Back to Journals » Journal of Pain Research » Volume 14
Health-Care Utilization and Outcomes with 10 kHz Spinal Cord Stimulation for Chronic Refractory Pain
Authors Gupta M , Ray M, Ladesich N, Gupta A
Received 10 February 2021
Accepted for publication 21 August 2021
Published 2 December 2021 Volume 2021:14 Pages 3675—3683
DOI https://doi.org/10.2147/JPR.S306126
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Krishnan Chakravarthy
Mayank Gupta, Mahoua Ray, Nicole Ladesich, Akshat Gupta
Neuroscience Research Center, LLC, Overland Park, KS, USA
Correspondence: Mayank Gupta 10995 Quivira, Overland Park, KS, 66201, USA
Tel +913 339-9437
Fax +913 339-9538
Email [email protected]
Background: Chronic pain is a common condition associated with decreased quality of life and increased health-care costs. Opioid analgesics are routinely used to treat chronic pain despite limited evidence of long-term efficacy. Spinal cord stimulation at a frequency of 10 kilohertz (10kHz-SCS) has been shown to be effective for treating chronic pain.
Objective: This study was conducted to evaluate the effects of 10kHz-SCS on patients’ pain intensity, volume of pain interventions, and opioid intake in a real-world setting.
Study Design: This study was a retrospective review of patient data.
Setting: The study was conducted at a single, community-based clinic.
Methods: Outcomes including pain relief, quality of life, opioid intake, and rate of health-care usage were evaluated using data from patients who were implanted with a 10kHz-SCS device to treat chronic pain. These outcomes were then compared for the pre- and post-implant periods.
Results: A total of 47 patients with a mean follow-up duration of 15.6 ± 6.2 months were included in this analysis. Mean pain relief was 73 ± 22% and 89% were responders at the final follow-up visit. The rate of medical interventions fell from 3.48± 3.05 per year before starting 10kHz-SCS to 0.49± 1.16 per year afterward (P < 0.001). Of 30 patients with available opioid consumption data, 89% maintained or decreased their intake after implant.
Conclusion: Retrospective data from a single center, with minimal exclusion criteria shows clinically significant pain relief with 10kHz-SCS, accompanied by significant indirect benefits including stable or reduced opioid use and reduced interventional procedures.
Keywords: chronic pain, pain management, spinal cord stimulation, health care costs, opioid analgesics
Introduction
Chronic pain is a common condition estimated to affect 50 million people in the US in 2016, or over 1 in 5 adults, and 19.6 million had pain that frequently limited work and life activities.1 Chronic pain is also associated with substantial health-care costs and decreased productivity, which were valued at up to $635 billion in 2011 or about $2000 per American.2 Non-steroidal anti-inflammatory drugs (NSAIDs) are most common first-line treatment for chronic pain, but if the pain cannot be managed with NSAIDs alone, opioid analgesics are often prescribed.3 However, there is little evidence supporting the efficacy of these drugs for treating chronic pain,4,5 and their long-term use can result in side effects and substance abuse disorders.6 Pain treatments including epidural steroid injections and nerve blocks have been used for chronic pain, including low back pain, but studies have shown these procedures to be ineffective over periods of a year or longer in a majority of patients.7–9
To avoid increased opioid utilization in patients who are refractory to conventional medical management, physicians are beginning to focus on minimally invasive, reversible interventional treatments such as spinal cord stimulation (SCS) for certain chronic pain populations.10–14 High-frequency SCS delivered at a frequency of 10 kilohertz (10kHz-SCS) has also proven to be effective for treating chronic pain with multiple etiologies,11,15–19 and this modality possesses the added benefit of producing paresthesia-independent pain relief.20 Previous research has also indicated 10kHz-SCS in patients with chronic pain is associated with maintenance, or even reductions, in opioid consumption over time.21,22 Many patients with chronic pain are already being treated with opioids by the time SCS is considered, so a treatment that addresses pain and simultaneously reduces or stabilizes patient risk from opioids would be doubly beneficial in this population.23
Several reviews have been published that analyze real-world outcomes in patients with chronic pain who receive 10kHz-SCS;24–26 however, more data is needed to evaluate not only the direct effects of high-frequency SCS on pain but also its indirect effects on health-care utilization and opioid consumption. The objective of this retrospective review is to determine if 10kHz-SCS is effective in minimizing the requirement for opioids and reducing health-care contacts and interventions.
Methods
Study Design
This single center retrospective analysis included patients who received 10kHz-SCS therapy between January 2, 2017 and December 29, 2019 at the Neuroscience Research Center in Overland Park, Kansas. The Midlands Investigational Review Board (Lenexa, Kansas) determined that the study met the criteria for an IRB exemption under the United States Code of Federal Regulations Title 45 Part 46 and a waiver of the informed consent requirement was given for the retrospective data collection, given that extracted data was deidentified protecting patient health information. The study was in compliance with the Declaration of Helsinki. Electronic medical record (EMR) data from Kansas Pain Management’s PrognoCIS (San Jose, California) EMR database were searched to identify patients with a diagnosis of chronic pain who were implanted with a 10kHz SCS device (Nevro, Redwood City, California) and whose EHR included data for at least 1 month after implant and an equivalent period of time before implant. Patients were excluded if their implant was removed for any reason or if they were involved with Workman’s Compensation cases.
Patient-Reported Outcomes
Self-reported data on pain intensity and quality, patient satisfaction, and quality of life were collected at baseline and at the final follow-up. Pain intensity and quality were assessed using an 11-point NRS scale with five custom items that included commonly reported neuropathic pain symptoms. Responders were defined as patients who reported ≥50% pain relief after implant, which is a threshold used in previous studies of 10kHz-SCS.11,16,24,27 Patient-reported quality of life was assessed using the Quality of Life Scale published by the American Chronic Pain Association,28 satisfaction was assessed on a 11-point scale, and percentage of pain relief reported using a visual analog scale. These items were collected per standard of care in our clinic to assess patient’s pain and response to SCS therapy, the questionnaire is available in Supplement 1.
Healthcare Utilization
Changes in health-care utilization following implant were assessed using the number of interventional pain procedures that were recorded in patients’ EHR including clinic visits, medical procedures, emergency room admissions, and surgeries. Types of medical procedures included radiofrequency ablation, medial branch block, transforaminal epidural steroid injection, and lumbar epidural steroid injection. The pre-implant procedure rate was calculated by the total pre-implant procedures recorded in the EMR divided by the months between the first clinic visit and the implant date. The post-implant procedure rate was similarly the total post-implant procedures recorded in the EMR divided by the months between the implant procedure and the database review. Finally, the total number of interventional pain procedures during the analyzed time periods was used to calculate a rate of contacts per year.
The average cost of medical procedures including radiofrequency ablation, medial branch block, transforaminal epidural steroid injection, and intralaminar epidural steroid injection was calculated using the national mean 2020 Medicare reimbursement levels. First, the mean reimbursement for ASCs, hospitals, and physicians was determined based on the above-mentioned procedure types performed and were then averaged to calculate a mean procedure cost.
Opioid Consumption
Data from the EHR for each patient were combined with data from the Kansas Tracking and Reporting of Controlled Substances (K-TRACS) database to determine whether individual patients used opioid analgesics and calculate their mean daily intake.29
Prospective Long-Term Follow-Up
Prospective surveys were sent to patients in the retrospective analysis who had been implanted for longer than 12 months. IRB approval was obtained to contact these patients both to get their consent to be included in the prospective analysis and to ask them to complete a questionnaire about their current pain. Individual pain scores determined by averaging the “best” and “worst” pain reported. They were also asked to complete a self-assessment with open text responses regarding activities they were not able to do before therapy versus after therapy. The prospective survey is included as Supplement 2.
Statistical Analysis
Data are presented throughout as means ± standard deviation (SD), or as a median and range, when test for normality was not met based on the Anderson-Darling test. Significance in differences between pre- and post-implant values were calculated using a paired t-test for data with normal distribution and the One-Way ANOVA test when normality criteria was not met. Significant differences were defined as those with p-values ≤0.05.
Results
Subject Demographics and Disposition
A search of the institution’s database identified EHRs for 66 patients who were implanted with 10kHz-SCS to treat a diagnosis of chronic pain from January 2, 2017, through January 31, 2019, and met all eligibility criteria. Of these, the EHRs for 47 patients included all necessary information and were included in the retrospective analysis, as shown in Figure 1. In addition, prospective surveys were sent to patients with more than 1 year since implant, and 10 patients were ultimately eligible, consented, and completed these surveys.
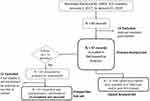 |
Figure 1 The selection of patient records for use in the retrospective analysis and prospective follow-up are shown in this flow chart. |
The demographics and baseline clinical characteristics of the 47 patients included in the analysis are summarized in Table 1. Patients ranged in age from 39 to 86 years old with a mean age of 65 years, and 27 (58%) were men. The most common areas associated with patients’ diagnoses of chronic pain were lower back (85%) and lower extremities (60%). The included patients had a mean follow-up interval of 15.6 ± 6.2 months and ranged from 2 to 28 months.
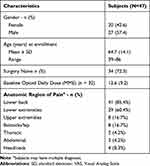 |
Table 1 Patient Demographics and Baseline Clinical Characteristics |
Pain
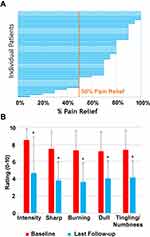
Post-implant pain relief was calculated using patient-reported pain intensity scores at last follow-up relative to baseline scores and is shown in Figure 2A. In total, 42 subjects (89%) were responders at last follow-up, and mean pain relief was 73% ± 22%, including 23 patients who reported >80% pain relief. Self-reporting by patients at the last follow-up visit also revealed significant reductions in multiple domains of pain relative to baseline, as shown in Figure 2B.
Healthcare Utilization
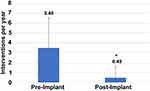
Data on all interventional pain procedures were analyzed for each patient and included procedures such as radiofrequency ablation, medial branch block, transforaminal epidural steroid injection, and lumbar interlaminar epidural steroid injection. The mean rate of medical interventions in this patient sample declined from a mean rate of 3.48 ± 3.05 interventions per year before implantation to 0.49 ± 1.16 interventions per year after implantation (One-way ANOVA, p < 0.001), a decline of 86% (Figure 3). The estimated cost savings for medical procedures only was calculated using 2020 Medicare reimbursement levels, and calculating a weight mean procedure cost for before and after SCS implant based on the proportion of different procedures that were performed. This resulted in a weighted mean procedure cost of $838.00 pre-implant and $790.83 post-implant due to a reduction in the frequency of use of the more expensive RFA procedure post-implant (Supplemental Table 1), which translates to approximate savings of $2528.74 per year per patient.
Opioid Consumption
Data on opioid use was obtained from the K-TRACS registry, which was available for 32 patient records. Among these patients, 28 (88%) decreased their daily opioid dose or remained on a stable dose of opioids from the pre- to the post-implant period, while daily opioid dose increased in 4 patients (13%) as shown in Figure 4A. None of the patients whose opioid consumption increased was taking more than 20 milligrams morphine equivalent (MME) by the last follow-up, and of 5 patients taking opioid doses of ≥20 MME daily before implant, 4 reduced their daily consumption under 20 MME by the last follow-up. The distribution of opioid doses among these 32 patients is shown in Figure 4B. The mean opioid dose was not significantly reduced following SCS implant with a change from an average of 9.6+/-13.4 to 7.0+/-9.3 MME (p = 0.310).
 |
Figure 4 The changes in patient opioid consumption after implant is shown in (A). (B) Shows, the pre- and post-implant distributions of opioid consumption in the patient sample. |
Quality of Life and Patient Satisfaction
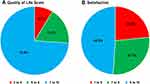
Patients’ quality of life was assessed at the most recent visit using the Quality of Life Scale,28 a descriptive scale with responses reported in whole numbers from completely non-functioning (0) to a normal quality of life (10). Among this patient population, the median response was 8, and responses ranged from 3 to 10. The results are summarized in Figure 5A and show that 34 patients (74%) reported quality of life scores of ≥7, which indicates they were able to participate in work, home, and social activities. Patient satisfaction was reported using a scale with a minimum score of 1, meaning not satisfied at all, and a maximum of 10, meaning extremely satisfied. The distribution of responses from patients in this sample is shown in Figure 5B, including 23 patients (49%) who reported satisfaction scores of 9 or 10, more than double the proportion who reported satisfaction scores of ≤6.
Prospective Assessments Regarding Long-Term Pain Relief
A total of 10 eligible patients had follow-up intervals of at least 12 months and responded to a prospective follow-up survey (Figure 1). The mean follow-up interval among this group was 20.8 ± 2.3 months, and their mean pain relief was 62%. All 10 were responders (≥50% pain relief), and 4 reported decreases in pain of 80% or more. Six out of 10 reported sustained improvement in function with at least one activity they can do now that they could not do prior to therapy. Results are shown in Supplemental Table 2.
Discussion
There is currently an unmet need for safe and effective treatment options for chronic pain. Multiple prospective clinical studies have been published demonstrating the efficacy of 10 kHz SCS,30–34 and observational data from community pain centers can add useful information on how this intervention affects real-world patient outcomes. However, previous observational, retrospective studies of patients treated with 10kHz-SCS for chronic pain have included limited sample sizes and shorter follow-up intervals than the prospective studies.24,25,35
Patients eligible for this retrospective review included all those at our institution who were treated with 10kHz-SCS for chronic pain, regardless of the cause, and we found significant and long-lasting reductions in patient-reported pain intensity after the initiation of stimulation. The mean pain relief of 73% and responder rate of 89% after a mean stimulation time of over 15 months compare favorably with results from previous clinical and real-world studies of 10 kHz SCS for treating chronic, refractory pain. The pivotal SENZA-RCT study and the prospective SENZA-EU study both reported pain relief in the back and legs ranging from 63% to 70% after 12 months of stimulation and responder rates from 65% to 79%.11,27 Other prospective studies reported similar results after 1 year of stimulation, including 72% pain relief and a responder rate of 90% in subjects with inoperable back pain15 and 82% pain relief and a 88% responder rate in patients with chronic postsurgical pain.16 In similar real-world studies, the much larger review by Stauss et al reported 63% pain relief and a responder rate of 74% after a mean treatment interval of less than 9 months,24 while another single-center review reported pain relief of 46% to 51% after 12 months of stimulation.25
It is important to consider cost as part of evaluating any new treatment for chronic pain, particularly in light of the expensive nature of chronic pain, which has been estimated to cost the US economy from $560 billion to $635 billion every year in direct health-care expenditures as well as lost productivity and reduced time at work.2 Recent reviews have concluded that SCS is a cost-effective option for treating patients with chronic, neuropathic low back pain,36,37 and although such a cost analysis was beyond the scope of this retrospective study, the estimated savings for each patient in this study due to reduced medical procedures alone was approximately $2500 per patient per year conservatively based on Medicare reimbursement.
We used the volume of interventional pain procedures as a proxy for health-care usage to indirectly examine the issue of cost. The number of procedures we observed during the post-implant period in this real-world patient population was substantially reduced from pre-implant levels, and are broadly similar to findings in previous real-world, single-site studies of 10 kHz SCS, which have found reductions of 39% to 84% in medical pain interventions such as epidural steroid injections, facet joint injections, radiofrequency ablations, and major joint injections following implant.25,38,39 EMR review allowed us to examine the type of procedures that were provided pre and post SCS implant, and showed the proportion of the more expensive RFA procedure decreased from 14.0% to 9.6%, while the frequency of the less expensive trigger point injection increased from 1.7% to 38.7%, which also contributes to cost reduction. Our results further support the conclusion that 10kHz-SCS can help to reduce health-care costs despite a larger initial investment by reducing follow-up care necessary for treating chronic, refractory pain with various etiologies.
It is also important to consider the effect of new, non-pharmacologic treatments for chronic pain on opioid consumption. The long-term use of opioids to treat chronic pain is associated with many risks, and guidelines from the Centers for Disease Control and Prevention recommend minimizing their use to the extent possible to reduce the danger to patients.40,41 Previous studies with 10 kHz SCS have shown stimulation is associated with stable or decreasing opioid consumption in patients with chronic pain,15,21,25,42–45 and our results likewise revealed stable or decreasing daily opioid doses in 89% of patients. Nearly a quarter decreased their opioid use, despite the lack of an active protocol of opioid reduction, supporting evidence that 10 kHz SCS could be an important tool in reducing opioid use in chronic pain patients. It is important to note that the risks presented by opioids increase in a dose-dependent manner,41 and 4 of the 5 patients in our study taking doses of more than 20 MME per day before implant reduced their intake below this threshold in response to 10kHz-SCS, which has been shown to reduce the risk of overdose and opioid-related mortality.46–48 Conversely, the 4 patients who increased their opioid intake during the study period were already at relatively low doses, and none increased their intake above 20 MME. The low average daily opioid prescription for our patients may be due to the fact that we attempt to wean them from high doses of opioids before considering SCS.
Limitations
This study was limited by its retrospective design and lack of randomization or control group, which could introduce bias into patients selected for treatment. The possibility of bias is partially mitigated by the use of broad eligibility criteria that included all patients who were implanted with a 10kHz-SCS device at the institution during the time period of interest, regardless of the cause of their chronic pain. Although there was no control group, the chronic nature of the patients’ pain means spontaneous recovery is unlikely and increases confidence that the observed, durable pain relief is associated with treatment. The study was also limited by its small size, and that the HCU data was collected through a retrospective EMR review, therefore patient recall was therefore not a factor. ER visits were included that were recorded in EMR which were generally pain-related visits. There was no attempt to determine if additional ER visits occurred that were not in the patient’s EMR. Office visits for healthcare outside of the clinical practice (such as physical therapy) were not analyzed. The patient’s interventional pain management was exclusively at Kansas Pain Management. Pain meds may have been prescribed by other care providers but the information for opioid use was extracted from KTRACS which is practice independent since it is based on state gathered prescription records. Another limitation is the small number of patients who we were able to contact and obtain consent to participate in prospective surveys makes those reported outcomes difficult to interpret except qualitatively. Finally, we produced an estimate of cost savings associated with reduced rates of interventional pain procedures in patients following initiation of 10kHz-SCS; however, a detailed cost analysis was beyond the scope of this review.
Conclusions
This retrospective review included data from patients at a single center who had chronic pain and were treated with 10kHz-SCS. Patients were selected using expansive eligibility criteria to include patients with pain in a variety of body regions and without regard to the etiology of the pain. The results show clinically significant and durable pain relief after the implant of the 10kHz-SCS device. In addition, we observed significant indirect benefits of treatment with 10kHz-SCS, including fewer interventional pain procedures as well as stable or reduced opioid use.
Acknowledgments
The authors thank Erik MacLaren, PhD of Galen Medical Writing for his assistance in language content and manuscript preparation, Anand Rotte, PhD and Rose Province-Azalde, MS of Nevro Corp. for manuscript review, and M. Bhandaru Ph.D. for preparing the illustrations for the manuscript.
Funding
This independent research study was funded by a grant from Nevro Corp.
Disclosure
This study was supported by an unrestricted grant from Nevro Corp.
Dr. Mayank Gupta reports grants from Nevro Corp., during the conduct of the study; personal fees from Nevro Corp., outside the submitted work; Consultant-Self Advisory/Medical Board-Self Investigator-Self from Averitas Pharma, Consultant-Self Investigator-Self from US WorldMeds, Consultant-Self Investigator-Self from Nalu Medical, Consultant-Self Advisory/Medical Board-Self from Foundation Fusion Solutions, Consultant-Self Investigator-Self from SPR Therapeutics, Inc., during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi:10.15585/mmwr.mm6736a2
2. Institute of Medicine. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press; 2011.
3. Schappert SM, Burt CW. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001-02. Vital Health Stat. 2006;13(159):1–66.
4. Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112(3):372–380. doi:10.1016/j.pain.2004.09.019
5. Busse JW, Wang L, Kamaleldin M, et al. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA. 2018;320(23):2448–2460. doi:10.1001/jama.2018.18472
6. Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116–127. doi:10.7326/0003-4819-146-2-200701160-00006
7. Lee JW, Shin HI, Park SY, Lee GY, Kang HS. Therapeutic trial of fluoroscopic interlaminar epidural steroid injection for axial low back pain: effectiveness and outcome predictors. AJNR Am J Neuroradiol. 2010;31(10):1817–1823. doi:10.3174/ajnr.A2180
8. Onafowokan OO, Fine NF, Brooks F, Stokes OM, Briggs TW, Hutton M. Multiple injections for low back pain: what’s the future? Eur Spine J. 2020;29(3):564–578. doi:10.1007/s00586-019-06258-w
9. Southern D, Lutz GE, Cooper G, Barre L. Are fluoroscopic caudal epidural steroid injections effective for managing chronic low back pain? Pain Physician. 2003;6(2):167–172.
10. Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669–681. doi:10.1097/j.pain.0000000000000814
11. Kapural L, Yu C, Doust MW, et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT Randomized Controlled Trial. Anesthesiology. 2015;123(4):851–860. doi:10.1097/ALN.0000000000000774
12. Kumar K, North R, Taylor R, et al. Spinal cord stimulation vs. conventional medical management: a Prospective, Randomized, Controlled, Multicenter Study of Patients with Failed Back Surgery Syndrome (PROCESS Study). Neuromodulation. 2005;8(4):213–218. doi:10.1111/j.1525-1403.2005.00027.x
13. Mekhail NA, Argoff CE, Taylor RS, et al. High-frequency spinal cord stimulation at 10 kHz for the treatment of painful diabetic neuropathy: design of a multicenter, randomized controlled trial (SENZA-PDN). Trials. 2020;21(1):87. doi:10.1186/s13063-019-4007-y
14. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98–106. doi:10.1227/01.NEU.0000144839.65524.E0
15. Al-Kaisy A, Palmisani S, Smith TE, et al. Long-term improvements in chronic axial low back pain patients without previous spinal surgery: a cohort analysis of 10-kHz high-frequency spinal cord stimulation over 36 months. Pain Med. 2018;19(6):1219–1226. doi:10.1093/pm/pnx237
16. Gupta M, Scowcroft J, Kloster D, et al. 10-kHz spinal cord stimulation for chronic postsurgical pain: results from a 12-month prospective, multicenter study. Pain Pract. 2020;20(8):908–918. doi:10.1111/papr.12929
17. Kapural L, Gupta M, Paicius R, et al. Treatment of chronic abdominal pain with 10-kHz spinal cord stimulation: safety and efficacy results from a 12-month prospective, multicenter, feasibility study. Clin Transl Gastroenterol. 2020;11(2):e00133. doi:10.14309/ctg.0000000000000133
18. Van Buyten JP, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation. 2013;16(1):59–65. doi:10.1111/ner.12006
19. Al-Kaisy A, Royds J, Al-Kaisy O, et al. Cascade programming for 10 kHz spinal cord stimulation: a single center case series of 114 patients with neuropathic back and leg pain. Neuromodulation. 2020;2:254.
20. De Carolis G, Paroli M, Tollapi L, et al. Paresthesia-independence: an assessment of technical factors related to 10 khz paresthesia-free spinal cord stimulation. Pain Physician. 2017;20(4):331–341.
21. Al-Kaisy A, Van Buyten JP, Amirdelfan K, et al. Opioid-sparing effects of 10 kHz spinal cord stimulation: a review of clinical evidence. Ann N Y Acad Sci. 2020;1462(1):53–64. doi:10.1111/nyas.14236
22. Amirdelfan K, Vallejo R, Benyamin R, et al. High-frequency spinal cord stimulation at 10 kHz for the treatment of combined neck and arm pain: results from a prospective multicenter study. Neurosurgery. 2020;87(2):176–185. doi:10.1093/neuros/nyz495
23. Gupta M, Abd-Elsayed A, Knezevic NN. Improving care of chronic pain patients with spinal cord stimulator therapy amidst the opioid epidemic. Neurol Sci. 2020;41(10):2703–2710. doi:10.1007/s10072-020-04435-0
24. Stauss T, El Majdoub F, Sayed D, et al. A multicenter real-world review of 10 kHz SCS outcomes for treatment of chronic trunk and/or limb pain. Ann Clin Transl Neurol. 2019;6(3):496–507. doi:10.1002/acn3.720
25. DiBenedetto DJ, Wawrzyniak KM, Schatman ME, Kulich RJ, Finkelman M. 10 kHz spinal cord stimulation: a retrospective analysis of real-world data from a community-based, interdisciplinary pain facility. J Pain Res. 2018;11:2929–2941. doi:10.2147/JPR.S188795
26. Russo M, Van Buyten JP. 10-kHz high-frequency SCS therapy: a clinical summary. Pain Med. 2015;16(5):934–942. doi:10.1111/pme.12617
27. Al-Kaisy A, Van Buyten JP, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15(3):347–354. doi:10.1111/pme.12294
28. Cowan P, Kelly N. Quality of life scale: a measure of function for people with pain; 2003. Available from: https://www.theacpa.org/wp-content/uploads/2017/08/Life_Scale_3.pdf.
29. Kansas Board of Pharmacy. K-TRACS. Available from: https://pharmacy.ks.gov/k-tracs.
30. Al-Kaisy A, Van Buyten JP, Kapural L, et al. 10 kHz spinal cord stimulation for the treatment of non-surgical refractory back pain: subanalysis of pooled data from two prospective studies. Anaesthesia. 2020;75(6):775–784. doi:10.1111/anae.15036
31. Galan V, Scowcroft J, Chang P, et al. 10-kHz spinal cord stimulation treatment for painful diabetic neuropathy: results from post-hoc analysis of the SENZA-PPN study. Pain Manag. 2020;10(5):291–300. doi:10.2217/pmt-2020-0033
32. Hagedorn JM, Layno-Moses A, Sanders DT, Pak DJ, Bailey-Classen A, Sowder T. Overview of HF10 spinal cord stimulation for the treatment of chronic pain and an introduction to the Senza Omnia system. Pain Manag. 2020;10(6):367–376. doi:10.2217/pmt-2020-0047
33. Luecke T, Edgar D, Huse D. 10 kHz spinal cord stimulation for the treatment of chronic back and/or leg pain: summary of clinical studies. SAGE Open Medicine. 2020;8:2050312120951369. doi:10.1177/2050312120951369
34. Tate JL, Stauss T, Li S, Rotte A, Subbaroyan J. A prospective, multi-center, clinical trial of a 10-kHz spinal cord stimulation system in the treatment of chronic pelvic pain. Pain Pract. 2020;2:458.
35. Sayed D, Salmon J, Khan TW, et al. Retrospective analysis of real-world outcomes of 10 kHz SCS in patients with upper limb and neck pain. J Pain Res. 2020;13:1441–1448. doi:10.2147/JPR.S257071
36. Hoelscher C, Riley J, Wu C, Sharan A. Cost-effectiveness data regarding spinal cord stimulation for low back pain. Spine. 2017;42(Suppl 14):S72–S79. doi:10.1097/BRS.0000000000002194
37. Odonkor CA, Orman S, Orhurhu V, Stone ME, Ahmed S. Spinal Cord stimulation vs conventional therapies for the treatment of chronic low back and leg pain: a systematic review of health care resource utilization and outcomes in the last decade. Pain Med. 2019;20(12):2479–2494. doi:10.1093/pm/pnz185
38. Mekhail NA, Aeschbach A, Stanton-Hicks M. Cost benefit analysis of neurostimulation for chronic pain. Clin J Pain. 2004;20(6):462–468. doi:10.1097/00002508-200411000-00012
39. Zucco F, Ciampichini R, Lavano A, et al. Cost-effectiveness and cost-utility analysis of spinal cord stimulation in patients with failed back surgery syndrome: results from the PRECISE Study. Neuromodulation. 2015;18(4):266–276. doi:10.1111/ner.12292
40. Dowell D, Compton WM, Giroir BP. Patient-centered reduction or discontinuation of long-term opioid analgesics: the HHS guide for clinicians. JAMA. 2019;1:1–3.
41. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA. 2016;315(15):1624–1645. doi:10.1001/jama.2016.1464
42. Al-Kaisy A, Van Buyten JP, Carganillo R, et al. 10 kHz SCS therapy for chronic pain, effects on opioid usage: post hoc analysis of data from two prospective studies. Sci Rep. 2019;9(1):11441. doi:10.1038/s41598-019-47792-3
43. Rapcan R, Mlaka J, Venglarcik M, Vinklerova V, Gajdos M, Illes R. High-frequency - spinal cord stimulation. Bratisl Lek Listy. 2015;116(6):354–356.
44. Salmon J. High-frequency spinal cord stimulation at 10 kHz for widespread pain: a retrospective survey of outcomes from combined cervical and thoracic electrode placements. Postgrad Med. 2019;131(3):230–238. doi:10.1080/00325481.2019.1587564
45. Sayed D, Kallewaard JW, Rotte A, Jameson J, Caraway D. Pain relief and improvement in quality of life with 10 kHz SCS therapy: summary of clinical evidence. CNS Neurosci Ther. 2020;26(4):403–415. doi:10.1111/cns.13285
46. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi:10.1001/jama.2011.370
47. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi:10.7326/0003-4819-152-2-201001190-00006
48. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686–691. doi:10.1001/archinternmed.2011.117
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.