Back to Journals » Clinical Ophthalmology » Volume 17
Head-to-Head Comparison of Intermediate Vision of Two Monofocal Intraocular Lenses
Authors Micheletti JM, Duncan NB, Hall B
Received 1 November 2023
Accepted for publication 14 December 2023
Published 21 December 2023 Volume 2023:17 Pages 3983—3990
DOI https://doi.org/10.2147/OPTH.S444696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
J Morgan Micheletti,1 Nicole B Duncan,2 Brad Hall3
1Berkeley Eye Center, Houston, TX, USA; 2Department of Ophthalmology and Visual Sciences, University of Wisconsin-Madison School of Medicine and Public Health, Madison, WI, USA; 3Sengi, Penniac, NB, Canada
Correspondence: J Morgan Micheletti, Berkeley Eye Center 3100 Weslayan, Suite 400, Houston, TX, 77027, USA, Tel +1-713-526-1600, Email [email protected]
Purpose: To compare intermediate visual outcomes in patients previously implanted with bilateral Clareon monofocal IOLs versus bilateral Eyhance IOLs.
Methods: This was a non-interventional, single-center, examiner-masked, comparative study. Participants were cataract patients presenting at least 3 months after uncomplicated, bilateral implantation of either Clareon or Eyhance non-toric and toric IOLs. Outcomes measures included binocular distance-corrected intermediate visual acuity (DCIVA), binocular corrected distance visual acuity (CDVA), binocular best-corrected defocus curve, postoperative mean residual spherical equivalent (MRSE), and residual astigmatism.
Results: A total of 620 eyes of 310 subjects (155 subjects per group) were evaluated. The mean difference in DCIVA was 0.05 logMAR between the Eyhance and Clareon IOLs which was significant (p < 0.01), but within the 0.1 logMAR non-inferiority margin. Mean CDVA of the Clareon group was 0.01 ± 0.03 logMAR compared to 0.02 ± 0.03 logMAR of the Eyhance Group (p > 0.05). Defocus curves from +1.0 D to – 3.0 D were not clinically nor statistically different between the Clareon and Eyhance groups (p > 0.05).
Conclusion: The results of this study show that bilateral implantation of Clareon monofocal IOLs and Eyhance monofocal IOLs lead to similar distance and intermediate visual outcomes.
Plain Language Summary: The natural lens inside the eye can become opaque. Cataract surgery removes this lens and replaces it with an artificial intraocular lens (IOL). The most often used type of intraocular lenses are monofocal lenses. These lenses provide clear vision for viewing distant objects. Increasing attention is also being paid to how these monofocal lenses perform for viewing objects at intermediate distances (such as using a digital device). The purpose of this study was to compare visual outcomes in patients with bilateral implantation of two different types of monofocal lenses, one of which was specifically designed to increase depth of focus. The results of this study suggest that implantation with these monofocal IOLs led to similar visual outcomes and that both monofocal IOLs may provide a similar potential to improve vision at the intermediate range.
Keywords: Clareon, Eyhance, monofocal, intermediate visual acuity
Introduction
The most widely utilized type of intraocular lens (IOL) in cataract surgery is a monofocal implant. While this type of IOL primarily improves vision at one distance based on refractive targeting, most patients require spectacles to obtain optimal intermediate and near vision. There is a growing desire to enhance the quality of spectacle-independent intermediate vision because many daily and meaningful activities, including grocery shopping, preparing meals, computer work, and using a tablet, are performed at this distance.1 Patients have also reported that freedom from spectacles while carrying out these activities can improve their quality of life.2
High levels of spectacle independence can be achieved with presbyopia-correcting IOLs, such as trifocals or extended depth of focus IOLs.3 However, these premium lenses come at a higher out-of-pocket cost to the patient and can involve undesirable photic phenomena.4–6 Importantly, monofocal IOLs may also achieve a slightly extended depth of focus, depending on their specific optical design,7,8 although these outcomes have not been a historically meaningful subject of discussion.
A prospective experimental study recently demonstrated that improved intermediate vision can be obtained by modifying the optic design of traditional monofocal IOLs.9 One Food and Drug Administration (FDA) approved monofocal IOL with modifications from the traditional design is the TECNIS Eyhance ICB00 (Johnson & Johnson Vision) IOL. Compared to its traditional monofocal analog, the TECNIS 1-piece IOL ZCB00, the Eyhance IOL has a modified aspheric anterior surface that facilitates a steady increase in lens power within the central 1 mm diameter of the IOL optic.10 This change in optical design intends to facilitate similar distance visual acuity and enhanced intermediate range of vision compared to ZCB00.10–13 Since the Eyhance was approved as a Level A Modification of the ZCB00 IOL, no clinical data was required to demonstrate a clinically meaningful extension of depth of focus. Therefore, no FDA registration study was performed to support the inference that this modified optic design provides a clinically significant extended range of vision. However, a European multicenter study reported that patients with bilateral Eyhance IOLs may experience improved intermediate vision compared to the ZCB00 monofocal IOL.10 It has also been reported that bilateral implantation with other monofocal IOLs can provide some intermediate vision.14,15 There is minimal data directly comparing visual outcomes between the Eyhance and newer monofocal IOL designs from different companies. The purpose of this study was to compare visual outcomes in patients with bilateral Clareon IOLs versus bilateral Eyhance IOLs.
Methods
This non-interventional prospective, single-center, bilateral, non-randomized, open-label, examiner-masked, two-arm comparative study was performed with the approval of an institutional review board (WIRB-Copernicus Group IRB). The study was conducted at a private practice, therefore an independent IRB was used. All subjects provided written informed consent before participation in the study. The study was conducted following the tenets of the 1964 Declaration of Helsinki, the appropriate US Health Insurance Portability and Accountability Act (HIPPA) regulations were followed, and it was registered at ClinicalTrials.gov (NCT05226884). Data are not available for sharing.
The study included subjects who previously underwent uncomplicated age-related cataract surgery with bilateral implantation of either Clareon monofocal IOLs or Eyhance monofocal IOLs at least three months prior to enrolment. Subjects who underwent surgery between March 2021 and September 2022 were eligible for participation, and recruitment was consecutive. Additional inclusion criteria were clear intraocular media (ie, no posterior capsular opacification) and a postoperative corrected distance visual acuity (CDVA) for each eye of logMAR 0.1 (20/25) or better after cataract removal. If an yttrium aluminum garnet (YAG) capsulotomy was performed to treat posterior capsular opacification (PCO) after surgery, a minimum of two weeks must have passed after the procedure to participate in the study. Subjects affected by ocular comorbidities, including glaucoma, strabismus, corneal dystrophy, corneal irregularities, retinal disease, optic nerve atrophy, or any degenerative visual disorder were excluded. Other exclusion criteria were previous anterior segment (other than cataract surgery) or posterior segment surgery, history of retinal detachment, a preexisting condition that may affect cataract removal (such as pseudoexfoliation syndrome, posterior polar cataract, or a prescription for Flomax), and any clinically significant acute or chronic illness that may have confounded the results of this investigation (such as diabetes, atopic disease, connective tissue disease, or immunocompromised state). Subjects with intraoperative complications, such as a capsular tear, or inadequate pupillary dilation, were excluded from the analysis.
The TECNIS Eyhance ICB00 IOL (Johnson & Johnson Vision) is a single-piece, hydrophobic acrylic, foldable monofocal lens with an overall length of 13.0 mm and an optic diameter of 6.0 mm. The anterior surface of the innermost 2.0 mm diameter is aspheric, designed to sustain a modest increase in central lens power and promote a slightly extended depth of focus.16 Both toric (DIUxxx) and non-toric (DIB00) versions of the Eyhance monofocal were included in the study.
The Clareon monofocal IOL (Alcon Laboratories, Inc.) is a one-piece, hydrophobic acrylic, foldable monofocal lens spanning 13.0 mm in overall length and 6.0 mm in optic diameter, with an aspheric anterior surface and a spherical posterior surface.17 Both models of the Clareon monofocal, the CCA0T0 (UV-blocking) and the CNA0T0 (blue light filtering chromophore), were included in the study as they have identical optical designs. Toric versions of the Clareon lens were not available at the time of implantation, therefore there were none included in this study.
Baseline preoperative characteristics were retrieved by chart review. These included anterior chamber depth (ACD), axial length (AL), pupil size, corneal astigmatism, keratometry readings in the vertical (K1) and horizontal (K2) corneal meridians, spherical equivalent (SE), IOL power, target, and predicted post-operative spherical equivalent, based off IOL calculations.
All cases included in the study were performed by four surgeons at a single private practice institution in Houston, Texas. The choice of IOL was decided before surgery after a discussion between the patient and the surgeon. Each surgeon performed their standard cataract extraction technique, phacoemulsification, and implanted one of the previously described IOLs in the capsular bag. The IOL power selection and outcome targets included bilateral emmetropia, mini-monovision, and monovision, and were individualized per patient based on a discussion with the surgeon. The second eye was implanted two weeks after the first eye for each patient.
All study measurements were taken at a single visit at least three months after cataract surgery had been performed to implant the same IOL in both eyes. The subject population for this study included patients who were not targeted for emmetropia in both groups (target ranged from −2.50 to 0.00 D). Therefore, visual acuities were collected with distance correction in place to eliminate residual postoperative refractive error as a confounding factor. Early Treatment Diabetic Retinopathy Study (ETDRS) charts were used to measure visual acuities based on cumulative letters, then subsequently converted to logMAR for statistical analysis. Distance and intermediate visual acuities were tested at 4m and 66 cm, respectively. Binocular defocus curve was done in −0.5 D increments over a range from +1.0 D to −3.0 D. No product complaints, adverse events, nor serious adverse events were reported to the principal investigators during this study.
The primary clinical outcome measure was the comparison of binocular distance-corrected intermediate visual acuity (DCIVA) in the Eyhance group to the binocular DCIVA in the Clareon group. Secondary outcome measures included the comparison of corrected distance visual acuity (CDVA), binocular distance corrected defocus curves, postoperative manifest refraction spherical equivalent (MRSE), and postoperative residual astigmatism (PRA) between the Eyhance group and the Clareon group.
Sample size calculations indicated that 155 subjects per arm (for a total of 310 subjects) were required to demonstrate non-inferiority with a 0.1 logMAR non-inferiority margin, assuming that the difference in binocular DCIVA between groups was 0.05 ± 0.15 logMAR, with 90% power and Type I error probability (alpha) of 0.05. Statistical analysis was performed using R (version 4.2.1; The R Foundation for Statistical Computing, Vienna, Austria). All analyses included data from all subjects who received either Clareon or Eyhance IOLs with no data imputation. Normality was assumed if skewness was ± 2 and kurtosis was ± 7. Welch Two Sample t-test was used for parametric data and the Wilcoxon rank-sum test was used for non-parametric data. For all statistical analyses, p < 0.05 was considered significant.
Results
A total of 315 cataract subjects were screened with five excluded because they did not meet the inclusion criteria. After screening, 310 subjects (620 eyes) were enrolled in the study, with 155 subjects per arm. Each subject had undergone implantation of the same IOL, either Clareon or Eyhance, bilaterally. Patient demographics and baseline characteristics were comparable between the two groups (Table 1).
 |
Table 1 Patient Demographics and Retrospective Pre and Post-Operative Data |
The refractive outcomes are also summarized in Table 1. Mean MRSE was −0.25 ± 0.49 in the Clareon group and −0.22 ± 0.71 in the Eyhance group (p = 0.01). Mean postoperative residual astigmatism was 0.36 ± 0.36 in the Eyhance group and 0.43 ± 0.44 in the Clareon group (p < 0.001). No subjects received a Clareon toric IOL, while 47.4% of eyes in the Eyhance group received a toric IOL. This was because the toric versions of the Clareon lens were not available at the time of implantation.
Binocular distance-corrected visual acuity at intermediate (DCIVA) and distance (CDVA) from both the Eyhance group (n = 155) and the Clareon group (n = 155) are summarized in Table 2. In the Clareon group, mean DCIVA was 0.24 ± 0.11 (n =155), which approximates a 2.5 letter (0.05 logMAR) difference from mean DCIVA in the Eyhance group (0.19 ± 0.11 logMAR). The 95% confidence interval was 0.02 to 0.07, and non-inferiority was confirmed for the Clareon group compared to the Eyhance group, based on a margin of 0.10 logMAR. Mean postoperative binocular CDVA was 0.01 ± 0.02 logMAR for the Clareon group and 0.02 ± 0.03 logMAR for the Eyhance group. The 95% confidence interval was −0.01 to 0.00, and non-inferiority was confirmed for the Clareon group compared to the Eyhance group, based on a margin of 0.10 logMAR. Figures 1 and 2 summarize the cumulative DCIVA and CDVA respectively. In the Clareon group, 43% of subjects had DCIVA of 20/30 or better, compared to 59% in the Eyhance group. All subjects in both groups reached a CDVA of 20/25 or better. Figure 3 summarizes the binocular distance-corrected defocus curve for the Clareon group and the Eyhance group. The binocular distance-corrected defocus curves yielded similar visual acuities (logMAR) between groups in the range from −3.00 D to +1.00 D defocus levels (p > 0.05).
 |
Table 2 Postoperative Visual Acuities (Clareon n = 155 Subjects; Eyhance n = 155 Subjects) |
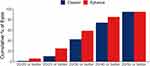 |
Figure 1 Cumulative postoperative binocular distance corrected visual acuity (DCIVA) between groups. |
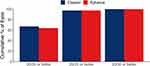 |
Figure 2 Cumulative postoperative binocular corrected distance visual acuity (CDVA) between groups. |
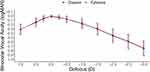 |
Figure 3 Binocular defocus curve (distance corrected) at least 3 months postoperatively for Clareon and Eyhance. D = diopters; logMAR = log of minimum angle of resolution. |
As an exploratory endpoint, subjects had DCIVA and CDVA retested in the Clareon group with an added −0.25 D offset. With the offset, mean DCIVA was 0.20 ± 0.10 logMAR for subjects, compared to 0.24 ± 0.11 logMAR for the Clareon group with no offset (p < 0.001). With the offset, mean CDVA was 0.03 ± 0.05 logMAR, compared to 0.01 ± 0.02 logMAR for the Clareon group with no offset (p < 0.001).
Discussion
Monofocal lenses remain the most common type of IOL to be implanted during cataract surgery, but there are rising expectations from both patients and surgeons to expand the range of spectacle-independent vision postoperatively. Given the ubiquitous usage of handheld digital devices, intermediate vision has become more important over the last decade which has driven the demand for functional intermediate vision.1,2 It should be noted that some intermediate vision is not a new feature of monofocal IOL technology. Previous studies showed that spherical or aspherical neutral IOLs provided more depth of focus than aspherical IOLs due to higher residual spherical aberration, and corneal aberration profile was a key parameter affecting the likelihood of attaining an extended depth of focus.7,8 In the Vivity FDA registration study, the comparator group was 111 patients with bilateral implantation of the AcrySof IQ SN60WF aspheric monofocal IOLs, and patients were found to have binocular mean distance corrected intermediate visual acuity of logMAR 0.196.14 Considering the similar optical design of SN60WF and the Clareon monofocal IOL, the AcrySof IQ-defocus curve data in the Vivity FDA registration study may support that the Clareon monofocal lens can provide some improvement in intermediate visual acuity while maintaining excellent distance visual acuity. Indeed, a recent study by Blehm and Hall15 reported some intermediate visual acuity with the Clareon monofocal IOL. In contrast, the Eyhance monofocal IOL specifically aims to extend the range of acceptable intermediate vision by modifying the anterior surface of its monofocal analog IOL (ZCB00).6 However, it is important to distinguish between the clinically validated functions of an IOL (or any medical device) and any additional design goals, commonly referred to as “designed to” or “aims to”. While these phrases may imply clinical value, they require cautious interpretation as they are not necessarily synonymous with empirically proven patient benefits. As such, IOLs may be engineered with the aim of offering extra advantages, such as expanding visual range; however, these aspirations attain substantiated status only when verified by clinical research. Caution is warranted when no significant drawbacks are apparent, given that the law of conservation of energy dictates that manipulation of light in one aspect will inevitably affect it in another. Importantly, if these design goals receive validation from adequately powered clinical studies, manufacturers would transition from promoting what a device is “designed to” clinically achieve to asserting that it ‘does’ or “has been shown to” fulfill those objectives. Fernandez et al18 recently conducted a literature review and concluded that Eyhance IOL does not meet the ANSI criteria for an EDOF. While the Eyhance IOL is categorized as a monofocal lens, the base cost of the IOL at the time of submission is approximately $50 more than that of the base ZCB00 IOL. There is no accompanying FDA trial to demonstrate a clinically significant increase in intermediate-range vision compared to a more economical monofocal IOL. The question remains whether the Eyhance IOL provides clinical benefits worth the higher healthcare costs compared to other available monofocal IOLs.
This is the first report of the intermediate visual outcomes of the Clareon and Eyhance monofocal IOLs in a head-to-head comparison and includes a large sample size (n=310). Non-inferiority of the Clareon monofocal IOL, relative to the Eyhance monofocal IOL was demonstrated for DCIVA, as the Clareon Group was within the logMAR 0.1 non-inferiority margin. Blehm and Hall15 reported a mean DCIVA for the Clareon monofocal IOL of 0.23 logMAR, which was similar to that reported in this study (0.24 logMAR). Previous reports of DCIVA with the Eyhance have been in the range of 0.01 to 0.20 logMAR,16,19,20 which is also similar to that reported in our study (0.19 logMAR). Mean CDVA were similar for the Clareon monofocal IOL and the Eyhance monofocal IOL. However, we would expect there to be minimal differences in CDVA for 2 monofocal IOLs. Mean CDVA for each group in our study were similar to previous reports.15,19,20 Cinar et al21 compared the Eyhance to the SN60WF IOL and reported that Eyhance had superior monocular intermediate vision and non-inferior distance and near vision compared to the SN60WF. However, the study by Cinar et al21 reported monocular outcomes (compared to binocular in our study) and had a much lower sample size (65 eyes in 65 patients per group versus 310 eyes in 155 patients per group).
We wanted to explore how shifting the correction of the Clareon monofocal group by −0.25 D would affect DCIVA and CDVA as an exploratory endpoint. There was an approximate mean letter gain of 2 in DCIVA, with a corresponding 1 letter loss in CDVA for the Clareon group with this offset, compared to no offset. This could suggest there is some benefit to targeting slight myopia, but further studies are needed.
Subjects who were enrolled in this study were those that had previous bilateral implantation with the Clareon or Eyhance IOLs. This was a limitation of this study, since the available patient population included subjects that were not targeted for plano (eg, mini-monovision and monovision patients). However, measuring visual acuities with distance correction allowed for a head-to-head comparison without residual postoperative refractive error as a confounding factor. Another limitation of this study was that both the toric and non-toric versions of the Eyhance IOL were included, while only the non-toric Clareon IOL was included. This was because the toric version of Clareon was not yet available at the time of implantation. Ideally, we would have been able to include Clareon toric IOLs, however, refractive correction eliminates the impact of other variables, including residual postoperative refractive error.
In conclusion, the results of this study showed that bilateral implantation of Clareon monofocal IOLs and Eyhance monofocal IOLs lead to similar visual outcomes. Both monofocal IOLs may provide a similar potential to improve vision at the intermediate range.
Acknowledgments
This paper was presented at the 2023 American Society of Cataract and Refractive Surgery (ASCRS) Annual Meeting as a conference talk with interim findings. An industry trade article was published that referenced some of the presented data (https://crstodayeurope.com/articles/monofocal-iols-impact-of-optical-design-on-intermediate-vision/monofocal-iols-impact-of-optical-design-on-intermediate-vision).
Funding
This study was supported with an investigator-initiated study grant (69901155) from Alcon Vision, LLC, Fort Worth, TX, USA.
Disclosure
J. Morgan Micheletti, MD, is a consultant for Alcon, and reports the following outside the submitted work: Alcon – Consultant, Speaker, Research Grant; Bausch & Lomb – Consultant; BVI – Consultant; Johnson & Johnson Vision – Research Grant; Lenstec – Speaker; RxSight – Consultant, Speaker; Zeiss – Consultant. Brad Hall reports that he has received consulting fees from Ace Vision Group outside the submitted work. The authors report no other conflict of interest for this work.
References
1. MacRae S, Holladay JT, Glasser A, et al. Special report: American Academy of Ophthalmology task force consensus statement for extended depth of focus intraocular lenses. Ophthalmology. 2017;124(1):139–141. doi:10.1016/j.ophtha.2016.09.039
2. Blancafort Alias S, Del Campo Carrasco Z, Salvador-Miras I, et al. Exploring vision-related quality of life: a qualitative study comparing patients’ experience of cataract surgery with a standard monofocal IOL and an enhanced monofocal IOL. Clin Ophthalmol. 2022;16:1641–1652. doi:10.2147/OPTH.S358386
3. Monaco G, Gari M, Di Censo F, Poscia A, Ruggi G, Scialdone A. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: trifocal versus extended range of vision. J Cataract Refract Surg. 2017;43(6):737–747. doi:10.1016/j.jcrs.2017.03.037
4. Fernández J, Rodríguez-Vallejo M, Martínez J, Burguera N, Piñero DP. What we have learnt from 30 years living with positive dysphotopsia after intraocular lens implantation?: a review. Expert Rev Ophthalmol. 2021;16(3):195–204. doi:10.1080/17469899.2021.1917997
5. Pedrotti E, Carones F, Talli P, et al. Comparative analysis of objective and subjective outcomes of two different intraocular lenses: trifocal and extended range of vision. BMJ Open Ophthalmol. 2020;5(1):e000497. doi:10.1136/bmjophth-2020-000497
6. Guo Y, Wang Y, Hao R, Jiang X, Liu Z, Li X. Comparison of patient outcomes following implantation of trifocal and extended depth of focus intraocular lenses: a systematic review and meta-analysis. J Ophthalmol. 2021;2021:1115076. doi:10.1155/2021/1115076
7. Rocha KM, Gouvea L, Waring G, Haddad J. Static and dynamic factors associated with extended depth of focus in monofocal intraocular lenses. Am J Ophthalmol. 2020;216:271–282. doi:10.1016/j.ajo.2020.04.014
8. Rocha KM, Soriano ES, Chamon W, Chalita MR, Nose W. Spherical aberration and depth of focus in eyes implanted with aspheric and spherical intraocular lenses: a prospective randomized study. Ophthalmology. 2007;114(11):2050–2054. doi:10.1016/j.ophtha.2007.01.024
9. Tognetto D, Cecchini P, Giglio R, Turco G. Surface profiles of new-generation IOLs with improved intermediate vision. J Cataract Refract Surg. 2020;46(6):902–906. doi:10.1097/j.jcrs.0000000000000215
10. Auffarth GU, Gerl M, Tsai L, et al. Clinical evaluation of a new monofocal IOL with enhanced intermediate function in patients with cataract. J Cataract Refract Surg. 2021;47(2):184–191. doi:10.1097/j.jcrs.0000000000000399
11. Azor JA, Vega F, Armengol J, Millan MS. Optical assessment and expected visual quality of four extended range of vision intraocular lenses. J Refract Surg. 2022;38(11):688–697. doi:10.3928/1081597X-20220926-01
12. Schmid R, Borkenstein AF. Analysis of higher order aberrations in recently developed wavefront-shaped IOLs. Graefes Arch Clin Exp Ophthalmol. 2022;260(2):609–620. doi:10.1007/s00417-021-05362-2
13. Steinmuller LN, Greve D, Rua Amaro D, Bertelmann E, von Sonnleithner C. Analysis of higher-order aberrations in relation to the clinical outcome of an enhanced monofocal IOL. Eur J Ophthalmol. 2022;11206721221134171.
14. McCabe C, Berdahl J, Reiser H, et al. Clinical outcomes in a United States registration study of a novel extended depth of focus intraocular lens with a nondiffractive design. J Cataract Refract Surg. 2022;48(11):1297–1304. doi:10.1097/j.jcrs.0000000000000978
15. Blehm C, Hall B. Evaluation of visual outcomes and 3-month refractive stability of a new hydrophobic acrylic intraocular lens. Clin Ophthalmol. 2023;17:1859–1864. doi:10.2147/OPTH.S415400
16. Unsal U, Sabur H. Comparison of new monofocal innovative and standard monofocal intraocular lens after phacoemulsification. Int Ophthalmol. 2021;41(1):273–282. doi:10.1007/s10792-020-01579-y
17. Stanojcic N, O’Brart D, Hull C, et al. Visual and refractive outcomes and glistenings occurrence after implantation of 2 hydrophobic acrylic aspheric monofocal IOLs. J Cataract Refract Surg. 2020;46(7):986–994. doi:10.1097/j.jcrs.0000000000000201
18. Fernandez J, Rocha-de-Lossada C, Zamorano-Martin F, Rodriguez-Calvo-de-Mora M, Rodriguez-Vallejo M. Positioning of enhanced monofocal intraocular lenses between conventional monofocal and extended depth of focus lenses: a scoping review. BMC Ophthalmol. 2023;23(1):101. doi:10.1186/s12886-023-02844-1
19. Huh J, Eom Y, Yang SK, Choi Y, Kim HM, Song JS. A comparison of clinical outcomes and optical performance between monofocal and new monofocal with enhanced intermediate function intraocular lenses: a case-control study. BMC Ophthalmol. 2021;21(1):365. doi:10.1186/s12886-021-02124-w
20. Nanavaty MA, Ashena Z, Gallagher S, Borkum S, Frattaroli P, Barbon E. Visual acuity, wavefront aberrations, and defocus curves with an enhanced monofocal and a monofocal intraocular lens: a prospective, randomized study. J Refract Surg. 2022;38(1):10–20. doi:10.3928/1081597X-20211109-02
21. Cinar E, Bolu H, Erbakan G, et al. Vision outcomes with a new monofocal IOL. Int Ophthalmol. 2021;41(2):491–498. doi:10.1007/s10792-020-01599-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
