. Enhanced area of secondary hyperalgesia in Women with multiple stressful life events: a pilot study. Pain Med. Epub 2016 Apr 7. | ||
Martinez V, Ben Ammar S, Judet T, Bouhassira D, Chauvin M, Fletcher D. Risk factors predictive of chronic postsurgical neuropathic pain: the value of the iliac crest bone harvest model. Pain. 2012;153(7):1478–1483. | ||
Dirks J, Petersen KL, Dahl JB. The heat/capsaicin sensitization model: a methodologic study. J Pain. 2003;4(3):122–128. | ||
Dirks J, Petersen KL, Rowbotham MC, Dahl JB. Gabapentin suppresses cutaneous hyperalgesia following heat-capsaicin sensitization. Anesthesiology. 2002;97(1):102–107. | ||
Cavallone LF, Frey K, Montana MC, et al. Reproducibility of the heat/capsaicin skin sensitization model in healthy volunteers. J Pain Res. 2013;6:771–784. | ||
Dirks J, Petersen KL, Rowbotham MC, Dahl JB. Effect of systemic adenosine on pain and secondary hyperalgesia associated with the heat/capsaicin sensitization model in healthy volunteers. Reg Anesth Pain Med. 2001;26(5):414–419. | ||
Jensen MT, Petersen KL. Gender differences in pain and secondary hyperalgesia after heat/capsaicin sensitization in healthy volunteers. J Pain. 2006;7(3):211–217. | ||
Mikkelsen S, Dirks J, Fabricius P, Petersen KL, Rowbotham MC, Dahl JB. Effect of intravenous magnesium on pain and secondary hyperalgesia associated with the heat/capsaicin sensitization model in healthy volunteers. Br J Anaesth. 2001;86(6):871–873. | ||
Petersen KL, Brennum J, Dahl JB. Experimental evaluation of the analgesic effect of ibuprofen on primary and secondary hyperalgesia. Pain. 1997;70(2–3):167–174. | ||
Petersen KL, Rowbotham MC. A new human experimental pain model: the heat/capsaicin sensitization model. Neuroreport. 1999;10(7):1511–1516. | ||
Reddy KS, Naidu MU, Rani PU, Rao TR. Human experimental pain models: a review of standardized methods in drug development. J Res Med Sci. 2012;17(6):587–595. | ||
Staahl C, Olesen AE, Andresen T, Arendt-Nielsen L, Drewes AM. Assessing efficacy of non-opioid analgesics in experimental pain models in healthy volunteers: an updated review. Br J Clin Pharmacol. 2009;68(3):322–341. | ||
Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology. 2004;100(1):115–119; discussion 115A. | ||
Manitpisitkul P, Mayorga A, Shalayda K, et al. Safety, tolerability and pharmacokinetic and pharmacodynamic learnings from a double-blind, randomized, placebo-controlled, sequential group first-in-human study of the TRPV1 antagonist, JNJ-38893777, in Healthy Men. Clin Drug Investig. 2015;35(6):353–363. | ||
Wang H, Papoiu AD, Coghill RC, Patel T, Wang N, Yosipovitch G. Ethnic differences in pain, itch and thermal detection in response to topical capsaicin: African Americans display a notably limited hyperalgesia and neurogenic inflammation. Br J Dermatol. 2010;162(5):1023–1029. | ||
Khambam SK, Naidu MU, Rani PU, Rao TR. A simple contact heat experimental pain model for evaluation of analgesic agents in healthy volunteers. Curr Ther Res Clin Exp. 2011;72(6):233–242. | ||
Gottrup H, Andersen J, Arendt-Nielsen L, Jensen TS. Psychophysical examination in patients with post-mastectomy pain. Pain. 2000;87(3):275–284. | ||
Wright A, Moss P, Sloan K, et al. Abnormal quantitative sensory testing is associated with persistent pain one year after TKA. Clin Orthop Relat Res. 2015;473(1):246–254. | ||
Hansen MS, Wetterslev J, Pipper CB, Asghar MS, Dahl JB. Is heat pain detection threshold associated with the area of secondary hyperalgesia following brief thermal sensitization? A study of healthy volunteers – design and detailed plan of analysis. BMC Anesthesiol. 2016;16(1):28. | ||
Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. | ||
Leung L. Pain catastrophizing: an updated review. Indian J Psychol Med. 2012;34(3):204–217. | ||
Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the pain catastrophizing scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002;96(3):319–324. | ||
Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. | ||
Norton S, Cosco T, Doyle F, Done J, Sacker A. The hospital anxiety and depression scale: a meta confirmatory factor analysis. J Psychosom Res. 2013;74(1):74–81. | ||
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. | ||
R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing V. Available from: http://www.R-project.org/. | ||
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. | ||
Rudin A, Eriksson L, Liedholm R, List T, Werner MU. Prediction of postoperative pain after mandibular third molar surgery. J Orofac Pain. 2010;24(2):189–196. | ||
Rudin A, Wolner-Hanssen P, Hellbom M, Werner MU. Prediction of post-operative pain after a laparoscopic tubal ligation procedure. Acta Anaesthesiol Scand. 2008;52(7):938–945. | ||
Ravn P, Frederiksen R, Skovsen AP, Christrup LL, Werner MU. Prediction of pain sensitivity in healthy volunteers. J Pain Res. 2012;5:313–326. | ||
Morris V, Cruwys S, Kidd B. Increased capsaicin-induced secondary hyperalgesia as a marker of abnormal sensory activity in patients with fibromyalgia. Neurosci Lett. 1998;250(3):205–207. | ||
Morris VH, Cruwys SC, Kidd BL. Characterisation of capsaicin-induced mechanical hyperalgesia as a marker for altered nociceptive processing in patients with rheumatoid arthritis. Pain. 1997;71(2):179–186. | ||
Salengros JC, Huybrechts I, Ducart A, et al. Different anesthetic techniques associated with different incidences of chronic post-thoracotomy pain: low-dose remifentanil plus presurgical epidural analgesia is preferable to high-dose remifentanil with postsurgical epidural analgesia. J Cardiothorac Vasc Anesth. 2010;24(4):608–616. | ||
Asghar MS, Pereira MP, Werner MU, Martensson J, Larsson HB, Dahl JB. Secondary hyperalgesia phenotypes exhibit differences in brain activation during noxious stimulation. PLoS One. 2015;10(1):e0114840. | ||
Granot M, Ferber SG. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity: a prospective study. Clin J Pain. 2005;21(5):439–445. | ||
Papaioannou M, Skapinakis P, Damigos D, Mavreas V, Broumas G, Palgimesi A. The role of catastrophizing in the prediction of postoperative pain. Pain Med. 2009;10(8):1452–1459. | ||
Vaughn F, Wichowski H, Bosworth G. Does preoperative anxiety level predict postoperative pain? AORN J. 2007;85(3):589–604. | ||
Hinrichs-Rocker A, Schulz K, Jarvinen I, Lefering R, Simanski C, Neugebauer EA. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) – a systematic review. Eur J Pain. 2009;13(7):719–730. | ||
Salomons TV, Moayedi M, Erpelding N, Davis KD. A brief cognitive-behavioural intervention for pain reduces secondary hyperalgesia. Pain. 2014;155(8):1446–1452. | ||
Hansen MS, Horjales-Araujo E, Dahl JB. Associations between psychological variables and pain in experimental pain models. A systematic review. Acta Anaesthesiol Scand. 2015;59(9):1094–1102. | ||
Alabas OA, Tashani OA, Tabasam G, Johnson MI. Gender role affects experimental pain responses: a systematic review with meta-analysis. Eur J Pain. 2012;16(9):1211–1223. | ||
Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–58. | ||
Campesi I, Fois M, Franconi F. Sex and gender aspects in anesthetics and pain medication. Handb Exp Pharmacol. 2012(214):265–278. | ||
Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–866. | ||
Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res. 2015;8:399–408. | ||
Iacovides S, Avidon I, Baker FC. Does pain vary across the menstrual cycle? A review. Eur J Pain. 2015;19(10):1389–1405. | ||
Lieberman HR, Corkin S, Spring BJ, Growdon JH, Wurtman RJ. Mood, performance, and pain sensitivity: changes induced by food constituents. J Psychiatr Res. 1982;17(2):135–145. | ||
Abbott FV, Etienne P, Franklin KB, Morgan MJ, Sewitch MJ, Young SN. Acute tryptophan depletion blocks morphine analgesia in the cold-pressor test in humans. Psychopharmacology (Berl). 1992;108(1–2):60–66. | ||
Choi JC, Chung MI, Lee YD. Modulation of pain sensation by stress-related testosterone and cortisol. Anaesthesia. 2012;67(10):1146–1151. | ||
Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14(1):135–143. | ||
Nielsen CS, Stubhaug A, Price DD, Vassend O, Czajkowski N, Harris JR. Individual differences in pain sensitivity: genetic and environmental contributions. Pain. 2008;136(1–2):21–29. | ||
Norbury TA, MacGregor AJ, Urwin J, Spector TD, McMahon SB. Heritability of responses to painful stimuli in women: a classical twin study. Brain. 2007;130(Pt 11):3041–3049. | ||
Tegeder I, Adolph J, Schmidt H, Woolf CJ, Geisslinger G, Lotsch J. Reduced hyperalgesia in homozygous carriers of a GTP cyclohydrolase 1 haplotype. Eur J Pain. 2008;12(8):1069–1077. | ||
Williams FM, Scollen S, Cao D, et al. Genes contributing to pain sensitivity in the normal population: an exome sequencing study. PLoS Genet. 2012;8(12):e1003095. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.