Back to Journals » Patient Preference and Adherence » Volume 17
Health-Care Resource Utilization and Treatment Patterns in Men with Erectile Dysfunction and Benign Prostatic Hyperplasia-Associated Lower Urinary Tract Symptoms in the United States: A Retrospective Database Study
Authors Goldstein I, Hassan T, Zou K , Divino V, DeKoven M, Imperato J , Anupindi VR, Li J
Received 26 May 2023
Accepted for publication 4 August 2023
Published 6 September 2023 Volume 2023:17 Pages 2187—2200
DOI https://doi.org/10.2147/PPA.S412969
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Irwin Goldstein,1 Tarek Hassan,2 Kelly Zou,3 Victoria Divino,4 Mitch DeKoven,4 Joseph Imperato,3 Vamshi Ruthwik Anupindi,4 Jim Li3
1Department of Sexual Medicine, Alvarado Hospital, San Diego, CA, USA; 2Department of Urology, Viatris Inc, Canonsburg, PA, USA; 3Global Medical Analytics and Real World Evidence, Viatris Inc, Canonsburg, PA, USA; 4Health Economics/Outcomes Research and Real-World Insights, IQVIA, Falls Church, VA, USA
Correspondence: Tarek Hassan, Global Medical Lead, Department of Urology, Global Medical and Clinical, Viatris Inc, 1000 Mylan Blvd, Canonsburg, PA, 15317, USA, Tel +1 3474432850, Email [email protected]
Objective: To compare health-care resource utilization (HCRU) outcomes in patients with erectile dysfunction (ED) and benign prostatic hyperplasia-associated lower urinary tract symptoms (BPH-LUTS) treated with tadalafil or non-phosphodiesterase-5 inhibitor (PDE5i), adherence to and persistence with tadalafil by dose in the United States (US).
Methods: This was a noninterventional, real-world evidence study of men (aged ≥ 45 years) with ED and BPH-LUTS treated with tadalafil or non-PDE5i. The IQVIA US PharMetrics Plus claims database was used. Outcomes included all-cause and disease-specific HCRU over a 12-month follow-up. Persistence with and adherence to tadalafil were evaluated stratified by dose (10 or 20 mg as needed; 2.5 or 5 mg as once daily [OD]).
Results: The final sample comprised 11,351 tadalafil and 48,722 non-PDE5i patients. For all-cause and disease-specific HCRU, including prescription fills, physician office visits, emergency room visits, laboratory tests, radiology examinations, outpatient surgical services, ancillary services, hospitalizations, mean number of utilizations, and proportions of patients with one or more utilizations, were lower for tadalafil compared with non-PDE5i patients. For all-cause HCRU, proportions of patients with one or more emergency room visits (18.6% vs 21.7%, p< 0.0001) and outpatient surgical visits (63.0% vs 68.8%, p< 0.0001) were significantly lower for tadalafil compared with non-PDE5i patients. For disease-specific HCRU, the proportion with one or more disease-specific physician office visits (55.1% vs 91.4%), laboratory tests (34.8% vs 58.2%), outpatient surgery (24.3% vs 38.9%), or outpatient ancillary services (18.0% vs 29.8%) were significantly lower for tadalafil compared with non-PDE5i patients (all comparisons, p< 0.0001). Mean persistence days (179.8 vs 61.2), proportion persistence (35.8% vs 6.5%), and mean adherence (0.5 vs 0.2) were higher for tadalafil OD doses than as-needed tadalafil doses.
Conclusion: Patients on tadalafil demonstrated less HCRU and higher persistence and adherence (OD versus as-needed tadalafil) than non-PDE5i patients, which demonstrates its benefit in the management of ED and BPH-LUTS in the US.
Plain Language Summary: Erectile dysfunction (ED) and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) conditions are more common in adult men and increase with age. These conditions affect their sexual satisfaction, create mental stress, and impact their interactions with family and associates. This study examined the patterns of health-care resource utilization (HCRU) in 11,351 patients treated with tadalafil versus 48,722 not treated with any PDE5i and compared treatment adherence to and persistence with tadalafil in patients with ED and comorbid BPH-LUTS in the US. All-cause and disease-specific HCRU were lower in patients treated with tadalafil than patients not treated with any PDE5i. The persistence with and adherence to therapy was higher with once-daily dose of tadalafil (2.5 or 5 mg) compared with as-needed dose of tadalafil (10 or 20 mg). Therefore, a lower dose of tadalafil demonstrated benefit in the management of men with ED and BPH-LUTS.
Keywords: medication adherence, persistence, phosphodiesterase-5 inhibitor, real-world evidence, tadalafil
Introduction
Erectile dysfunction (ED) is a condition in which adult men fail to achieve and maintain sufficient erection as part of the multifaceted process of male sexual function.1–6 In the US, ED affects 18 million men aged 20 years or older.7,8 By 2025, it is estimated that the global prevalence of ED will be 322 million.9 Diabetes mellitus, cardiovascular disease, hypertension, obesity, smoking, depression, and lower urinary tract symptoms (LUTS) have an impact on sexuality and are major risk factors for development of ED.5,8,10,11
Patients with LUTS are associated with a wide range of diagnoses, one of which is benign prostatic hyperplasia (BPH). Men with BPH-LUTS may have an increased smooth-muscle tone and reduced level of nitric oxide, resulting in ED.11–13 The LUTS are associated with urinary obstruction caused by benign enlargement of the prostate. Epidemiological studies have demonstrated a strong correlation between ED and LUTS in men with BPH.14
BPH is another most common disease in aging men.15 The prevalence of BPH increases with age from 14.8% to 36.8% in males aged 40–80 years and above.13 The prevalence of moderate–severe LUTS increases from 22% to 45% in men between 50 and 80 years of age.1 Men with ED are twice as likely to suffer from BPH-LUTS than men without ED. While both ED and BPH-LUTS are highly correlated with age and other comorbidities, the relationship between ED and BPH-LUTS remains significant even after controlling for these factors, suggesting that BPH-LUTS is an independent risk factor for sexual dysfunction in older men.16
Pharmacotherapies for BPH-LUTS include both α1-adrenoreceptor antagonists (α-blockers), and 5α reductase inhibitors (5α-RIs). However, a significant number of men still require invasive therapy due to inadequate symptom relief, unacceptable side effects, or complications of the disease.17 Currently, PDE5Is such as sildenafil, tadalafil or vardenafil are considered the first-line therapeutic agents for the management of ED due to their established efficacy and favorable safety profile.8,14,18
The efficacy and safety of tadalafil once daily in the treatment of patients with ED and/or the treatment of signs and symptoms of BPH was established in several pivotal randomized double-blind placebo-controlled studies.3 Tadalafil was approved by the US Food and Drug Administration (FDA) in 2003 to treat ED.3 Then, the FDA expanded the approved indications to include BPH, either alone or in combination with ED.19 For the treatment of BPH-LUTS, there are various therapies and relevant clinical practice guidelines that help and guide physicians in making better disease-management decisions. However, evidence on the comparisons of health-related outcomes between patients treated with tadalafil and those who not receiving any PDE5i therapy in a real-world setting are limited. This study aimed to compare the patterns of health-care resource utilization (HCRU) in patients treated with tadalafil or not treated with any PDE5i and to compare treatment adherence to and persistence with tadalafil, stratified by dose, in patients with ED and comorbid BPH-LUTS in the US.
Methods
Data Source
This was a cross-sectional real-world evidence (RWE) study based on analysis of data obtained from IQVIA US PharMetrics Plus claims database.20 A retrospective database analysis was conducted among ED and BPH-LUTS patients treated with tadalafil or not treated with any PDE5i in the US. Data were collected from January 1, 2014 to October 31, 2019, and all available data from eligible subjects were used. The study did not involve any primary data collection.
The PharMetrics Plus database is a patient-centric, closed-claims database of fully adjudicated pharmacy, hospital, and medical claims anonymized at the patient level that captures the complete patient journey for all services billed to and covered by the patient’s health plan. The study subjects are from commercial plans with medical and pharmacy benefits with a small group of commercial Medicare and commercial Medicaid patients. Subject informed consent or institutional review board approval was not obtained. Since this study used only deidentified patient records and did not involve the collection, use, or transmittal of individually identifiable data, approval to conduct it was not required.
Study Design
The study period included the preindex period, the index date, and postindex period. Patients had a fixed 12-month preindex or baseline period and a fixed 12-month postindex or follow-up period. The preindex period was the 12 months immediately before the index date. This period was between January 1, 2014 and the index date (first treatment claim or second diagnosis of ED and BPH-LUTS). The index date was the first pharmacy claim of tadalafil or the date of second claim of an ED and BPH-LUTS diagnosis, during the selection window. The selection window was January 1, 2015 to October 31, 2018. The postindex follow-up period was from the index date to the most recent claims data available, which was until October 31, 2019 (Figure 1). Sample-size calculation was not performed, since this was a retrospective observational study using all available retrospective data.
Inclusion Criteria
Inclusion criteria were: ≥45 years of age, one or more pharmacy claims for tadalafil, with one or more confirmatory claims of diagnosis for ED and BPH-LUTS (with one or more treatment claims for an α1-blocker, 5α-RIs. or combination) for both tadalafil and non-PDE5i treatment groups during the preindex period or on the index date (day −360 through day 1); continuous enrollment (CE) ≥12 months before and after the index date (pre- and postindex periods); and CE ≥12 months after the index date (postindex period).
Exclusion Criteria
Exclusion criteria were: one or more claim of any PDE5i indicated for pulmonary arterial hypertension (PAH); one or more claim for any nonindex PDE5i indicated for ED (for tadalafil group); one or more claim for the index PDE5i in the 1-year preindex period (for tadalafil group); one or more claim for any PDE5i indicated for ED (for non-PDE5i treatment group); one or more diagnoses of PAH; incomplete data or data-quality issues (invalid or missing year of birth, region or health-plan enrollment dates, or with Medicare cost coverage or State Children’s Health Insurance Program) or ≥65 years at the index date and not covered by Medicare Risk).
Outcome Measures
This study analyzed the proportion of patients who utilized the various health-care resources, the proportion who continued their tadalafil, and the proportion who discontinued tadalafil for the treatment of ED and BPH-LUTS. Demographic characteristics were measured as of the patient’s index date, while clinical characteristics were measured over the 12-month preindex period. Demographic variables were age and age-group, geographic region, payer type, health-plan type and index year. Clinical variables were physician/health-care provider specialty with and without office visit, Charlson Comorbidity Index (CCI), comorbidities of interest, medications of interest, and procedures of interest.
Outcomes in the postindex period were all-cause and disease-specific (ED- and BPH-LUTS-related) HCRU and persistence with and adherence to the index therapy. Disease-specific HCRU was determined based on claims with diagnosis codes of ED, BPH-LUTS and/or therapy used in the treatment of ED or BPH-LUTS (PDE5i, α1-blocker, 5α-RIs, or their combination). HCRU was expressed as both the proportion of patients with such utilization and per-patient mean, SD, and median for utilization.
Persistence on the index therapy was calculated based on the time (in consecutive days) from treatment initiation until discontinuation or end of the 12-month follow-up period, whichever occurred first and was reported categorically (percentage persistent over the 12-month follow-up period) and as a continuous variable (persistence days, mean, median, and SD). Discontinuation was defined as a gap in index therapy of 1.5 times the days’ supply of the prior fill. The date of discontinuation was defined as the last day of supply prior to the gap. Adherence to the index therapy was measured and reported as proportion of days covered (PDC) over the 12-month postindex period (mean PDC as a point estimate with 90% CI). PDC was calculated as the number of days with drug on hand during the 360-day period divided by 360.
Statistical Methods
All statistical analyses were performed using SAS version 9.4 (SAS, Cary, NC). Missing values for categorical and descriptive outcomes are reported as a separate category for categorical variables. Imputation was applied, using the mean of the days’ supply of claims for the same national drug code (NDC) with days of supply >0. For categorical measures, data include the frequency and percentage of total study subjects observed in each category. For continuous and count variables, findings are presented as means, SD, and medians. For baseline characteristics, postindex HCRU, persistence, and adherence, two-sample comparisons between tadalafil and non-PDE5i group were conducted using two-sample t-test (mean) and Wilcoxon rank-sum test for continuous variables and χ2 test or Fisher’s exact test for categorical variables, as appropriate. Two-sided p-values are reported.
Results
A total of 273,857 tadalafil patients and 187,182 non-PDE5i therapy patients were identified during the selection window. The final eligible sample comprised 11,351 (4.1%) tadalafil patients and 48,722 (26.0%) non-PDE5i therapy patients (Figure 1).
Demographic Characteristics
Patients in both the groups showed variation in region and slight variation in other demographic characteristics (Table 1). Mean age was similar for tadalafil and non-PDE5i therapy groups (mean 57.1 and 57.9 years, respectively). Most patients were in the southern US: 45.3% for tadalafil and 52.0% for non-PDE5i therapy. Both tadalafil patients (58.7%) and non-PDE5i therapy patients (58.7%) had similar commercial insurance payer type. Most patients had a preferred provider organization (PPO) type of health plan, but this was higher for tadalafil than non-PDE5i therapy (87.4% vs 82.4%, p<0.0001). The index year was most commonly 2015 for both tadalafil and non-PDE5i therapy (36.5% vs 35.2%) patients, and few patients had an index year of 2018 (10.3% vs 14.0%, respectively).
 |
Table 1 Baseline patient demographic characteristics |
Clinical Characteristics
The baseline clinical characteristics showed that a primary care physician was most associated with the office visit on or closest (prior) to the index date for the tadalafil (42.2%) patients compared with non-PDE5i (30.7%) patients. A urologist was associated with the index date for 54.1% of the non-PDE5i patients. Patients had a mean CCI score of 1.0 and 1.1 for tadalafil and non-PDE5i, respectively, and the proportion with a CCI score of 0 ranged from 50.7% (non-PDE5i treatment) to 55.0% (tadalafil). Common baseline comorbidities in tadalafil patients and non-PDE5i patients included hypertension (51.4% and 55.6%), dyslipidemia (53.2% and 54.0%), osteoarthritis (22.8% and 22.3%), sleep disorders (22.2% and 20.6%), and diabetes (18.1% and 22.9%). A majority of tadalafil patients used an α1 blocker (most commonly tamsulosin) in the preindex (64.2%) period compared to 39.3% of non-PDE5i treatment patients. Measurement of postvoiding residual urine was more common among tadalafil (22.6%) patients compared with non-PDE5i patients (15.1%). In the preindex period, the proportion with one or more hospitalization (10.8% vs 10.4%) was infrequent, but the proportion with one or more emergency room visit was less (19.5% and 22.8%, p<0.0001) for tadalafil patients compared with non-PDE5i patients. Mean physician office visits were 11.5 and 11.0 for tadalafil and non-PDE5i patients (Table 2).
 |
Table 2 Baseline clinical characteristics |
All-Cause HCRU over the 1-Year PostIndex
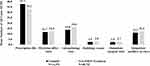
Mean numbers of many all-cause HCRU were lower for tadalafil patients than non-PDE5i patients, including physician office visits (11.7 vs 12.4, p<0.0001), laboratory/pathology tests (13.8 vs 16.4, p<0.0001), radiology exams (2.5 vs 2.9, p<0.0001), surgical services (2.4 vs 2.7, p<0.0001), and ancillary services (11.1 vs 12.3, p<0.0001; Figure 2). Mean emergency room visits (0.3 vs 0.4, p<0.0001) and hospitalizations (1.4 vs 1.5, p=0.1118) were low for both treatment groups.
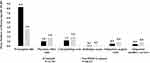
Similarly, the proportions of patients with one or more all-cause HCRU were also lower for tadalafil patients than non-PDE5i patients for many HCRU services, including ER visits (18.6% vs 21.7%, p<0.0001), laboratory/pathology tests (91.6% vs 95.9%, p<0.0001), radiology exams (59.3% vs 63.4%, p<0.0001), outpatient surgical visits (63.0% vs 68.8%, p<0.0001), and hospitalizations (7.5 vs 9.3%, p<0.0001) (Figure 3). For the first hospitalization, the most common primary discharge diagnosis (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] three-digit level) was M17 — osteoarthritis of the knee (7.4% vs 8.4%) followed by C61 — malignant neoplasm of prostate (6.6% vs 5.5%) in the tadalafil and non-PDE5i groups, respectively.
Disease-Specific HCRU over the 1-Year Postindex
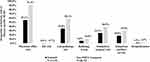
The mean number of disease-specific prescription fills was higher for tadalafil patients compared with non-PDE5i patients (8.3 vs 3.6, p<0.0001). The mean numbers of other disease-specific HCRUs were small for both treatment groups, but the differences were still statistically significant (p<0.0001), due to the large samples (Figure 4).
The proportion of patients with one or more disease-specific physician office visits (55.1% vs 91.4%, p<0.0001), proportion with one or more disease-specific laboratory/pathology tests (34.8% vs 58.2%, p<0.0001), proportion with one or more disease-specific radiology exams (5.8% vs 10.8%, p<0.0001), proportion with one or more disease-specific outpatient surgeries (24.3% vs 38.9%, p<0.0001), and proportion with one or more disease-specific outpatient ancillary services (18.0% vs 29.8%, p<0.0001) were significantly lower for tadalafil patients than non-PDE5i patients. The proportions of patients with one or more disease-specific emergency room visits (0.6% vs 0.7%, p=0.04) and one or more disease-specific hospitalizations (1.5% vs 2.5%, p<0.0001) were small (Figure 5). For the first hospitalization, the most common primary discharge diagnosis (ICD-10-CM three-digit level) was C61 (malignant neoplasm of prostate) in the tadalafil and non-PDE5i (32% vs 30%) groups, respectively.
Persistence and Adherence over the 1-Year Postindex
Treatment patterns were also descriptively assessed for the tadalafil group, stratified based on dose prescribed over the 1-year follow-up (Table 3). During the follow-up, 67.3% of tadalafil patients (7636/11,351) were identified with use of tadalafil 2.5 or 5 mg only, while 28.9% of tadalafil patients (3286/11,351) were identified with use of tadalafil 10 or 20 mg only (corresponding to prescribed indication for ED). Many of the patients experienced discontinuation over the 1-year postindex, but this was higher for tadalafil patients treated with 10 or 20 mg only than patients treated with 2.5 or 5 mg only (93.5% vs 64.2%).
 |
Table 3 Persistence and adherence over the 1-year postindex period by tadalafil dose |
Persistence days were fewer for patients treated with 10 or 20 mg (61.2 vs 179.8 days). For PDC over the 1-year follow-up, the mean point estimate (90% CI) was lower for patients treated with 10 or 20 mg (0.20 [0.198–0.211]) than patients treated with 2.5 or 5 mg (0.50 [0.495–0.508]). Tadalafil patients treated with 10 or 20 mg, compared to patients treated with 2.5 or 5 mg, also had fewer mean tadalafil prescription claims (3.0 vs 5.8) and fewer mean total days of tadalafil supply over the 1-year follow-up (25 vs 33). A lower proportion of patients treated with 10 or 20 mg had more than one tadalafil prescription claim (57.0% vs 79.8%) compared to patients treated with 2.5 or 5 mg, and of these patients, the former had a longer time between claims (100.8 vs 64.3 days).
Discussion
This RWE study comprehensively evaluated all-cause and disease-specific HCRU of patients with ED and comorbid BPH-LUTS treated with tadalafil or not treated with any PDE5i. A previous study by Ilo et al evaluated HCRU in terms of general practitioner visits using the Clinical Practice Research Datalink (CPRD) among incident ED, ED patients with a prior BPH diagnosis (BPH-ED), incident LUTS secondary to BPH (referred to as BPH), and BPH patients with a prior ED diagnosis (ED-BPH).21 It was observed that more than 89% of patients across all study cohorts visited the general practitioner in the 12 months after diagnosis, and the median number of consultations per patient ranged from three (ED only) to seven (ED-BPH).
A review of the literature on PDE5i treatment patterns primarily yielded results among patients with ED only, highlighting a gap in knowledge among patients with ED and comorbid BPH-LUTS. In the study of Ilo et al, approximately 25% of the patients who had initiated treatment with tadalafil among both incident ED and BPH-ED cohorts, and only 15% of the patients persisted on treatment within 1 year of treatment initiation.21
A meta-analysis of 22 observational studies of PDE5is among patients with ED found a mean discontinuation rate of almost 50% after 1 year.22 Studies that evaluated persistence and adherence at 6 months showed higher persistence than our current study, but this was related to use of different definitions. In a prospective, noninterventional, observational study among African and Middle Eastern men with ED, 68.8% of tadalafil patients were persistent and 59.6% adherent at 6 months.23 The definitions differed from the current study due to the observational nature of the study and patient-reported outcomes were collected, which made it possible to define persistence as the use of one or more dose during the prior 4 weeks while adherence was defined as compliance with dosing instructions during the most recent medication. Such definitions cannot be implemented using a claims database. Similarly, another observational study among Brazilian men with ED that used the same definitions as the prior study found overall persistence to be 69.2% and adherence 70.2%, respectively, with both numerically higher for tadalafil patients.24
Further research is needed to evaluate the persistence and adherence among patients with ED and comorbid BPH-LUTS treated with PDE5i.
Limitations
This study has several limitations inherent to RWE studies involving claims data, such as missing data and study-design limitations. Primarily, results from retrospective studies must be interpreted with caution and in the context of results from similar studies, because they can only establish associations and not causal relationships. Administrative claims data do not provide as much clinical detail as medical records as they are primarily collected for the purposes of payment. Therefore, there is a potential for miscoding or misclassification. The assessment of persistence with and adherence to tadalafil based on observed prescription fills is somewhat limited, given that per label, tadalafil can be used either as needed or daily, while non-PDE5i therapy is to be used daily for most patients. Finally, the results may not be generalizable to a broader population of individuals with comorbid ED and BPH-LUTS in the US or globally.
Conclusion
Disease-specific HCRU was lower in patients treated with tadalafil than patients not treated with any PDE5i. Variation in persistence with and adherence to therapy was observed among tadalafil patients based on prescribed dose. Persistence and adherence were higher in patients treated once daily with tadalafil (2.5 or 5 mg) than patients treated with as-needed doses of tadalafil (10 or 20 mg), which demonstrates its benefit in the management of men with ED and BPH-LUTS.
Data Sharing
Data and materials are not available to be shared publicly.
Ethics Approval and Consent to Participate
Because this study was conducted using only deidentified data and did not involve use or transmittal of individually identifiable data, institutional review board approval was not necessary. Analysis of commercially available deidentified secondary data sources is considered exempt from the requirements for “human subjects research” in the US.
Consent for Publication
Consent for publication was not required or collected due to the retrospective claims database, given the data were already deidentified due to the retrospective nature of the study.
Acknowledgments
Medical writing assistance and editorial support was provided by Shantha Kumar and Aswin Kumar from Viatris. The abstract titled “Health-Care Resource Utilization (HCRU) and Treatment Patterns in Men with Erectile Dysfunction (ED) and Benign Prostatic Hyperplasia-Associated Lower Urinary Tract Symptoms (BPH-LUTS) in the United States (US): A Retrospective Database Study” was accepted as an unmoderated poster at the 22nd World Meeting of the ISSM (WMSM Congress), which was held virtually from November 19 to 21, 2021.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, interpretation, or all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by Pfizer and Upjohn, a legacy division of Pfizer, merged with Mylan to form Viatris.
Disclosure
IG reports nonfinancial support from Viatris during the conduct of the study and personal fees from Coloplast outside the submitted work. TH, KZ, and JL are employees and own stocks of Viatris. TH, KZ and JL are former employees and own stocks from Pfizer. JI is a former employee of Viatris and owns stocks. MD, VD, and VRA are paid consultants for Viatris and other pharmaceutical companies. They are employees of IQVIA, which received funding from Upjohn — A legacy Pfizer Division — for this study. The authors report no other conflicts of interest in this work.
References
1. McVary KT, Monnig W, Camps JL, et al. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol. 2007;177(3):1071–1077. doi:10.1016/j.juro.2006.10.055
2. Anonymous. NIH consensus conference. Impotence. NIH consensus development panel on impotence. JAMA. 1993;270(1):83–90. doi:10.1001/jama.1993.03510010089036
3. Eli Lilly and Company. Cialis [Prescribing Information]. Indianapolis, IN: Eli Lilly and Company; 2018.
4. Laumann EO, West S, Glasser D, et al. Prevalence and correlates of erectile dysfunction by race and ethnicity among men aged 40 or older in the United States: from the male attitudes regarding sexual health survey. J Sex Med. 2007;4(1):57–65. doi:10.1111/j.1743-6109.2006.00340.x
5. Marumo K, Nakashima J, Murai M. Age-related prevalence of erectile dysfunction in Japan: assessment by the international index of erectile function. Int J Urol. 2001;8(2):53–59. doi:10.1046/j.1442-2042.2001.00258.x
6. Corona G, Lee DM, Forti G, et al. Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS). J Sex Med. 2010;7(4 Pt 1):1362–1380. doi:10.1111/j.1743-6109.2009.01601.x
7. Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120(2):151–157. doi:10.1016/j.amjmed.2006.06.010
8. Mulhall JP, Luo X, Zou KH, Stecher V, Galaznik A. Relationship between age and erectile dysfunction diagnosis or treatment using real-world observational data in the USA. Int J Clin Pract. 2016;70:1012–1018. doi:10.1111/ijcp.12908
9. Shiferaw WS, Akalu TY, Aynalem YA. Prevalence of erectile dysfunction in patients with diabetes mellitus and its association with body mass index and glycated hemoglobin in Africa: a systematic review and meta-analysis. Int J Endocrinol. 2020;2020:1–10. doi:10.1155/2020/5148370
10. Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol. 1994;151(1):54–61. doi:10.1016/S0022-5347(17)34871-1
11. Wylie K, Kenney G. Sexual dysfunction and the ageing male. Maturitas. 2010;65(1):23–27. doi:10.1016/j.maturitas.2009.10.018
12. Camacho ME, Reyes-Ortiz CA. Sexual dysfunction in the elderly: age or disease? Int J Impot Res. 2005;17(1):S52–6. doi:10.1038/sj.ijir.3901429
13. Lee SWH, Chan EMC, Lai YK. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta-analysis. Sci Rep. 2017;7(7984):1–10. doi:10.1038/s41598-016-0028-x
14. Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers. 2017;2:16003. doi:10.1038/nrdp.2016.3
15. Roehrborn CG, McConnell JD. Etiology, pathophysiology, epidemiology, and natural history of benign prostatic hyperplasia. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Campbell’s Urology. Philadelphia, PA: WB Saunders Company; 2002:1297–1330.
16. Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol. 2003;44(6):637–649. doi:10.1016/j.eururo.2003.08.015
17. Lee C, Cockett A, Cussenot K, et al. Regulation of prostate growth benign prostatic hyperplasia.
18. Kirby MG, Schnetzler G, Zou KH, Symonds T. Prevalence and detection rate of underlying disease in men with erectile dysfunction receiving phosphodiesterase type 5 inhibitors in the United Kingdom: a retrospective database study. Int J Clin Pract. 2011;65(7):797–806. doi:10.1111/j.1742-1241.2011.02693.x
19. U.S. Food and Drug Administration. Cialis (tadalafil) tablets, 5 mg [Internet]. U.S. FDA (U.S.): Cialis supplemental new drug application; 2011 [updated 2011 Oct; cited 2023 Aug 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/021368Orig1s020.pdf.
20. IQVIA. IQVIA PharMetrics® Plus Database. United States: IQVIA Inc; 2022.
21. Ilo D, Raluy-Callado M, Graham-Clarke P, et al. Patient characteristics, and treatment patterns for patients with benign prostatic hyperplasia, erectile dysfunction or co-occurring benign prostatic hyperplasia and erectile dysfunction in general practices in the UK: a retrospective observational study. Int J Clin Pract. 2015;69(8):853–862. doi:10.1111/ijcp.12657
22. Corona G, Rastrelli G, Burri A, et al. First-generation phosphodiesterase type 5 inhibitors dropout: a comprehensive review and meta-analysis. Andrology. 2016;4(6):1002–1009. doi:10.1111/andr.12255
23. El-Meliegy A, Rabah D, Al-Mitwalli K, et al. A 6-month, prospective, observational study of PDE5 inhibitor treatment persistence and adherence in Middle Eastern and North African men with erectile dysfunction. Curr Med Res Opin. 2013;29(6):707–717. doi:10.1185/03007995.2013.791263
24. Cairoli C, Reyes LA, Henneges C, et al. PDE5 inhibitor treatment persistence and adherence in Brazilian men: post-hoc analyses from a 6-month, prospective, observational study. Int Braz J Urol. 2014;40(3):390–399. doi:10.1590/S1677-5538.IBJU.2014.03.14
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.