Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Hearing Loss Secondary to Systemic Sclerosis Vasculopathy: Case Study with a Short Review
Authors Bobeica C , Niculet E , Craescu M, Parapiru EL, Musat CL, Dinu C , Chiscop I, Nechita L, Stefanescu V , Stefanopol IA , Pelin AM , Nechifor A , Balan G, Tatu AL
Received 2 January 2022
Accepted for publication 15 April 2022
Published 30 May 2022 Volume 2022:15 Pages 967—973
DOI https://doi.org/10.2147/CCID.S356818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Carmen Bobeica,1,* Elena Niculet,1,2 Mihaela Craescu,1 Elena-Laura Parapiru,3,* Carmina Liana Musat,1,* Ciprian Dinu,4,* Iulia Chiscop,5,* Luiza Nechita,3,* Victorita Stefanescu,6,* Ioana Anca Stefanopol,1,7,* Ana Maria Pelin,8,* Alexandru Nechifor,3,* Gabriela Balan,3,9,10,* Alin Laurentiu Tatu2,3,11
1Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, Romania; 2Multidisciplinary Integrated Center of Dermatological Interface Research MIC-DIR (Centrul Integrat Multidisciplinar de Cercetare de Interfata Dermatologica - CIM-CID), ‘Dunărea de Jos’ University, Galați, Romania; 3Clinical Medical Department, Faculty of Medicine and Pharmacy, Dunărea de Jos University, Galați, Romania; 4Dental Department, Faculty of Medicine and Pharmacy, Dunărea de Jos University, Galați, Romania; 5Clinical Surgical Department, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, Romania; 6Medical Department, Faculty of Medicine and Pharmacy, Dunărea de Jos University, Galați, Romania; 7Department of Pediatrics, Clinical Emergency Hospital for Children “Sf. Ioan”, Galati, Romania; 8Department of Pharmaceutical Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, Romania; 9Department of Gastroenterology, “Sf. Apostol Andrei” County Emergency Clinical Hospital, Galați, Romania; 10Research Center in the Field of Medical and Pharmaceutical Sciences, “Dunărea de Jos” University, Galați, Romania; 11Dermatology Department, “Sf. Cuvioasa Parascheva” Clinical Hospital of Infectious Diseases, Galați, Romania
*These authors contributed equally to this work
Correspondence: Elena Niculet; Mihaela Craescu, Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, 35 Alexandru Ioan Cuza Street, Galați, 800008, Romania, Tel +40741398895 ; +40751869864, Email [email protected]; [email protected]
Abstract: Systemic sclerosis (SSc) is a collagenosis with a substrate of chronic inflammation, which is determined by autoimmunity. The pathogenesis of this disease involves microvasculopathy (small vessel pathology) followed by excessive cutaneous and visceral fibrosis. Although acoustic and vestibular impairment is not classified as being a secondary pathology of SSc, several studies have identified cases of SSc that associate hearing loss and especially vertigo and tinnitus. This paper presents data from the medical literature that have identified vestibular and auditory symptoms among patients with SSc, associating the clinical case presentation of a patient suffering from SSc, which is associated with hearing loss. The need for additional studies on larger groups of patients is underlined, in order to clarify the impact of vasculopathy and fibrosis on the acoustic and vestibular analyzer in patients with SSc.
Keywords: systemic sclerosis, hearing loss, vasculopathy, fibrosis, capillaroscopy
Introduction
Systemic sclerosis (SSc) is a connective tissue disease in which skin induration is accompanied by multiorgan damage. The pathogenic process is a complex issue based on a substrate of vasculopathy and excessive fibrosis. The multiorgan damage found in SSc mainly targets the lung, digestive tract, kidney and heart.1 Although hypoacusis is not classified as being a secondary pathology of SSc, studies have shown that a fairly high percentage of patients with SSc report vestibular and auditory symptoms, with symptoms being as severe as hearing loss.2 Bassyouni et al found that 33% of patients with SSc have a mild degree of vestibular dysfunction. The vestibular damage was found to be directly proportional to the severity of the vascular alterations shown on capillaroscopy and to the Rodnan score of cutaneous induration. No associations were observed between vestibular impairment and the disease subsets of SSc.3
In 2008, Agrawal and his research team analyzed acoustic and vestibular symptoms on a group of SSc patients, in comparison to a group of patients without SSc, with different age groups. They found that patients with SSc associated hearing loss much more frequently than patients without SSc.4 Later in 2011, Maciaszczyk recorded 40% hearing loss on a group of 20 patients diagnosed with SSc.5 Between 2012 and 2015, Silva et al conducted a study of 50 patients with SSc. At the end of the study, they noticed that most of the patients had vertigo and tinnitus, and 46% of the patients included in the study had hearing loss.2
Their study was initiated based on the observations of Maciaszczyk and his collaborators and obtained a similar result. As early as 2011, research conducted by Maciaszczyk noted that dizziness and especially tinnitus are the most common acoustic-vestibular symptoms identified in SSc.5 Another study team, Bevilacqua et al, conducted a study analyzing the prevalence of hearing loss in the general population in a group of 2427 Brazilians in Rondônia. According to the study, they were included without being diagnosed with SSc and a percentage of 26.1% of people studied were registered with low hearing acuity, without being diagnosed with SSc. However, the share of people with dizziness and tinnitus was higher than those with hearing loss.6
Also, Beria (2007) identified in Brazil an even lower percentage of people with hearing loss - only 15.2% of the general population. Analyzing the prevalence of hearing loss among the population without SSc, one can highlight that aging does not associate hearing loss in a percentage that is as high as in those suffering from SSc.2,6,7
Other studies have revealed that hearing loss is the most common vestibular symptom in SSc.8,9 Berrettini and Bondali specified that the vasculopathy which is characteristic for SSc affects the artery that ensures the vascularization of the labyrinth found in the inner ear. The labyrinthine lesion seems to be caused by the limitation of arterial blood flow at this level.2,9
Deroee (2009) observed that high-frequency sounds are affected in the initial phase of hearing loss. Several authors found to be of use the evaluation of the central and peripheral segment of the acoustic and vestibular analyzer in patients with SSc. They concluded that the inner ear is affected by the restriction of blood flow to the cochlea by microvascular inflammation of the cochlear nerve in the context of autoimmunity.5,8–11 Similar to the pattern of nail fold capillary damage, Deroee found that low cochlear capillary density and sinuous capillaries produce local ischemia and hypoxia that will further induce cell apoptosis.10,12
Pereira’s (2006) study showed that local inflammation spreads to the middle and outer ear and causes recurrent polychondritis with transmission hearing loss.12,13 Moreover, the study of Kastanioudakis (2001) which was done on a group of 30 SSc patients, found a number of 3 cases suffering from inflammation extending to the Eustachian tube.12,14
The occurrence of hearing loss in the course of SSc has been hardly studied. Therefore, more research is needed in order to gather clear and concrete evidence of vascular and fibrotic damage to the inner and middle ear.2
Case Report
Subject
The current study aims to present the clinical case of a 66-year-old female patient who was diagnosed with SSc associated hearing loss. The patient signed the informed consent for the publication of personal pathological data and for the publication images. Ethics approval was obtained from the Ethics Committee of the Clinical Hospital “Sf. Maria” in Bucharest, Romania, with the decision number 5213, from 04.04.2019.
The patient was hospitalized in August 2019, complaining of small and large joint polyarthralgias having an inflammatory character; these symptoms were accompanied by prolonged morning stiffness, hand digital ulcers with inflammatory signs and partial hearing loss. The patient comes from the rural area and is an active smoker for almost 20 years, smoking 10 cigarettes a day.
The patient was diagnosed in December 2015 with SSc, the limited skin type, associated with vascular damage. The positive diagnosis was made in the clinical context of a deep digital ulceration found on the plantar side of the left hallux. On the background of this ulcerative lesion, the patient was referred to the vascular surgery ward. The patient associates the development of this ulceration with a psychological stressful event in her family (her son’s death). Currently, she presents for the recurrence of digital ulcers accompanied by inflammatory signs.
The patient’s personal history identifies the long-term occupational exposure to toxic agents (approximately 10 years, to the following substances: carbon monoxide (CO), nitrogen dioxide (NO2), sulfur dioxide (SO2), lead). Starting with 1980, the patient worked in a special cast iron foundry, in a high-temperature environment (200°C). The patient has as associated pathologies: chronic obliterative arterial disease of the lower limbs (stage III–IV Fontaine), deep vein thrombosis of the popliteal artery and of the right posterior tibial artery. Over the course of the disease, recurrent digital ulcers have progressed to one Staphylococcus aureus superinfection episode, for which antibiotic treatment was initiated according to the patient’s antibiogram; she also associated treatment with endothelin-1 receptor antagonist (bosentan) and a vasodilator (alprostadil). Among systemic complications, the patient presented moderate diffuse pulmonary sclero-emphysema and chronic erosive gastritis “watermelon stomach”. For associated comorbidities, the patient received statins known to have an anti-cytokine effect, despite the side effects.15,16
Clinically, the general condition is slightly influenced, the patient suffering from discrete asthenia quantified by the score of 20/100 evaluated by the patient using the analog visual scale. There was a gradual weight decrease of 10 kg over the span of 3 years. Arthralgias were present at the bilateral distal interphalangeal joints (8 painful joints with the pain score quantified by the patient using the visual analog scale = 10/100), without joint contracture of the small joints in the hands, with discrete phalangeal muscle weakness at this level (score = 1) and with muscle atrophy of the dorsal interosseous muscles, scleroderma facies with slight microstomy (5 cm oral opening), sclerodactyly (Figure 1) and acrocyanosis of the left IInd finger (Figure 2). Multiple stellar scars with the characteristic appearance of “rat bite” and active digital ulcerations have been identified on the pulp of the fingers. Some ulcers of the IInd and IVth left fingers and Ist right finger showed signs of infection (Figure 3). The skin had “salt and pepper” pigmentation disorders, the hyperpigmented areas alternating with the hypopigmented ones. Skin induration was quantified as being 4, calculated as the Rodnan score and obtained by summing the partial scores equal to 1 (for mild thickening of the hands and feet). Multiple acroosteolysis of the fingers showed an obvious shortening of the IInf and IIIrd fingers, bilaterally (Figure 4). Concerning the peripheral vascular compartment, the patient presents triphasic Raynaud phenomena, having present pulsations of the radial artery, with slightly diminished ones at the pedal artery level and an absent pulse of the posterior tibial artery.
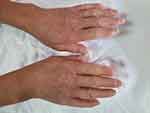 |
Figure 1 Sclerodactyly and digital contracture in flexion. |
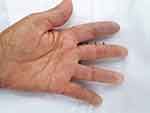 |
Figure 2 Acrocyanosis left IInd finger. |
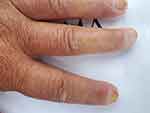 |
Figure 3 Infected digital ulcer. |
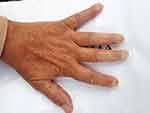 |
Figure 4 Acroosteolysis of the fingers. |
Subcrepitant rales have been identified in both lung bases without the patient suffering from dyspnea. Radiological examination suggested interstitial pulmonary fibrosis, which was confirmed by computed tomography examination. The patient was normotensive and presented hearing impairment. Hearing acuity has progressively decreased during the disease development and evolution.
Biologically, there were significant signs of an inflammatory syndrome expressed by increased values of erythrocyte sedimentation rate (ESR = 76 mm/h) and C reactive protein (CRP = 61.92 mg/l) which directed towards the active form of the disease. The immunological profile showed an increased titer of antinuclear antibodies (ANA). Analysis of the extended ANA profile revealed increase in anticentromere antibodies (ACA). Data from the literature indicate that ACAs have high specificity for the limited subset of SSc. The absence of other autoantibodies suggests that the patient does not have other autoimmune diseases, although sometimes the immunological profile of SSc or other autoimmune diseases is negative or atypically. Medication indicated for existing comorbidities may be a source of adverse reactions or may have an anti-cytokine benefit with anti-inflammatory potential in SSc.15–20
In dynamics, the evolution of ulcers was favorable, with the complete resolution of the Staphylococcus aureus infectious process under a combination of antibiotic therapy (ciprofloxacin and cefuroxime, according to the antibiogram). Vasodilator medication was combined with alprostadil in order to relieve peripheral vasospasm.
Discussion
The patient was diagnosed with SSc according to the 2013 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR).21,22 Depending on the extent of the skin damage, the patient was included in the limited subset of SSc. Smoking and exposure to occupational toxic substances (lead, CO, NO2, SO2) are considered as risk factors. The patient claims that the high temperatures caused her acroparesthesias and the triphasic Raynaud’s phenomenon on her hands. Very interesting is the patient’s statement regarding a major psychological event that took place 6 months before the onset of the digital ulceration found on the left hallux. She considers that the death of her son was the determining factor for the appearance of the ulcerative lesion of the hallux. Subsequently, the patient considers that the unfavorable evolution towards infection was determined by her mental suffering caused by the aggravation of her mother’s health. The literature data supports this observation (the existence of a psychological stress factor prior to the development of SSc), a study by Chen et al and one by Faravelli et al reporting that patients registered important life events one year prior to SSc initiation (deaths, recent stressful events, undesirable life events, uncontrolled events). Even childhood adverse life events were incriminated in the initiation of SSc.23,24
In addition to skin damage, the patient presented interstitial pulmonary fibrosis with the absence of dyspnea; gastroparesis, macrovascular damage to the popliteal artery and posterior tibial artery were also present. Although dyspnea was absent, subcrepitant rales were present at the base of the lung, suggesting that pulmonary fibrosis is symptomatic from the early stages. As a particular feature of the case, the patient presented with hearing loss, probably due to vasculitic damage and secondary fibrosis of the middle and inner ear. Some comments can be made on this aspect. Data from the literature report that hearing loss is not known to be a typical manifestation of SSc, but some studies show that the inner and middle ear may be affected in the course of this disease due to microvascular destruction. Shenavandeh and his study team reported 10 cases of subjective hearing loss and 36 cases of objective hearing loss in patients with SSc, as compared to the control group of 6 and 10 patients, respectively.25
By making a correlation with the capillaroscopic pattern of this patient (Figure 5), one can highlight that the hearing loss could be determined by the microvasculopathy characteristic of SSc. The patient has a nonspecific capillaroscopic pattern for SSc, characterized by a slightly reduced capillary density and frequent dilated capillaries. Some aspects characteristic of SSc are missing, such as: megacapillaries, hemorrhages and neovascularization, but there is a slight structural disorganization and digital necrosis.2,5 Maciaszczyk and Silva claim that the microvascular damage of the inner ear is due to the vasospasm characteristic of the Raynaud phenomenon associated with fibrosis of the auditory organ.2,5 They appreciate that hearing impairment depends on the duration of the disease and also on its severity.2,5 Contrary to these observations, Bassyouni did not notice any correlation between vestibular function and the duration of the disease.3
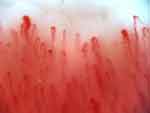 |
Figure 5 Capillaroscopy – nonspecific pattern, with dilated vessels. |
The presence of recurrent, persistent ulcerations in the studied patient can indicate an elevated degree of microvasculopathy, revealed by the capillaroscopic examination. Therefore, one can report that the presence of hearing loss is the expression of microvascular alterations in the context of autoimmune alterations.
Moreover, studies conducted by Bevilacqua and Beria show that hearing loss is much less common in the population without SSc and over the age of 65, as compared to groups of patients with SSc.6,7 This finding suggests that SSc appears to be associated with hearing loss. There are few studies on SSc secondary hearing loss. For this reason, it is necessary to carry out additional studies to clarify the role of vasculopathy in affecting the middle and inner ear.2,26
Conclusion
Vasculopathy characteristic to SSc precedes the process of excessive fibrosis and is responsible for skin and internal organ damage. Moreover, both microvascular damage to the structures in the middle and inner ear, and consequent fibrosis, appear to be responsible for the progressive onset of hearing loss.
It is estimated that the accentuation of the hearing deficit is proportional to the intensity of the vasculitic damage. In the context of vasculopathy, vasodilator medication may have a net benefit in preventing hearing loss in patients with SSc. Data on SSc secondary hearing loss are limited. As a result, more studies are needed for the optimal management of this pathology.
Abbreviations
SSc, systemic sclerosis; CO, carbon monoxide, NO2, nitrogen dioxide, SO2, sulfur dioxide; ESR, erythrocyte sedimentation rate; CRP, C reactive protein; ANA, antinuclear antibodies; ACA, anticentromere antibodies; ACR, American College of Rheumatology; EULAR, European League Against Rheumatism.
Ethics Approval
The patient approved and signed the informed consent for the publication of personal data and for the publication images, information, which can be found in the patient’s personal medical chart. At the same time, the information provided in the current manuscript is in concordance with the principles of the Declaration of Helsinki. Ethics approval was obtained from the Ethics Committee of the Clinical Hospital “Sf. Maria” in Bucharest, Romania, with the decision number 5213, from 04.04.2019.
Acknowledgments
The authors wish to acknowledge that the present study was academically supported by the “Dunărea de Jos” University of Galați, Romania, through the research center – Multidisciplinary Integrated Center of Dermatological Interface Research MIC-DIR (Centrul Integrat Multidisciplinar de Cercetare de Interfata Dermatologica - CIM-CID).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The current paper's article publishing charge was paid by the “Dunarea de Jos” University of Galati, Romania.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Barsotti S, Orlandi M, Codullo V, et al. One year in review 2019: systemic sclerosis. Clin Exp Rheumatol. 2019;37(Suppl119(4)):3–14.
2. Silva MM, Araújo RPC, Araujo FAGDR, Valente JS, Corona AP. Hearing alterations in systemic sclerosis. Codas. 2019;31(1):e20170119. doi:10.1590/2317-1782/20182018119
3. Bassyouni IH, Emad Y, Rafaat HA, Dabbous AO. Relationship between nailfold capillary abnormalities and vestibular dysfunction in systemic sclerosis. Joint Bone Spine. 2011;78(3):266–269. doi:10.1016/j.jbspin.2010.07.019
4. Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med. 2008;168(14):1522–1530. doi:10.1001/archinte.168.14.1522
5. Maciaszczyk K, Waszczykowska E, Pajor A, Bartkowiak-Dziankowska B, Durko T. Hearing organ disorders in patients with systemic sclerosis. Rheumatol Int. 2011;31(11):1423–1428. doi:10.1007/s00296-010-1503-5
6. Bevilacqua MC, Banhara MR, de Oliveira AN, et al. Survey of hearing disorders in an urban population in Rondonia, Northern Brazil. Rev Saude Publica. 2013;47(2):309–315. doi:10.1590/S0034-8910.2013047004059
7. Béria JU, Raymann BC, Gigante LP, et al. Hearing impairment and socioeconomic factors: a population-based survey of an urban locality in southern Brazil. Rev Panama Public Health. 2007;21(6):381–387.
8. Amor-Dorado JC, Arias-Nuñez MC, Miranda-Filloy JA, Gonzalez-Juanatey C, Llorca J, Gonzalez-Gay MA. Audiovestibular manifestations in patients with limited systemic sclerosis and centromere protein-B (CENP-B) antibodies. Medicine. 2008;87(3):131–141. doi:10.1097/MD.0b013e318173aa56
9. Berrettini S, Ferri C, Pitaro N, et al. Audiovestibular involvement in systemic sclerosis. ORL J Otorhinolaryngol Relat Spec. 1994;56(4):195–198. doi:10.1159/000276655
10. Deroee AF, Huang TC, Morita N, Hojjati M. Sudden hearing loss as the presenting symptom of systemic sclerosis. Otol Neurotol. 2009;30(3):277–279. doi:10.1097/MAO.0b013e31819bda52
11. Gilliland BC. Esclerosis sistémica (esclerodermia) y trastornos relacionados. In: Kasper DL, editor. Tratado de Medicina Interna. Río de Janeiro: McGraw-Hill; 2006:2076–2087.
12. Rabelo MB, Corona AP. Auditory and vestibular dysfunctions in systemic sclerosis: literature review. Codas. 2014;26(5):337–342. doi:10.1590/2317-1782/20140201475
13. Pereira DB, Amaral JLA, Szajubok JCM, Lima SMAL, Chahade AH. Otolaryngological manifestations in autoimmune rheumatic diseases. Rev Bras Rheumatol. 2006;46(2):118–125. doi:10.1590/S0482-50042006000200006
14. Kastanioudakis I, Ziavra N, Politi EN, Exarchakos G, Drosos AA, Skevas A. Hearing loss in progressive systemic sclerosis patients: a comparative study. Otolaryngol Head Neck Surg. 2001;124(5):522–525. doi:10.1067/mhn.2001.115092
15. Gonçalves RSG, Dantas AT, Pereira MC, et al. Statins inhibit cytokines in a dose-dependent response in patients with systemic sclerosis. Inflammation. 2019;42(2):407–411. doi:10.1007/s10753-018-0907-3
16. Nwabudike LC, Elisei AM, Buzia OD, Miulescu M, Tatu AL. Statins. A review on structural perspectives, adverse reactions and relations with non-melanoma skin cancer. RevChim. 2018;69(9):2557–2562.
17. Tatu AL, Ionescu MA. Multiple autoimmune syndrome type III-thyroiditis, vitiligo and alopecia areata. Acta Endocrinol. 2017;13(1):124–125. doi:10.4183/aeb.2017.124
18. Goussot R, Francès C, Cury K, et al. Prospective evaluation of the frequency of genital lichen sclerosus in 79 patients with systemic sclerosis. Br J Dermatol. 2018;179(4):999–1000. doi:10.1111/bjd.16898
19. Tatu AL, Nwabudike LC. The treatment options of male genital lichen sclerosus et atrophicus. short title for a running head: treatments of genital lichen sclerosus.
20. Tatu AL, Nwabudike LC. Male genital lichen sclerosus - A permanent therapeutic challenge. J Am Acad Dermatol. 2018;79(3 Suppl 1):AB185.
21. Masi AT, Medsger TA
22. van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72(11):1747–1755. doi:10.1136/annrheumdis-2013-204424
23. Chen Y, Huang JZ, Qiang Y, Wang J, Han MM. Investigation of stressful life events in patients with systemic sclerosis. J Zhejiang Univ Sci B. 2008;9(11):853–856. doi:10.1631/jzus.B0820069
24. Faravelli C, Pietrini F, Rotella F, et al. Stressful life events at the onset and during the evolution of systemic sclerosis. Int J Clin Rheumatol. 2019;14(5):171–178.
25. Shenavandeh S, Hashemi SB, Masoudi M, et al. Hearing loss in patients with scleroderma: associations with clinical manifestations and capillaroscopy. Clin Rheumatol. 2018;37(9):2439–2446. doi:10.1007/s10067-018-4162-7
26. Valente JPS, Corona AP. Retrocochlear impairments in systemic sclerosis: a case report study. Codas. 2017;29(6):e20160238. doi:10.1590/2317-1782/20172016238
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
