Back to Journals » International Journal of Nanomedicine » Volume 15
Healthcare Applications of pH-Sensitive Hydrogel-Based Devices: A Review
Authors Hendi A, Umair Hassan M, Elsherif M , Alqattan B , Park S, Yetisen AK, Butt H
Received 13 January 2020
Accepted for publication 17 March 2020
Published 2 June 2020 Volume 2020:15 Pages 3887—3901
DOI https://doi.org/10.2147/IJN.S245743
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Asail Hendi,1 Muhammad Umair Hassan,2 Mohamed Elsherif,3 Bader Alqattan,4 Seongjun Park,5,6 Ali Kemal Yetisen,7 Haider Butt4
1School of Engineering, University of Birmingham, Birmingham B15 2TT, UK; 2Optoelectronics Research Lab, COMSATS University, Islamabad 45550, Pakistan; 3Department of Experimental Nuclear Physics, Nuclear Research Center, Egyptian Atomic Energy Authority, Cairo, Egypt; 4Department of Mechanical Engineering, Khalifa University, Abu Dhabi, United Arab Emirates; 5Department of Bio and Brain Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Republic of Korea; 6KAIST Institute for Health Science and Technology (KIHST), Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Republic of Korea; 7Department of Chemical Engineering, Imperial College London, London SW7 2AZ, UK
Correspondence: Mohamed Elsherif
Department of Experimental Nuclear Physics, Nuclear Research Center, Egyptian Atomic Energy Authority, Cairo, Egypt
Email [email protected]
Haider Butt
Department of Mechanical Engineering, Khalifa University, Abu Dhabi, United Arab Emirates
Email [email protected]
Abstract: pH-sensitive hydrogels have been developed greatly over the past few years. This has been possible due to the synthesis of new hydrogel systems with increased sensitivity – a sensitivity of up to 10− 5 pH units has already been established. Recently, pH-sensitive hydrogels have shown to be very useful in biomedical applications, such as targeted cancer treatment and treatment of skin lesions. Prolonged drug release has been made available through the use of such hydrogels. The synthesis of pH-sensitive hydrogels is also quick and cost-effective. This review presents a background on the properties of pH-sensitive hydrogels and discusses some of the hydrogels with different sensitivity ranges and their possible applications. A range of synthesis processes have also been briefly introduced along with the fabrication of different structures such as microcantilevers and contact lenses.
Keywords: pH-sensitive, sensors, hydrogels, biomedical applications, biocompatible
Introduction
Hydrogels are networks of water-soluble polymers containing unique properties which make them ideal for use in biomedical applications.1 Hydrogels can be made of synthetic or natural polymers and they are capable of imbibing large quantities of water or fluids. Due to their considerable water content, hydrogels are flexible and similar to natural tissues which make them ideal for tissue engineering applications.2 Their ability for absorbing water results from the hydrophilic functional groups attached to the hydrogel backbone. Also, hydrogels exhibit high permeability for oxygen and nutrients which make them attractive for contact lens manufacturing.3 Hydrogels are valuable due to their easy fabrication process, small size, mouldability, immunity to electromagnetic radiation, and biocompatibility.4 More importantly, the physical characteristics of hydrogels can be tuned to provide specifications to match various applications. Hence, their applications cover a broad range, from drug delivery to the treatment of skin lesions (Figure 1). Hydrogel sensors for environmental applications such as temperature, humidity, pH, metal ions, and gases were reported.2,5-7 Furthermore, their applications extend to biomarker detection such as lactate, glucose, and proteins.6,8-10 More recently, synthetic hydrogels have been gradually replacing natural hydrogels due to their high potential for retaining water, high mechanical strength, and long functioning life; in addition, their degradability can be tailored. pH-sensitive hydrogels are able to alter their volume in response to a change in the pH of the environment they are placed in. They are able to swell significantly with high sensitivity – detection of minute pH changes of as small as 10−5 pH units. Hydrogels are promising for pH sensing due to their wide measuring range.4,11 Hydrogel-based sensors may be miniaturized and hence are of great importance when combined into microsystems.12,13 Microcantilevers fabricated using silicon wafers have shown significant sensitivity with the detection of microscale changes in pH.14 This review aims to provide a brief introduction to the behavior of pH-sensitive hydrogels as well as the materials of various hydrogel compositions with their various applications.
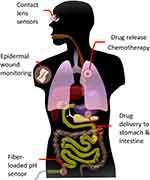 |
Figure 1 An illustration of possible health applications of pH-sensitive hydrogel sensors/drug deliverers in the human body. |
Working Principle of pH-Sensitive Hydrogels
Stimuli-responsive hydrogels alter their volume in response to the variation in the environment they are exposed to. These volumetric changes have been reported to be more than a hundredfold.15,16 The amount of water in the polymer matrix varies from at least 20% to 99% by weight.17 The biocompatibility of hydrogels is directly linked to the degree of water retention within their volume – more than 95% of water retention is considered to have high biocompatibility. pH-sensitive hydrogels consist of a backbone polymer either containing weak acidic groups or basic groups which become more ionized in higher or lower pH environments, respectively.17 Mechanical stability is attributed to the backbone polymer while the ionizable component provides the pH sensitivity.15 Mechanical, responsive, and diffusive properties may be controlled by tailoring the molecular structure of the hydrogels.18 In addition, the properties also depend on the type of crosslinking. Generally, hydrogels exhibit low tensile strength which is the bottleneck for their use in load-bearing applications, and there is a large area of research in this particular field.1 Usually, pH-sensitive hydrogels exhibit significant sensitivity and have a working range which is determined by the ionizable component.15 When the pH-sensitive hydrogel is in contact with a solution, depending on the pH of the solution, the quantity of dissociated carboxylic ions changes, which alters the volume and refractive index of the hydrogel – this is the working principle of the pH-sensitive hydrogel sensors.4,19 The variation in the size and refractive index is expected due to the varying water content in the swollen hydrogel. With swelling, the refractive index generally undergoes a negative shift as pure water usually possesses a lower refractive index compared to the hydrogel in its shrunken unexposed form.15
Figure 2 illustrates the swelling behavior of the hydrogel in basic pH obtained from the work of Tomar et al.20 Figure 2A shows the swelling observed at pH 7.4 while Figure 2B shows the shrunken state of the particles at pH 1.5. The particles were kept in the acidic (pH 1.2) and basic (pH 7.4) pH mediums for 6 hours. Tomar et al attributed the swelling of the particles to the carboxylic group ionization at pH 7.4 which leads to water uptake with the particles coalescing with each other. It was observed that the storage modulus was higher than the loss modulus at pH 1.2 indicating the elastic behavior at acidic pH, in comparison to the viscous property seen at basic pH.
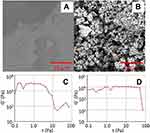 |
Figure 2 Hydrogel in different pH environments. Scanning electron microscopy of hydrogel particles presented by Tomar et al at (A) pH 7.4; (B) pH 1.2.20 Strain dependence of G’ at constant angular frequency (1 rad/sec) at (C) pH 7.4; (D) pH 1.2. Reprinted from Tomar LK, Tyagi C, Choonara YE, Kumar P, Pillay V. Rheological and swelling behavior of pH sensitive hydrogel particles. APCBEE Procedia. 2014;9:192–196. Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported (CC BY-NC-ND 3.0) (https://creativecommons.org/licenses/by-nc-nd/3.0/legalcode).20 |
Hydrogel Compositions and Synthesis
Hydrogel preparation processes are generally simple and cost-effective. Cross-linking reactions between polymer molecules can be used to synthesize these stimuli-responsive hydrogels.18 Cross-linked polymers are insoluble but swell via solvent absorption because of the interconnections amongst the polymer chains. Various crosslinking methods are summarized in Table 1 and some references are cited for deep further details.18 The ionizable component within the polymer determines the working range of the hydrogel. Many different compositions of hydrogels exist, each with a varying sensitivity range (Table 2). Chitosan which is a natural polymer has been presented in many papers due to the useful properties it posses such as biodegradability and biocompatibility.21–24
 |
Table 1 Various Crosslinking Methods for Synthesizing pH-Responsive Hydrogels |
 |
Table 2 Overview of Compositions and Applications Presented in a Few Published Papers |
Recently, developments have seen in the usage of graphene oxide (GO) as a key ingredient in the fabrication of a pH-sensitive hydrogel for drug delivery.32 More et al developed a hydrogel using GO and para-aminosalicylic acid (PAS) which was lyophilized to give air-dried hydrogel (ADH). The hydrogel was optimized using varying concentrations of GO and PAS as well as drug content and it was found that equal concentrations of GO and PAS along with a drug (ratio of 1:1) led to optimal results. The supramolecular hydrogel was explored for the management of Mycobacterium tuberculosis. The fabricated hydrogel was found to have both pH-sensitive properties and different drug release profiles in neutral and acidic conditions, representing physiological pH of the blood and internal pH of the infected macrophages.
Polyethylene glycol dimethacrylate (PEGDMA) and methacrylic acid (MAA) in molar feed ratio of 1:2 with ammonium sulfate (1% of monomer concentration) as a free radical initiator were used to develop pH-sensitive hydrogel particles by Tomar et al (Figure 3A).20 To synthesize the particles, a polymerization reaction was performed for 4h at 60°C with the use of water as the continuous medium. The particles obtained were washed with deionized water and were then placed separately in the acidic and basic buffer for 6h to analyze their response to pH. Dai et al fabricated a hydrogel from dibenzaldehyde-functionalized polymer (DF-PEG) and polyaspartylhydrazide (PAHy) polymer with the incorporation of black phosphorus nanosheets (BPNSs) – Figure 3B illustrates the composition of the injectable DF-PEG-PAHy/BPNSs hydrogel.31 The fabricated hydrogel is capable of responding to both pH and near-infrared (NIR) light and showed ideal mechanical and swelling properties. The chemotherapy drug (doxorubicin (DOX)) was efficiently contained in the hydrogel structure for cancer therapy. The fabricated DF-PEG-PAHy/BPNSs composite hydrogel was tested in vivo by conducting experiments in tumor-bearing nude mice.
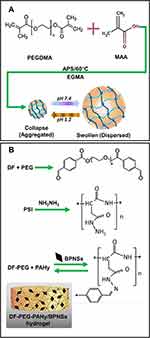 |
Figure 3 (A) Materials and preparation method presented by Tomar et al.20 Reprinted from Tomar LK, Tyagi C, Choonara YE, Kumar P, Pillay V. Rheological and swelling behavior of pH sensitive hydrogel particles. APCBEE Procedia. 2014;9:192–196. Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported (CC BY-NC-ND 3.0) (https://creativecommons.org/licenses/by-nc-nd/3.0/legalcode).20 (B) Schematic diagram showing the composition of DF-PEG-PAHy/BPNSs hydrogel presented by Wu et al. Reprinted from Wu RS, Lin J, Xing YM, Dai ZL, Wang LW, Zhang XP. pH-sensitive black phosphorous–incorporated hydrogel as novel implant for cancer treatment. J Pharm Sci. 2019;108(8):2542–2551. Copyright 2019, with permission from Elsevier.31 |
Fabrication Processes
Long-period fiber grating (LPFG) coated with a pH-sensitive hydrogel has been presented by Mishra et al.39 The LPFG used was written in a germanium-boron co-doped fiber using a CO2 laser. The fiber was then coated with the hydrogel by dipping it into the solution for 5 minutes and leaving it overnight to dry. The hydrogel was made of acrylamide and ammonium, persulphate was used as an initiator, and N-tetramethylethylenediamine (TEMED) was used as a catalyst. The pH sensing capability of the fabricated hydrogel-coated LPFG was investigated through the comparison of the transmission spectra obtained for the varying pH values, using the setup in Figure 4. Upon introducing pH solutions into the fiber’s attached hydrogel, the hydrogel swelled/deswelled resulting in a change in its refractive index, consequently the effective refractive index of the fiber cladding altered. Thus, the resonance wavelength of the fiber shifted. Swelling of the hydrogel is resulting from dissociation of the carboxylic ions immobilized in the hydrogel that in turn increases the electrostatic repulsion due to generating more ionized carboxylic groups. A wide operating range from pH 2 to 12 was reported.
 |
Figure 4 (A) Experimental setup used by Mishra et al showing the grating.39 (B) Graph showing the variation of resonance wavelength with pH for the hydrogel-coated LPFG. © 2016 IEEE. Reprinted, with permission, from Mishra SK, Zou B, Chiang KS. Wide-range pH sensor based on a smart-hydrogel-coated long-period fiber grating. IEEE J Sel Top Quantum Electron. 2016;23(2):284–288.39 |
Hydrogel contact lenses sensitive to pH have been looked at for drug delivery utilizing the pH changes in tears. Noh et al (2017) prepared the hydrogel solution by adding a cross-linking agent (EGDMA) and initiator (AIBN) to HEMA.40 Two different ionic polymer groups (poly(vinylpyrrolidone) PVP, poly(N-isopropyl acrylamide NIPAAm) were used in this study (Figure 5). This solution was then filled onto plastic molds and the polymerization took place using an oven at 100 °C. The tested drug was hydroxypropyl methylcellulose (HPMC) which is commonly used to treat dry eye disease. The drug was stored onto the contact lenses by immersing the dried contact lenses into 1% HPMC for 3 h. The expansion of the lens structure regulates the drug diffusion with drug release, activated in basic conditions for the p-HEMA-VP (20 wt%) lens. The p-HEMA-NIPAAm (20 wt%) lens was found to de-swell in basic solution, decreasing drug release. The prepared artificial tears had a pH value of between 5.8 and 8.35.
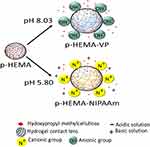 |
Figure 5 Schematic showing the swelling of the lens in solutions with different pH. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature; Kim G, Kim HJ, Noh H. pH sensitive soft contact lens for selective drug-delivery. Macromol Res. 2018;26(3):278–283. Copyright 2018. https://www.springer.com/journal/13233.40 |
Microcantilevers coated with a pH-sensitive hydrogel solution have ultrahigh sensitivity enabling microscale detection of changes in pH (Figure 6).14 Bashir et al (2002) fabricated cantilevers using silicon wafers on which the prepared hydrogel solution was patterned. SOI wafers, with a 1 μm silicon layer and 1 μm oxide layer, were used to fabricate cantilevers on which the prepared polymer was patterned. An oxide layer was grown and the photoresist mask was used to anisotropically etch the wafer. A further oxide layer was grown on the sidewall and the oxide from the substrate-exposed silicon surface was etched using a dry anisotropic etch. Finally, a release etch was performed to free the cantilever and the wafers by immersion in a 10 wt% acetone solution. A mole ratio of 80:20 methacrylic acid:poly(ethylene glycol) 200 dimethacrylate was prepared with DMPA as the initiator. The prepared mixture of poly(methacrylic acid) (PMAA) and poly(ethylene glycol) dimethacrylate was spin-coated onto the cantilever samples and patterned using UV polymerization.14 As the pH around the structure increased, the polymer expanded resulting in a change in surface stress and hence causing the microcantilever to bend. A maximum deflection sensitivity of 1 nm/5 × 10−5 ΔpH was reported.
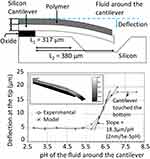 |
Figure 6 (A) Schematic of the cantilever/polymer structure. (B) Equilibrium cantilever deflection as a function of pH around the polymer. Reprinted from Bashir R, Hilt JZ, Elibol O, et al. Micromechanical cantilever as an ultrasensitive pH microsensor. Appl Phys Lett. 2002;81(16):3091–3093. With the permission of AIP Publishing. © 2002 American Institute of Physics.14 |
The usage of 3D printing has slowly evolved to generate structures and devices for a wide range of industrial applications. 3D printing enables the production of components with complex geometries at high precision and low cost.41 Recent work has mainly focused on 3D printing of natural polymers in the field of tissue engineering, such as gelatin/chitosan and alginate.21,42 Yan et al developed a cell assembling machine that enabled the formation of 3D living tissue/organ analogies.21 Gelatin/chitosan at a ratio of 10:1 was used to form the 3D structure. Biodegradable oxidized alginate was used as a bioink for bioprinting by Jia et al.42 RGD-modified oxidized alginate hADSCs bioink was printed to form a lattice structure on a substrate made of gelatin. The printing of synthetic hydrogels has been limited due to the lack of suitable bioinks available.43 Panhuis et al reported the fabrication of degradable hydrogels using 3D printing. Bioinks based on hyaluronic acid (HA) have been investigated by Burdick et al for the use in cartilage or cardiac tissue engineering.44 A 3D printing system was used along with the use of standard software to generate the 3D CAD model. The printing process used by Burdick et al can be seen in Figure 7. A stepper motor-driven nozzle suitable for extrusion on a 3D printer was fabricated and used. The ink was loaded into the syringe and once printing commenced, UV light was focused onto the printing area. Postprocessing included UV curing after the printing stage was complete in order to stabilize the printed structures.44
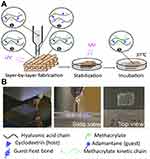 |
Figure 7 3D printing of hydrogels. (A) Schematic of printing stabilization and incubation processes. The fabrication steps involved: (1) hydrogel assembly with guest–host bonds inside the syringe chamber, (2) disruption of the guest–host bonds at the syringe neck due to shearing effect, (3) fast healing of the guest-hold bonds after passing the gel through the syringe neck and the gel is deposited, (4) UV curing of the deposited methacrylate gel, (5) stabilization to enhance the polymerized network and incubation. (B) Images of the printing process and printed multilayer structure. Reprinted with permission from Ouyang L, Highley CB, Rodell CB, et al. 3D printing of shear-thinning hyaluronic acid hydrogels with secondary cross-linking. ACS Biomater Sci Eng. 2016;2(10):1743–1751. Copyright © 2016, American Chemical Society.44 |
Stereolithography (SLA) is a 3D printing technique that has previously been used to develop a wide range of temperature and pH-sensitive hydrogel structures by Cohn et al.45 F127 dimethacrylate (FDMA) hydrogels were 3D printed using a photopolymerization process using an SLA 3D printer. Following the printing stage, the hydrogel structures were rinsed with water and dried in the vacuum. The hydrogel’s response to pH was studied by carrying out swelling-deswelling experiments. Buckyball structures of the hydrogel were printed, and the effect of both temperature and pH was demonstrated (Figure 8).45
 |
Figure 8 (A) Buckyball structure at 37 °C and 6 °C at pH 2.0 and pH 7.4 as shown by Dutta and Cohn.45 (B–C) Water uptake curves of 3D printed hydrogels immersed in different pH solutions: (B) Water absorption versus time for the hydrogel made of monomer concentration of 80 wt% in the aqueous solution while concentrations of the monomers, F127 dimethacrylate (F) relative to acrylic acid (A), varied between 70:30% and 10:90%, respectively, and (C) Water absorption against time for the hydrogel made of the monomers of concentration 30 wt% in the aqueous solution. Scale bar: 2 cm. Republished with permission of Royal Society of Chemistry, from Dutta S, Cohn D. Temperature and pH responsive 3D printed scaffolds. J Mater Chem B. 2017;5(48):9514–9521. Copyright 2017; permission conveyed through Copyright Clearance Center, Inc.45 |
Applications
Many papers have previously looked at the properties of a variety of different pH-sensitive hydrogels with various sensitivity ranges suitable for a wide range of applications such as drug delivery in the stomach.24 The success of these numerous hydrogels has been investigated by evaluating the swelling capability as well as the drug release for hydrogels with drug delivery capability.20,23-25,28 Others have investigated sensitivity by monitoring changes in SPR wavelength.4 Below are a few examples of some of the applications of pH-sensitive hydrogels.
Remote pH Monitoring
pH-sensitive hydrogels suitable for biological use have previously been investigated in the application of monitoring epidermal wounds as presented by Tamayol et al (2016).46 Healthy skin is slightly acidic while during healing the pH of the wound (7.15–8.93) shifts from an alkaline to the neutral state. Tamayol et al developed hydrogel microfibers incorporated with a pH-responsive dye which changes color based on the pH of the wound indicating the condition and healing of the wound. Alginate-based microfibers were loaded with pH-responsive mesoporous silica beads. Sodium-alginate (2%w/v) mixed with glycerol (0–60% w/v), and bead density (0–0.5 million/mL) was injected through the core channel with a flow of CaCl2 (2% w/v) around the core. The use of hydrogels for epidermal applications is beneficial as they enable the wound to be maintained in a moist environment. The pH range studied was from 6.5 to 9 to assess the response of the fiber to the pH variation of the environment. Figure 9 shows the fabrication of the microfibers presented by Tamayol et al.
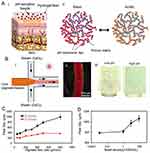 |
Figure 9 Fabrication process of microfibers. (A) Schematic illustration of hydrogel microfiber, pH-response of the dye loaded beads in basic (i) and acid media (ii). (B) Schematic of the fiber fabrication process. The fiber core made of sodium alginate and dye loaded beads and calcium alginate plays the fiber cladding role (i). A typical model of the alginate fiber fabricated by the microfluidic chip (ii). A micrograph of the fabricated hydrogel microfibers (iii). (C) Effect of core flow rate on fiber diameter. (D) Effect of bead density on fiber diameter while the core and cladding flowrates remained constant at 600 µL/min and 2 mL/min, respectively. Reprinted with permission from Tamayol A, Akbari M, Zilberman Y, et al. Flexible pH‐sensing hydrogel fibers for epidermal applications. Adv Healthc Mater. 2016;5(6):711–719. © 2016 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.46 |
Hydrogels based on pH-sensitive hydroxyethyl cellulose (HEC)/hyaluronic acid (HA) fabricated by Kwon et al are skin-compatible and may be used for the treatment of skin lesions.25 Maintaining the pH of the skin is important in order to provide an effective barrier. The hydrogels were tested in vitro for the release of isoliquiritigenin (ILTG) drugs. The swelling properties of the hydrogels were investigated at pH values in the range 1–13. The efficiency of drug release was found to be greater than 70% at pH 7 due to the larger pore size. As the pH increases, the hydrogel swelling ratio also increases which has been attributed to electronic repulsion and the nature of HA.
Additionally, Schulz et al investigated and tested a hydrogel-based sensor in vitro in the physiological pH range and found that a sharp transition period was seen between pH 7.8 and pH 6.8.30 The application presented combined the tested hydrogel with a micromachined piezoresistive pressure transducer for the application of online pH monitoring. This combination provides a cost-efficient design with the capability of miniaturization. The properties of the sensor depend heavily on the properties of the hydrogel layer. The hydrogel used was based on hydroxypropyl methacrylate (HPMA)/N,N-dimethylaminoethyl methacrylate (DMA)/tetra-ethyleneglycol dimethacrylate (TEGDMA) and shows high sensitivity in the physiological pH range.
A polyacrylamide coated no-core fiber (NCF) pH sensor was fabricated by Pathak et al (2017).47 The hydrogel was made of the aqueous solution combining acrylamide and bisacrylamide (19:1) – forming a 3D structured mesh-gel network. This sensor was tested at increasing pH (3–10) and comparisons between the transmission spectra were conducted with the sensor showing a good linear response in acidic and basic ranges.47 With increasing pH, the hydrogel swells due to electrostatic repulsion which is produced from the ionization of the carboxylic group. Consequently, the refractive index (RI) of the hydrogel decreases. Increasing wavelength was observed over the range investigated by Pathak et al as seen in Figure 10. A sensitivity of 1.94 nm/pH was reported for the fabricated sensor as well as good stability and repeatability.
 |
Figure 10 (A) Schematic diagram of the pH-responsive fiber. SMA-905 is a connector attached to the end of the fiber to facilitate the light coupling process and the fiber connection with the spectrometer. (B) Change of the resonance wavelength with increasing pH as illustrated by Pathak and Singh. Reprinted from Pathak AK, Singh VK. A wide range and highly sensitive optical fiber pH sensor using polyacrylamide hydrogel. Opt Fiber Technol. 2017;39:43–48. Copyright 2017, with permission from Elsevier.47 |
Many published papers have focused on the use of hydrogels in the human body for applications in drug delivery, particularly those hydrogels' response in the acidic region. A limited number of articles reported on the synthesis of hydrogels with sensitivity around the physiological range. The hydrogel compositions suitable for the possible application of monitoring changes in the pH of blood have been presented by Liechty and Peppas as well as Schulz et al.27,30 Schulz et al presented a sensor with an ideal pH range for the application, as they achieved sufficient sensitivity within the pH range of 7.4 to 7.0, while Liechty and Peppas investigated the release of chemotherapy drugs using different hydrogel compositions in conditions where pH was less than 6.5.
Drug Delivery
For the application of drug delivery, the drug is incorporated into the polymer backbone.26,48-52 pH-sensitive hydrogels have been investigated frequently for the use in drug delivery of peptide drugs. This is essentially due to the hydrogels protecting the peptide drugs in the acidic environment as suggested by Tomar et al (2014).20 Yadav and Shivakumar (2012) used a derivative of chitosan that is soluble in water to enable drug delivery to the intestine as it offers sufficient sensitivity to pH.23 From their work, it was concluded that the hydrogel swelling is dependent on the concentration of the cross-linking agent. Carboxymethyl chitosan (CMC) and carbopol were used to formulate the pH-sensitive hydrogels containing theophylline. The study showed that the fabricated hydrogel could successfully be used to release theophylline in the intestine, making it useful for treating symptoms of nocturnal asthma. Prolonged drug release was shown in the in vitro and in vivo studies which is ideal.
Recently, the use of pH-sensitive hydrogels for cancer therapy has been investigated as a method of improving the efficiency of the released drugs. Dai et al (2019) developed a hydrogel for delivering doxorubicin (Dox), an anti-cancer drug, to tumor sites where the pH has been shown to decrease below the physiological range.31 The composition of this hydrogel was mentioned in section 2. In vitro tests were used to identify the drug release profile of DOX from the DF-PEG-PAHy/BPNSs hydrogel (Figure 11). A slow and stable release of DOX was shown in this study and it was found that NIR irradiation accelerated the release of the drug. The release of Dox drug was also investigated in the work of Etrych et al.11 using water-soluble polymer N-(2-hydroxypropyl)methacrylamide (HPMA). Dox attached to an HPMA polymer backbone, through degradable hydrazone linkages combined with spacers stable in physiological conditions, was shown to release DOX sufficiently in the mild acidic environment.11 The effective release of DOX was found at pH 5.0 while a slight release was seen at the physiological pH, demonstrating that the hydrogel is more stable in physiological conditions.
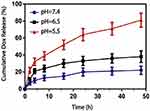 |
Figure 11 Results obtained from Dai et al showing the dependence of the rate of the anticancer drug (DOX) release on the pH of the medium. Reprinted from Wu RS, Lin J, Xing YM, Dai ZL, Wang LW, Zhang XP. pH-sensitive black phosphorous–incorporated hydrogel as novel implant for cancer treatment. J Pharm Sci. 2019;108(8):2542–2551. Copyright 2019, with permission from Elsevier.31 |
Dong et al (2017) fabricated a Salecan-based pH-sensitive hydrogel for controlled insulin delivery.53 The Salecan-based pH-sensitive hydrogel was prepared through a graft copolymerization reaction between Salecan and 2-acrylamido-2-methyl-1-propanesulfonic acid. Poly(2-acrylamido-2-methyl-1-propanesulfonic acid) is a water-soluble polymer that is able to accept or donate protons depending on the pH environment. The preparation process of the Salecan-g-PAMPS hydrogel can be seen in Figure 12A. A swelling-diffusion approach was used to load insulin into the prepared hydrogels. In vitro release experiments were conducted and it was validated that the release of insulin could be tailored according to the pH of the medium. Figure 12B and C illustrates the cumulative insulin release with time showing suppressed release at acidic pH and enhanced release at neutral pH.53 Buffer solution with a pH of 7.4 was used to model the pH of the colon and a pH of 1.2 to model the gastric environment at 37 °C. Applications of pH-responsive hydrogels extended to be utilized for measuring the response of cancer cells to anticancer drugs and to record the metabolism of cancer cells.54 Cancer cells consume glucose releasing lactate which leads to increase in the medium’s pH with a value 0.4 units while normal cells cause no pH change. In the presence of doxorubicin, multidrug-resistant cancer cells caused a drop in the pH of the medium.
 |
Figure 12 (A) Preparation process of the Salecan-g-PAMPS hydrogel. (B–C) In vitro insulin release profiles; (B) at pH 1.2 and (C) at pH 7.4 as presented by Qi et al. Reprinted with permission from Qi X, Wei W, Li J, et al. Salecan-based pH-sensitive hydrogels for insulin delivery. Mol Pharm. 2017;14(2):431–440. Copyright © 2017, American Chemical Society.53 |
Food Industry
Targeted delivery of bioactive food components considers encapsulation processes that are pH and time-dependent and hence pH-sensitive hydrogels are of interest in this field (Figure 13). Encapsulation of these components protects the functionality and may also allow the release to specific areas of the gut. P. de Vos et al briefly introduced the use of Chitosan for this application, which is highly compatible with living cells and dissolves at low pH.22 Chitosan is applied in combination with another polymer, such as alginate, that withstands the acidic environment in the stomach.
 |
Figure 13 Protection of bioactive food components using capsules. Adapted from de Vos P, Faas MM, Spasojevic M, Sikkema J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int Dairy J. 2010;20(4):292–302. Crown copyright © 2009 Published by Elsevier Ltd. All rights reserved. With permission from Elsevier.22 |
Wound Dressing
Wound medication represents a great challenge and poses a huge financial burden on the global healthcare system. Chronic diseases such as aging, diabetes, and obesity make handling wounds even harder. According to the World Health Organization (WHO) records, more than 30,000 deaths per year occur due to burns and scalds.55
The skin repairment for adults and overage individuals naturally occur; however, wound healing is accompanied by scarring and the traditional approaches cannot achieve the requirements. There was a speculate that the dry and uncovered wounds recover faster. This speculation was diminished when Winter and his group introduced the first generation of wound dressings. The dressings repaired wounded pig skin twice faster as compared to air-exposed wounds.56 Since then, dried wound dressings were developed such as woven cotton gauze, and non-woven blends of rayon with other fibers. Dried wound dressings were the most traded dressings till mid of 1970.57 Dried dressings maintain the wound area dry due to absorbing the wound’s exudates and fluids. These fluids leak out the dressings resulting in wound contamination. In addition, dried dressings cause strong pains during removing time as they adhere to wound surface and suffer from low oxygen permeability. Furthermore, they decrease the epithelialization rate and cell proliferation due to maintaining the wound in dry conditions.57 In 1970, moisten wound dressing were introduced and showed faster healing rate than dried dressings. Also, the wound healing was not accompanied with inflammation and stigma.58 The high water content and oxygen permeability of moisten dressing are considered the most interesting characteristics. In addition, their wet nature allows to maintain wound surface moist all the time, which enhance the epithelialization rate and cell growth.
Hydrogels were found to be the best choice for wet wound dressings application as they offer many advantages such as keeping the wound moisten, permeating oxygen, removing wound’s exudates, protecting the wound from infection and contamination, and easy and comfortable to be removed.59 Among wet wound dressings, hydrogel wound dressings are the market leader sharing with 43% of the consumed wet dressings. Hydrogel dressings are sold under various brand names, including GRX wound Gel, Biolex, TegaGel, NuGel, Carrasyn, 2nd Skin Fexderm, Exu Dry Dressing, and Cultinova Gel.59
Among the hydrogels used for wet dressings, Poly vinyl alcohol (PVA) has been found to be the most frequently used hydrogel because of the superior performance of the PVA-based hydrogels as compared to their counterparts of other hydrogels. To overcome the mechanical properties' issue of PVA, it is blended with some natural polysaccharides or some synthetic ones.59
The pH of the skin is slightly acidic and varies in the range of 4–6.60 However, skin injuries expose the skin to the internal body fluids that have physiological pH (7.4) – resulting in an increase of the pH of the damaged skin. pH of wounds was found to vary in the range of 6.5–8.5 depending on the wound conditions.61 For wound healing, the wound cells interact with a number of bioactive molecules: cytokines, extracellular matrix proteins, and growth factors. The interaction, is directly or indirectly influenced by the wound’s pH.62 Hence, pH-responsive hydrogels can play a significant role in cell growth reaction and monitoring the wound status as well. Banerjee et al introduced a pH-responsive hydrogel that sustained releasing growth factors for accelerating the wound healing.61 The pH-responsive hydrogel was made of poly(N-isopropylacrylamide-co-acrylic acid) via radical copolymerization. The hydrogel was loaded with bovine serum albumin (BSA), vascular endothelial growth factor, and epithermal growth factor which have been released at a different rate for 14 days within the range of the wound’s pH (6.7–7.9). Releasing the growth factors were progressive with an increase in wound pH. The loaded hydrogel with growth factors was tested on murine excisional wound model and showed an enhancement of wound healing compared to conventional sustained release growth factor therapy. The capability of pH-responsive hydrogels to monitor the wound status and to release drug simultaneously was recently reported.63 The pH-hydrogel released antibiotic agents and monitored the bacterial infections of wound sites using the pH detection as an indicator (Figure 14). The hydrogel accuracy for detecting bacterial infections was comparable with commercial systems and the smartphone was used as a reader for the wound conditions.
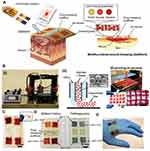 |
Figure 14 Wound dressings for monitoring and healing wounds. (A) Schematic of the wound dressing include pH-sensitive and drug release system. (B-i) A photo of the system used for fabricating the wound dressing. The system combines 3D printer and microfluidic nozzle. (B-ii) Schematic of the layer deposition by the co-axial flow system. (B-iii) Arrays of the pH-responsive hydrogel show the ability of the production system to produce the wound dressings on large scale. (C) A photo for the lyophilized and sterilized and wound dressing. (D) Naturally extracted cabbage juice and synthetic Brilliant Yellow used a pH indicator in the hydrogel matrix. (E) Conformal contact of the wound dressing with irregular surfaces. Reprinted with permission from Mirani B, Pagan E, Currie B, et al. An advanced multifunctional hydrogel‐based dressing for wound monitoring and drug delivery. Adv Healthc Mater. Epub 2017 Sep 25;6(19). © 2017 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.63 |
Conclusions
Hydrogels sensitive to pH have been investigated significantly over the past years due to the properties they exhibit which make them useful for a wide range of applications. Hydrogel-based sensors are being developed for a wide range of applications which include traditional fields of medicine. Their biocompatibility and high sensitivity are some of the properties which make them ideal candidates for such applications. Bashir et al reported a sensitivity of 5 × 10−5 pH and Zhao et al reported a sensitivity at 13 nm/pH at higher pH region (8–10 pH).4,14 pH-sensitive hydrogels have been used for a wide range of applications such as in contact lenses, targeted drug delivery, and food protection/monitoring. Many synthesis processes have been reported – from their preparation using silicon wafers to 3D printing. The interest in pH-sensitive hydrogels is still growing due to the range of applications it can be used for and is seen to have great utilization in our daily life in the near future.
Acknowledgments
This work was supported by Smart Project Program through KAIST-Khalifa Joint Research Center (KK-JRC) (N11190250).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49(8):1993–2007. doi:10.1016/j.polymer.2008.01.027
2. Bacelar AH, Cengiz IF, Silva-Correia J, et al. “Smart” hydrogels in tissue engineering and regenerative medicine applications. In: Khang G, editor. Handbook of Intelligent Scaffolds for Tissue Engineering and Regenerative Medicine. Jenny Stanford Publishing; 2017:333–367.
3. Badawy AR, Hassan MU, Elsherif M, et al. Contact lenses for color blindness. Adv Healthc Mater. 2018;7(12):1800152. doi:10.1002/adhm.201800152
4. Zhao Y, Lei, M., Liu, S.X., et al. Smart hydrogel-based optical fiber SPR sensor for pH measurements. Sens Actuators B Chem. 2018;261:226–232. doi:10.1016/j.snb.2018.01.120
5. Hunt JA, Chen R, van Veen T, et al. Hydrogels for tissue engineering and regenerative medicine. J. Mater. Chem. B. 2014;2(33):5319–5338. doi:10.1039/C4TB00775A
6. Elsherif M, Moreddu R, Hassan MU, et al. Real-time optical fiber sensors based on light diffusing microlens arrays. Lab Chip. 2019;19(12):2060–2070. doi:10.1039/C9LC00242A
7. Moreddu R, Elsherif M, Butt H, et al. Contact lenses for continuous corneal temperature monitoring. RSC Adv. 2019;9(20):11433–11442. doi:10.1039/C9RA00601J
8. Elsherif M, Hassan MU, Yetisen AK, et al. Wearable contact lens biosensors for continuous glucose monitoring using smartphones. ACS Nano. 2018;12(6):5452–5462. doi:10.1021/acsnano.8b00829
9. Elsherif M, Hassan MU, Yetisen AK, et al. Hydrogel optical fibers for continuous glucose monitoring. Biosens Bioelectron. 2019;137:25–32. doi:10.1016/j.bios.2019.05.002
10. Elsherif M, Hassan MU, Yetisen AK, et al. Glucose sensing with phenylboronic acid functionalized hydrogel-based optical diffusers. ACS Nano. 2018;12(3):2283–2291. doi:10.1021/acsnano.7b07082
11. Riaz RS, Elsherif M, Moreddu R, et al. Anthocyanin-functionalized contact lens sensors for ocular pH monitoring. ACS Omega. 2019;4(26):21792–21798. doi:10.1021/acsomega.9b02638
12. Jiang N, Ahmed R, Rifat AA, et al. Functionalized flexible soft polymer optical fibers for laser photomedicine. Adv Opt Mater. 2018;6(3):1701118. doi:10.1002/adom.201701118
13. Yetisen AK, Jiang N, Fallahi A, et al. Glucose‐sensitive hydrogel optical fibers functionalized with phenylboronic acid. Adv Mater. 2017;29(15):1606380. doi:10.1002/adma.201606380
14. Bashir R, Hilt JZ, Elibol O, et al. Micromechanical cantilever as an ultrasensitive pH microsensor. Appl Phys Lett. 2002;81(16):3091–3093. doi:10.1063/1.1514825
15. Richter A, Paschew G, Klatt S, et al. Review on hydrogel-based pH sensors and microsensors. Sensors. 2008;8(1):561–581. doi:10.3390/s8010561
16. Yetisen AK, Butt H, Volpatti LR, et al. Photonic hydrogel sensors. Biotechnol Adv. 2016;34(3):250–271. doi:10.1016/j.biotechadv.2015.10.005
17. Deligkaris K, Tadele TS, Olthuis W, et al. Hydrogel-based devices for biomedical applications. Sens Actuators B Chem. 2010;147(2):765–774. doi:10.1016/j.snb.2010.03.083
18. Peppas NA, Hilt J, Khademhosseini A, et al. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345–1360. doi:10.1002/adma.200501612
19. Yetisen AK, Butt H, da Cruz Vasconcellos F, et al. Light‐directed writing of chemically tunable narrow‐band holographic sensors. Adv Opt Mater. 2014;2(3):250–254. doi:10.1002/adom.201300375
20. Tomar LK, Tyagi C, Choonara YE, Kumar P, Pillay V. Rheological and swelling behavior of pH sensitive hydrogel particles. APCBEE Procedia. 2014;9:192–196. doi:10.1016/j.apcbee.2014.01.034
21. Yan Y, Wang X, Pan Y, et al. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials. 2005;26(29):5864–5871. doi:10.1016/j.biomaterials.2005.02.027
22. de Vos P, Faas MM, Spasojevic M, Sikkema J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int Dairy J. 2010;20(4):292–302. doi:10.1016/j.idairyj.2009.11.008
23. Kumar Singh Yadav H, Shivakumar H. In vitro and in vivo evaluation of ph-sensitive hydrogels of carboxymethyl chitosan for intestinal delivery of theophylline. ISRN Pharm. 2012;2012:1–9. doi:10.5402/2012/763127
24. Rizwan M, Yahya R, Hassan A, et al. pH sensitive hydrogels in drug delivery: brief history, properties, swelling, and release mechanism, material selection and applications. Polymers. 2017;9(4):137. doi:10.3390/polym9040137
25. Kwon SS, Kong BJ, Park SN. Physicochemical properties of pH-sensitive hydrogels based on hydroxyethyl cellulose–hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur J Pharm Biopharm. 2015;92:146–154. doi:10.1016/j.ejpb.2015.02.025
26. Etrych T, Jelı́nková M, Řı́hová B, et al. New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: synthesis and preliminary in vitro and in vivo biological properties. J Control Release. 2001;73(1):89–102. doi:10.1016/S0168-3659(01)00281-4
27. Liechty WB, Peppas NA. Expert opinion: responsive polymer nanoparticles in cancer therapy. Eur J Pharm Biopharm. 2012;80(2):241–246. doi:10.1016/j.ejpb.2011.08.004
28. Park SY, Bae YH. Novel pH‐sensitive polymers containing sulfonamide groups. Macromol Rapid Commun. 1999;20(5):269–273. doi:10.1002/(SICI)1521-3927(19990501)20:5<269::AID-MARC269>3.0.CO;2-3
29. Du L, Wang C, Meng L, et al. The study of relationships between pKa value and siRNA delivery efficiency based on tri-block copolymers. Biomaterials. 2018;176:84–93. doi:10.1016/j.biomaterials.2018.05.046
30. Schulz V, Gerlach, G., Günther, M, et al. Piezoresistive pH microsensors based on stimuli-sensitive polyelectrolyte Hydrogels piezoresistive pH-mikrosensoren auf der basis stimuli-sensitiver polyelektrolytischer hydrogele. tm-Technisches Messen Plattform für Methoden, Systeme und Anwendungen der Messtechnik. 2010;77(3):179–186.
31. Wu RS, Lin J, Xing YM, Dai ZL, Wang LW, Zhang XP, et al. pH-sensitive black phosphorous–incorporated hydrogel as novel implant for cancer treatment. J Pharm Sci. 2019;108(8):2542–2551. doi:10.1016/j.xphs.2019.03.003
32. More MP, Chitalkar RV, Bhadane MS, et al. Development of graphene-drug nanoparticle based supramolecular self assembled pH sensitive hydrogel as potential carrier for targeting MDR tuberculosis. Mater Technol. 2019;34(6):324–335. doi:10.1080/10667857.2018.1556468
33. Odian G. Principles of Polymerization. John Wiley & Sons; 2004.
34. Carraher
35. Matyjaszewski K. Cationic Polymerizations: Mechanisms, Synthesis & Applications. CRC Press; 1996.
36. Grubbs RB. Nitroxide-mediated radical polymerization: limitations and versatility. Polym Rev. 2011;51(2):104–137. doi:10.1080/15583724.2011.566405
37. Lee SB, Russell AJ, Matyjaszewski K. ATRP synthesis of amphiphilic random, gradient, and block copolymers of 2-(dimethylamino) ethyl methacrylate and n-butyl methacrylate in aqueous media. Biomacromolecules. 2003;4(5):1386–1393. doi:10.1021/bm034126a
38. Demirci S, Kinali‐Demirci S, Caykara T. Novel pH‐responsive mixed‐charge copolymer brushes based on carboxylic acid and quaternary amine monomers. J Polym Sci a Polym Chem. 2013;51(7):1612–1619. doi:10.1002/pola.26532
39. Mishra SK, Zou B, Chiang KS. Wide-range pH sensor based on a smart-hydrogel-coated long-period fiber grating. IEEE J Sel Top Quantum Electron. 2016;23(2):284–288. doi:10.1109/JSTQE.2016.2629662
40. Kim G, Kim HJ, Noh H. pH sensitive soft contact lens for selective drug-delivery. Macromol Res. 2018;26(3):278–283. doi:10.1007/s13233-018-6029-9
41. Wang X, Jiang M, Zhou Z, et al. 3D printing of polymer matrix composites: a review and prospective. Compos B Eng. 2017;110:442–458. doi:10.1016/j.compositesb.2016.11.034
42. Jia J, Richards DJ, Pollard S, et al. Engineering alginate as bioink for bioprinting. Acta Biomater. 2014;10(10):4323–4331. doi:10.1016/j.actbio.2014.06.034
43. Murphy R, Walsh DP, Hamilton CA, et al. Degradable 3D-printed hydrogels based on star-shaped copolypeptides. Biomacromolecules. 2018;19(7):2691–2699. doi:10.1021/acs.biomac.8b00299
44. Ouyang L, Highley CB, Rodell CB, et al. 3D printing of shear-thinning hyaluronic acid hydrogels with secondary cross-linking. ACS Biomater Sci Eng. 2016;2(10):1743–1751. doi:10.1021/acsbiomaterials.6b00158
45. Dutta S, Cohn D. Temperature and pH responsive 3D printed scaffolds. J Mater Chem B. 2017;5(48):9514–9521. doi:10.1039/C7TB02368E
46. Tamayol A, Akbari M, Zilberman Y, et al. Flexible pH‐sensing hydrogel fibers for epidermal applications. Adv Healthc Mater. 2016;5(6):711–719. doi:10.1002/adhm.201500553
47. Pathak AK, Singh VK. A wide range and highly sensitive optical fiber pH sensor using polyacrylamide hydrogel. Opt Fiber Technol. 2017;39:43–48. doi:10.1016/j.yofte.2017.09.022
48. Vashist A, Kaushik A, Alexis K, et al. Bioresponsive injectable hydrogels for on-demand drug release and tissue engineering. Curr Pharm Des. 2017;23(24):3595–3602. doi:10.2174/1381612823666170516144914
49. Onaciu A, Munteanu RA, Moldovan AI, Moldovan CS, Berindan-Neagoe I. Hydrogels based drug delivery synthesis, characterization and administration. Pharmaceutics. 2019;11(9):432. doi:10.3390/pharmaceutics11090432
50. Vemula PK, Wiradharma N, Ankrum JA, et al. Prodrugs as self-assembled hydrogels: a new paradigm for biomaterials. Curr Opin Biotechnol. 2013;24(6):1174–1182. doi:10.1016/j.copbio.2013.02.006
51. Wang Y, Cheetham AG, Angacian G, et al. Peptide–drug conjugates as effective prodrug strategies for targeted delivery. Adv Drug Deliv Rev. 2017;110:112–126. doi:10.1016/j.addr.2016.06.015
52. Oliva N, Conde J, Wang K, et al. Designing hydrogels for on-demand therapy. Acc Chem Res. 2017;50(4):669–679. doi:10.1021/acs.accounts.6b00536
53. Qi X, Wei W, Li J, et al. Salecan-based pH-sensitive hydrogels for insulin delivery. Mol Pharm. 2017;14(2):431–440. doi:10.1021/acs.molpharmaceut.6b00875
54. Shaibani PM, Etayash H, Naicker S, et al. Metabolic study of cancer cells using a pH sensitive hydrogel nanofiber light addressable potentiometric sensor. ACS Sens. 2017;2(1):151–156. doi:10.1021/acssensors.6b00632
55. Kamoun EA, Chen X, Mohy Eldin MS, et al. Crosslinked poly (vinyl alcohol) hydrogels for wound dressing applications: a review of remarkably blended polymers. Arab J Chem. 2015;8(1):1–14. doi:10.1016/j.arabjc.2014.07.005
56. Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193(4812):293–294. doi:10.1038/193293a0
57. Edwards JV, Yager DR, Cohen IK, et al. Modified cotton gauze dressings that selectively absorb neutrophil elastase activity in solution. Wound Repair Regen. 2001;9(1):50–58. doi:10.1046/j.1524-475x.2001.00050.x
58. Kokabi M, Sirousazar M, Hassan ZM. PVA–clay nanocomposite hydrogels for wound dressing. Eur Polym J. 2007;43(3):773–781. doi:10.1016/j.eurpolymj.2006.11.030
59. Kamoun EA, Kenawy E-RS, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res 2017;8(3):217–233. doi:10.1016/j.jare.2017.01.005
60. Schneider LA, Korber A, Grabbe S, et al. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res. 2007;298(9):413–420. doi:10.1007/s00403-006-0713-x
61. Banerjee I, Mishra D, Das T, et al. Wound pH-responsive sustained release of therapeutics from a poly (NIPAAm-co-AAc) hydrogel. J Biomater Sci Polym Ed. 2012;23(1–4):111–132. doi:10.1163/092050610X545049
62. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi:10.1152/physrev.2003.83.3.835
63. Mirani B, Pagan E, Currie B, et al. An advanced multifunctional hydrogel‐based dressing for wound monitoring and drug delivery. Adv Healthc Mater. 2017;6(19):1700718. doi:10.1002/adhm.201700718
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
