Back to Journals » Journal of Inflammation Research » Volume 15
HDL-Associated Lipoproteins: Potential Prognostic Biomarkers for Gram-Negative Sepsis
Authors Zou G , Zhu Q, Ren B, Guo Q, Wu Y, He J, Wu Y, Luo Z
Received 29 November 2021
Accepted for publication 28 January 2022
Published 17 February 2022 Volume 2022:15 Pages 1117—1131
DOI https://doi.org/10.2147/JIR.S350737
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Guoying Zou,1 Qing Zhu,1 Biqiong Ren,1 Qi Guo,1 Yuanyuan Wu,1 Junyu He,1 Ying Wu,1 Zhihong Luo2
1Department of Clinical Laboratory, The Second People’s Hospital of Hunan Province, Changsha, Hunan, 410007, People’s Republic of China; 2Office of the Party Committee, The Second People’s Hospital of Hunan Province, Changsha, Hunan, 410007, People’s Republic of China
Correspondence: Zhihong Luo, Office of the Party Committee, The Second People’s Hospital of Hunan Province, Furong Middle Road 427, Yuhua District, Changsha, Hunan, 410007, People’s Republic of China, Tel +86 19848029533, Email [email protected]
Purpose: To determine the levels of serum HDL-associated apolipoproteins (apoM and apoC) and HDL-binding receptor (scavenger receptor BI, SR-BI) in patients with gram-negative bacteria sepsis (G-sepsis) and to evaluate the value of lipoproteins in the diagnosis, severity and prognosis of G-sepsis.
Patients and Methods: A total of 128 patients with sepsis, 40 patients with system inflammatory reaction syndrome (SIRS) and 40 healthy subjects were enrolled in the Second People’s Hospital of Hunan Province from September 2019 to September 2020. The levels and the correlation of lipoproteins were detected and dynamically monitored by enzyme-linked adsorption method, ROC curve for the diagnostic, severity and prognostic value of lipoproteins in G-sepsis.
Results: The levels of serum HDL-associated lipoproteins in patients with G-sepsis were significantly decreased (P < 0.05), and the ROC curve showed that HDL-C, SR-BI, apoM and apoC had cut-off values of 0.915 mmol/L, 122.100 pg/mL, 102.400 ug/mL and 17.55 mg/mL, respectively, for the diagnosis of G-sepsis, with the sensitivity was 85.56%, 97.78%, 93.33% and 73.03%, and the specificity was 95.0%, 82.50%, 61.54% and 82.50%, respectively. There was a correlation between HDL-associated apolipoproteins. Changes in serum HDL-associated lipoproteins were more obvious in shock group than classic inflammation indicators, such as PCT, IL-6 and CRP. They showed a trend change on day 3, with the levels of SR-BI and apoC changing 2– 3 times, and the sensitivity of HDL-C, SR-BI, apoM and apoC for the diagnosis of G-septic shock were 32.43%, 72.97%, 65.75%, and 43.24%, and specificity of 94.34%, 81.13%, 83.07%, and 86.79%, respectively. The AUC, sensitivity and specificity of apoM combined with SR-BI were improved.
Conclusion: HDL-associated lipoproteins were correlated with bacterial-infected types, and serum levels of HDL-associated lipoproteins can be used as potential biomarkers for early diagnosis and progress of G-sepsis. ApoM combined with SR-BI could improve the sensitivity and specificity of prognosis assessment.
Keywords: apolipoprotein, high-density lipoprotein, gram-negative sepsis, biomarker, prognosis
Introduction
Bacterial infection is a common condition that often needs to be ameliorated with antibiotics. However, for unknown reasons, a proportion of infected patients still develop sepsis, which is organ dysfunction due to malfunctioning host response.1 Sepsis is the leading cause of death in intensive care units (ICU) and accounts for one of the five deaths worldwide.2 Early detection of sepsis is critical to the timely initiation of appropriate treatment.3 Despite the significant advances in the underlying mechanisms of sepsis pathogenesis, there are currently no clinically effective treatments or specific diagnostic biomarkers.
Sepsis can be divided into gram-negative sepsis (G-sepsis) and gram-positive sepsis (G+sepsis) according to the type of bacteria infected. Infections due to gram-negative microorganisms have higher mortality rates (10–40%) than gram-positive ones (7–27%).4 While the ability to clear pathogens in G-sepsis patients was significantly reduced, patients suffered persistent infection and a unique cascade reaction, presenting a “cytokine storm”, which could develop into septic shock and even death in a short time.5 Blood culture is still the gold standard for sepsis diagnosis. However, it does not provide clinicians with timely results within 24 hours. What is more, there are many problems in laboratory diagnosis and examination, such as complicated blood culture sampling, low positive rate, leading to poor feasibility of early diagnosis, easy to miss the golden time of sepsis rescue, and difficult to reduce fatality rate. Therefore, rapid and straightforward laboratory biomarkers for G-sepsis are urgently indispensable.
Previously, heart rate changes, nutritional indicators (such as serum protein), acute reactive proteins [such as C-reactive protein (CRP), procalcitonin (PCT)], cytokines [such as interleukin 6 (IL-6)] and immune function indicators (human leukocyte antigen-DR, neutrophil CD64) were used as indicators to predict the occurrence of sepsis; nevertheless, none of them played a warning role before or in the first few hours of disease. In recent years, extracellular heat shock protein 72 (eHSP72), extracellular heat shock protein 90α (eHSP90α), cortisol, and human glucocorticoid receptor (hGR) were used to identify early-onset sepsis from the system inflammatory reaction syndrome (SIRS) (<24 h).6 A multicenter prospective study suggested that soluble CD14 subtype (sCD14-ST), also known as presepsin, was an effective marker for the diagnosis of sepsis, more quickly than blood cultures; however, it could not distinguish between gram-negative and gram-positive bacterial infections, local infections and systemic infections,7 which would limit the correct choice of antibiotics.
Although the inflammatory mechanisms that initially drive the onset of sepsis are well understood, downstream pathways including those driving metabolic dysregulation remain elusive. Pathogen-associated lipids (PALs), including lipopolysaccharide (LPS) from Gram-negative and lipoteichoic acid (LTA) from Gram-positive bacterial cell walls, are thought to be the main drivers of the change from a simple infection to sepsis.8 In a mice model of sepsis, the marked decrease in carbohydrate oxidation and increase in fatty acid oxidation observed within 48 h after LPS administration revealed a change from glucose to predominantly lipid metabolism, depending on the degree of sepsis, especially in the acute phase, lipids were the predominant energy source in the acute phase of sepsis when protein metabolism was relatively low.9 In our early study of bacterial infectious diseases, it was found that the change in serum HDL-C and apoA1 (one of the HDL-associated apolipoproteins) levels had a good predictor of gram-negative bacterial infection.10–12 In this study, we attempted to further use the conventional detection methods to monitor lipid metabolism indicators, HDL-associated apolipoproteins (apoM and apoC) and HDL binding receptor (scavenger receptor BI, SR-BI) in patients with G-sepsis, and to explore the predictive abilities of HDL-associated lipoproteins in the early stage of occurrence, and the prognosis of disease, which makes it possible to carry out effective intervention and appropriate selection of antibiotics in the golden time of rescue, and provides ideas for the treatment of G-sepsis. To our knowledge, this is the first study that highlights differential serum lipid profiles for diagnosing, assessing and predicting G-sepsis.
Materials and Methods
Bioinformatic Analysis
First, we used public databases to understand the relationship between sepsis, especially gram-negative sepsis, and lipid metabolism. Comparative Toxicogenomics Database (CTD, http://ctdbase.org/) is a public resource for how environmental exposures affect human health. It provides manually curated information about chemical–gene/protein interactions, chemical–disease and gene–disease relationships.13 GeneCards (https://www.genecards.org/) is a searchable, integrative database providing all annotated and predicted human genes from ~150 web sources, including genomic, transcriptomic, proteomic, genetic, clinical and functional information.14 DISEASES (https://diseases.jensenlab.org/) is a weekly updated web resource that integrates evidence on disease-gene associations from automatic text mining, manually curated literature, cancer mutation data, and genome-wide association studies.15 We extracted related data from the three databases mentioned above using the sepsis or gram-negative sepsis keyword to find the involved genes and/or proteins. Next, the common sepsis-related genes from these 3 databases were obtained using the Venn Diagram software (http://bioinformatics.psb.ugent.be). The STRING database (https://string-db.org, version 11.0) was used for the analysis of co-expression genes from these 3 databases (The confidence score >0.4 was considered statistically significant),16 and performed Cytoscape (version 3.8.2) for 20 top hub genes.17 We then performed functional analysis using R software package (version 3.6.3),18 the enrichment analysis of lipid metabolism associated with sepsis was plotted.
Inclusion Criteria of Study Subjects
According to of the sepsis guidelines version 3.0 the diagnostic criteria: infection (positive culture of blood, urine, cerebrospinal fluid, wound discharge, respiratory secretions, other body fluids, etc.) and patients with the sequential organ failure assessment (SOFA) score ≥2. Patients with adequate fluid resuscitation still need a vasoconstrictor to achieve a mean arterial pressure (MAP) of 65 mmHg or more, and LAC ≥ 2 mmoL was diagnosed as septic shock.19 The G-sepsis patients were divided into survival and death groups according to the outcome of 28 days after ICU admission.
SIRS diagnostic criteria: SIRS can be diagnosed if two or more of the following criteria are met. ①Body temperature >38°Cor <36°C. ②Heart rate >90/min or hypotension (systolic blood pressure <90 mmHg, or lower than baseline >40 mmHg). ③Shortness of breath (>20/min) or excessive ventilation (PaCO2<32mmHg). ④ Peripheral blood white blood cell count >12.0×109/L or <4.0×109/L, or the proportion of immature white blood cells >10%. However, others causing the above acute abnormal changes were excluded.
Exclusion criteria: ①Patients admitted to the ICU for less than 24 hours; ② Patients with acute and chronic infectious diseases, tumors and autoimmune diseases; ③Patients with diabetes, coronary heart disease, and hyperlipidemia; ④Patients with incomplete clinical data. There was no statistically significant difference in gender and age among the four groups of study subjects (P>0.05), and they were comparable.
Study Subjects
From September 2019 to September 2020, 90 patients with gram-negative sepsis in the ICU 48 h of the Second People’s Hospital of Hunan Province (hereinafter referred to as “the hospital”) were enrolled as the G-sepsis group, including 49 males and 41 females, the average age is 63.3 ± 6.8 years. Another 38 patients with gram-positive bacteria sepsis were enrolled as the G+sepsis group, including 16 males and 22 females, with an average age of 60.8 ± 8.6 years. Forty fever patients with negative blood culture were enrolled as the SIRS group, including 22 males and 18 females, with an average age of 64.8 ± 7.2 years. In the same period, 40 healthy people in the physical examination center of the hospital were enrolled as the healthy control group, including 20 males and 20 females, with an average age of 63.8 ± 8.4 years. There were 37 patients with G-septic shock, including 16 males and 21 females, with an average age of 66.3 ± 7.4 years. Sixty-nine patients with G-sepsis survived and 21 died. This study complied with the guidelines of the Declaration of Helsinki, informed consent was obtained from every individual, and the consent process was approved by the Ethics Council of the Second People’s Hospital of Hunan Province (No. L2017014).
Specimen and Data Collection
(1) Patients enrolled: infection (positive culture of blood, urine, cerebrospinal fluid, wounds, respiratory secretions, other body fluids, etc.), and patients with SOFA score ≥2. We prospectively recorded clinical data of patients 48 hours after admission to ICU, including gender, age, medical history, treatment process, time of admission to ICU, culture-positive time and 28-day case fatality rate, APACHE II score and SOFA score (take the first day of admission to ICU. The worst parameter is calculated by APCHEII score and SOFA score). At the same time, serum samples were collected on the 1st, 3rd and 5th day after admission to the ICU. We excluded patients who stayed in ICU for less than 24 hours, who suffered from acute and chronic transfection diseases, tumors and autoimmune diseases.
(2) Retention of test specimens: Collect fasting venous blood of all subjects on 1 d, 3 d, and 5 d after the occurrence, centrifuged at 3000 r/min for 10 min, and aspirate 3 mL in EP tube and store at −80°C for later use.
(3) Detection of specimens:
ELISA Analysis
Serum HDL-associated lipoproteins (HDL-C, SR-BI, apoM and apoC) levels were determined by ELISA method, strictly in accordance with the kit instructions (Jianglai Biotechnology Co., LTD, China). Followed these steps: The kits were placed in the refrigerator at 4°C overnight, balanced at room temperature for 60 min before using. After the crystallization was completely dissolved, the washing solution was configured (1:20 dilution), then prepared the standard according to the number of reagent instructions; Added 100 μL horseradish peroxidase (HRP) labeled detection antibody to the blank, standard and sample wells, shaked and mix slightly, then sealed and incubate in a 37°C incubator (Changjin Technology Co. LTD., China) for 30 min; washed 5 times, 1 min every time with the washing machine (Shenzhen Healas Technology Co., LTD., China), and then thrown off and dried; Added 50 μL substrate A and substrate B to every well, incubate for 15 min at 37°C; Added 50μL stop solution to every well, the OD value of each well within 15 min using 450nm wavelength (Multiskan MK3, Thermo Fisher Shanghai Instruments Co., China); After the absorption value of standard and sample wells minus the absorption value of blank wells, took OD value as ordinate and concentration as abscissa to draw the standard curve; Calculated the concentration of these parameters according to the standard curve equation.
Biochemical Analysis
Automatic biochemical analyzer (Siemens Healtheomo Diagnostics InC, Germany) detects biochemical indicators [serum creatinine (SCR), uric acid (UA), total Bilirubin (TBIL) and alanine transaminase (ALT) were provided by Siemens Healtheomo Diagnostics InC, Germany, and lactic acid (LAC) was provided by Yonghe Sun Biotechnology Co., LTD, China] and blood lipid parameters [high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC) and triglyceride (TG) were provided by Siemens Health Diagnostics InC, Germany] and CRP (Beijing Strong Biotechnology Co., LTD, China) levels, automatic electrochemiluminescence analyzer (Roche, Germany) were detected inflammation parameters PCT and IL-6 (Roche, Germany) levels.
Bacterial Culture and Identification
The blood samples of the patients were placed in blood culture bottles, scanned and cultured in an automatic blood culture instrument (BACTEC FX, Becton, Dickinson and Company, USA), and then transferred after positive alarm. The samples were inoculated in chocolate culture dishes and blood culture dishes, placed overnight in a carbon dioxide incubator (Thermo Fisher Scientific Instruments Co., USA) at 37°C, and the colonies were identified by an automatic bacterial culture system (VITEK 2 Compact, Biomerieux Diagnostic Products Co., LTD, Shanghai).
We analyzed the difference in the concentration of the above indicators between the groups, and the relationship with the disease and prognosis.
Statistical Analysis
SPSS25.0 software was used for statistical analysis of the experimental data. The measurement data conforming to the normal distribution were expressed as mean ± standard deviation, t-test was used for comparison between two groups, and univariate analysis for comparison between multiple groups. The non-conforming normal distribution counting data were represented by [M (P25, P75)], Mann–Whitney U-test was used for comparison between two groups, and Kruskal–Wallis H-test for comparison between multiple groups. Pearson correlation coefficient was used for the correlation of each parameter, logistic analysis for the risk factors affecting the prognosis of G-sepsis, and the receiver operating curve (ROC) and AUC analysis for the role of HDL-associated lipoproteins in the diagnosis, severity and prognosis of G-sepsis (AUC of 0.5–0.7 indicated low diagnostic value, 0.7–0.9 for medium diagnostic value, and above 0.9 for high diagnostic value). P<0.05 was considered statistically significant.
Results
Bioinformatic Analysis of Lipid Metabolism Associated with Sepsis/ G-Sepsis
We combined related genes on sepsis or gram-negative sepsis from CTD, GeneCards and DISEASES databases using Venn Diagram software, with a total of 164 common genes (Figure 1A). We screened hub genes, and among the 20 top genes, there were genes related to inflammatory cytokines and lipid metabolism (Figure 1B). The enrichment analysis of lipid metabolism associated with sepsis/G-sepsis showed that the common genes were significantly enriched in biological processes such as lipid localization, transport and storage, cellular components such as plasma lipoprotein (HDL, VLDL, TG) particle and protein-lipid complex, molecular functions such as intermembrane lipid transfer activity and protein-lipid complex binding, and the enriched KEGG pathways included IL-17 signaling pathway, cytokine–cytokine receptor interaction, Toll-like receptor signaling pathway, cholesterol metabolism and sphingolipid signaling pathway (Figure 1C and Table 1).
The Demographic Data of Subjects
Next, we verified the disorder of lipid metabolism in sepsis, especially in G-sepsis, through clinical study data. In this study, 128 sepsis patients with positive blood culture were included, of which 105 cases had body temperature (T) >38.3°C (82.0%), 16 cases T <36.0°C (12.5%), 121 cases pulse (P) >90 times/min (94.5%), 48 cases respiratory rate (R) >30 times/min (37.5%), 45 cases blood glucose >7.7 mmol/L without diabetes (35.2%), 26 cases abnormal creatinine (20.3%). 78 cases white blood cell count >12×109/L (60.9%) and 17 cases <4×109/L (13.3%). 25 cases suffered from abnormal consciousness (19.5%), 28 cases hypotension (21.9%), 36 cases hypoxemia (28.1%), 48 cases abnormal liver function (37.5%), 24 cases abnormal coagulation function (18.8%), 87 cases dyslipidemia (70%), 41 cases hyperlactemia (32.0%), 6 cases abdominal distension (4.6%), and 4 cases hyperbilirubinemia (3.1%) (Table 2).
 |
Table 1 Top 5 of Functional Analysis of Lipid Metabolism Associated with Sepsis in Which Common Genes Extracted from the 3 Databases |
 |
Table 2 Clinical Characteristics of Subjects |
A pathogen analysis of 128 blood culture-positive samples was carried out. 90 strains of Gram-negative bacteria were mainly Escherichia coli (37.5%), Klebsiella pneumoniae (16.4%) and Acinetobacter baumannii (6.3%), Escherichia coli accounted for the highest proportion. There were 38 strains of Gram-positive bacteria, Staphylococcus aureus (10.9%) and Staphylococcus haemolyticus (3.1%) were the main bacteria (Table 3).
 |
Table 3 Distribution and Composition of Pathogenic Bacteria |
The Physical Data of Subjects
Compared with the control group, the levels of HDL-C, LDL-C, TC, TG and TBIL in infection subjects were significantly decreased, while the levels of PCT, IL-6, CRP, SCR and ALT were significantly increased (P < 0.05), the level of UA increased with severity of infectious diseases (P < 0.05). Compared with SIRS and G+ sepsis group, the levels of serum PCT, IL-6, CRP, SCR and ALT in G-sepsis group were significantly increased, while levels of HDL-C, LDL-C, TC, TG and TBIL were significantly decreased (P < 0.05), and the infection mainly occurred in the lung, abdomen, urinary and trauma in G-sepsis patients (Table 4).
 |
Table 4 The Physical Data of Subjects |
The Changes and Correlation of the Levels of HDL-Associated Lipoproteins in Inflammatory Diseases
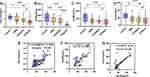
Compared with the control group, serum SR-BI, apoM, apoC and HDL-C levels were successively decreased (P < 0.05) in infection subjects. The serum levels of SR-BI, apoM and HDL-C were decreased in G-sepsis group significantly higher than those in SIRS group, while serum apoC had no difference (P > 0.05). Compared with G+sepsis group, HDL-C and SR-BI levels were significantly decreased in G-sepsis group (P < 0.05) (Figure 2A–D).
The levels of SR-BI, apoM, apoC, and HDL-C were changed in G-sepsis patients, so the correlation was further analyzed. The results showed that serum SR-BI was positively correlated with HDL-C, apoM, and apoC in G-sepsis patients, with correlation coefficients r = 0.507, r = 0.727 and r = 0.828, respectively (P < 0.001), which suggested that there was a certain correlation between each parameter (Figure 2E–G).
Diagnostic Efficacy of Serum HDL-Associated Lipoproteins in G-Sepsis
From the above observation, we found that the levels of serum HDL-associated lipoproteins were altered in inflammatory diseases, and then we wondered if these changes could be useful for diagnosing. ROC curve analysis showed that the AUC of serum SR-BI, apoM, apoC and HDL-C in distinguishing G-sepsis from control subjects were 0.966, 0.816, 0.812 and 0.914, respectively. When the optimal critical values were 122.10pg/mL, 102.40 ug/mL, 17.55 mg/dL and 0.915 mmol/L, the sensitivity was and 97.78%, 93.33%, 73.03% and 85.56%, respectively; the specificity was 82.50%, 61.54%, 82.50% and 95.00%, respectively; These differences were statistically significant (P < 0.001) (Figure 3A). Among the inflammatory indicators, we observed, the AUC of serum PCT, IL-6, CRP and LAC were 0.903, 0.725, 0.767 and 0.823, respectively, the sensitivity was 89.92%, 84.16%, 82.16% and 84.52%, and the specificity was 83.72%, 64.14%, 63.48% and 70.31%, respectively.
In distinguishing G-sepsis from SIRS subjects, the AUC of serum SR-BI, apoM, apoC and HDL-C were 0.962, 0.665, 0.655 and 0.703, respectively. When the optimal critical values were 109.70 pg/mL, 38.69 ug/mL, 15.83 mg/dL and 0.685 mmol/L, the sensitivity was and 86.66%, 38.89%, 71.91% and 50.00%, respectively; the specificity was 82.50%, 90.00%, 60.00% and 80.00%, respectively (Figure 3B).
In distinguishing G- sepsis from G+ sepsis subjects, the AUC of serum SR-BI, apoM and HDL-C were 0.939, 0.624 and 0.639, respectively. When the optimal critical values were 175.64pg/mL, 39.03 ug/mL and 0.94 mmol/L, the sensitivity was and 83.33%, 39.89% and 85.56%, respectively; the specificity was 92.31%, 97.87% and 43.37%, respectively. The value of apoC in differentiating G- sepsis from G+ sepsis was not significant (P > 0.05) (Figure 3C).
The Decrease of Serum HDL-Associated Lipoproteins Indicated the Aggravation of G-Sepsis
In order to understand the role of serum HDL-associated lipoproteins in evaluating the severity of G-sepsis, we analyzed the changers in G-sepsis shock patients. The scores of APACHE II and SOFA in G- sepsis shock subjects were significantly higher than those in G-sepsis (P < 0.001), while the levels of SR-BI, apoM, apoC and HDL-C were significantly decreased (P < 0.001), which suggested that apolipoprotein and HDL receptor were closely related to severity of G-sepsis, and the levels of SR-BI, apoM, apoC and HDL-C decreased with the aggravation of the disease (Figure 4A–D), while among the inflammatory indicators we observed, only PCT and LAC had differential variation (P < 0.001).
While dynamically observing the severity, we tested the specimens required for clinical diagnosis and treatment every day, and found that the third day and the fifth day were the time of high frequency of examination, so we monitored the results of 15 patients (No patients at all three time points were excluded) with G-sepsis on 1 d, 3 d and 5 d after ICU admission. The results showed that the levels of serum HDL-associated lipoproteins changed as the disease progressed. The results showed that serum SR-BI and apoC appeared to have an upward trend (P < 0.05), and the level of apoM reached the lowest value on the 3rd day after ICU admission, and then elevated (P < 0.05). During the monitoring process, it was found that the extent of altering in SR-BI and apoC levels reached 2–3 times, which could give prominent indication of disease progress (Figure 4E–H).
APACHE II score and SOFA score were clinical indicators to evaluate the severity of sepsis, so we analyzed the correlation between the scores and HDL-associated lipoproteins. The levels of serum SR-BI, apoC, and apoM were negatively correlated with APACHE II score and SOFA score [the correlation coefficients with APACHE II score were r=−0.720, r=−0.593, r=−0.379, respectively (Figure 5A–D); the correlation coefficients with SOFA score were r=−0.624, r=−0.457, r=−0.343, respectively (Figure 5E–H).].
Further, we evaluated the diagnostic efficacy of HDL-associated lipoproteins for disease severity. The results showed that the AUC of the levels of serum SR-BI, apoM and apoC in G-sepsis patients were 0.788, 0.748 and 0.660, respectively. When the optimal thresholds were 41.230 pg/mL, 41.730 ug/mL and 5.337 mg/dL, the sensitivity were 72.97%, 65.75%, 43.24%, respectively; and specificity were 81.13%, 83.07% and 86.79%, respectively (Figure 5I).
While among the inflammatory indicators we observed, the AUC of serum PCT and LAC were 0.702 and 0.792. When the optimal thresholds were 10.23 ug/mL and 3.45 mmol/L, the sensitivity were 72.25% and 81.53%, and specificity were 68.72% and 78.42%.
The above results suggested that HDL-associated lipoproteins were closely related to the progression of G-sepsis.
The Prognostic Effect of Serum HDL-Associated Lipoproteins on Patients with G-Sepsis
The levels of HDL-C, SR-BI, apoM and apoC in G-sepsis death were much more decreased than those in the survival (P < 0.001), which was further suggested that the lower the serum levels of HDL-C, SR-BI, apoM and apoC, the more severe the disease. Among inflammatory factors, only LAC had prognostic significance (4.72 mmol/L vs 2.76mmol/L, P < 0.001), while PCT, IL-6 and CRP had no prognostic significance (P > 0.05) (Figure 6A–D).
According to logistic regression analysis, serum SR-BI and apoM were risk factors affecting the prognosis of patients with G-sepsis (Table 5). ROC curve results showed that the AUC of serum SR-BI and apoM for predicting death from G-sepsis were 0.923 and 0.887, respectively. When the optimal critical values were 39.480 pg/mL and 44.750 ug/mL, respectively, the sensitivity and specificity were 86.36%, 89.86% and 85.71%, 85.51%, respectively. The AUC, sensitivity and specificity of apoM and SR-BI combination in predicting death from G-sepsis were 0.977, 95.24% and 89.88%, respectively, which was greater than the predictive value of a single indicator, and the predictive sensitivity and specificity of the combined diagnosis were higher than that of a single indicator (Figure 6E).
 |
Table 5 Prognostic Risk Factors for G- Sepsis |
Discussion
Sepsis has the characteristics of risk and high fatality rate, can easily damage the host tissues and organs and develop into multiple organ failure syndrome (MODS).20 Timely identification of infected patients and antibiotic treatment are key to prevent the development of sepsis. 29 studies noted decreased HDL, TC and LDL, and elevated TG concentrations in SIRS and/or sepsis patients. In particular, HDL appears to be an inflammatory marker, as the reduction in its levels reflects the intensity of the underlying inflammatory process.21 The results of blood lipid detection are easy to obtain in clinical laboratory, and little affected by antibiotic drugs in the treatment process, which can genuinely reflect morbid state. Most previous studies on sepsis have focused on the role of HDL or apoA1 and comparative analyses between survivor and non-survivor groups or sepsis and sepsis shock groups.22–24 In this study, we analyzed the differences in serum HDL-associated lipoprotein levels in patients with G-sepsis and G+sepsis and the association of lipid levels with severity and prognosis.
Plasma lipids and apolipoproteins undergo comprehensive changes in sepsis; what is more, lipid mediators play a distinct role in innate immune signaling, which provide new frontiers for targeting the septic response. While the changes in lipid and lipoprotein metabolism during sepsis are well characterized whether they are related to the pathogen type remains unclear. HDL is a complex biological molecule, with a surface of phospholipids, free cholesterol and specialized apolipoproteins surrounding a neutral lipid core, consisting of apoA1, apoC1, apoM, etc. Lipoproteins neutralize Pathogen-associated lipids (PALs) and LPS from Gram-negative bacterial cell walls in the blood, thereby reducing the pro-inflammatory response of immune cells. Of all lipoprotein classes, HDLs bind PALs with the most excellent affinity.25 Our results also suggested alterations to HDLs in patients with G-sepsis.
In this study, levels of serum HDL-C, LDL-C, TC, TG were significantly decreased in sepsis patients, 70% of G-sepsis patients were dyslipidemia (Table 2), Escherichia coli accounted for the highest proportion of infected bacteria (Table 3), and the infection mainly occurred in the lung, abdomen, urinary and trauma in G-sepsis patients (Table 4). In our early study of bacterial infectious diseases, it was found that the serum HDL and apoA1 levels of patients with G-bacterial infection were lower than those with G+ bacterial infection,10–12 further study found that serum HDL-associated lipoproteins were correlated with the progression and prognosis of G-sepsis.
In plasma, apoM, an apolipoprotein primarily present in HDL, is transported with HDL, and apoM+ HDL accounts for 5% of the total HDL, and is also the main carrier of plasma sphingosine-1-phosphate (S1P). Recently, the anti-inflammatory functions of apoM mediated by HDL have received increasing attention.26,27 ApoC1 circulates in plasma with a concentration of 6 mg/dL and is mainly bound to the lipoproteins HDL and VLDL,28 makes up about 2% of that of HDL, and is also the major plasma inhibitor of cholesteryl ester transfer protein (CETP). SR-BI is a multiligand membrane protein receptor that binds to apolipoproteins on HDL to promote selective uptake and reverse transport of cholesterol. ApoM, apoC and SR-BI are involved in HDL cholesterol metabolism and are closely related to the formation of atherosclerosis.
The relationship between these HDL-associated lipoproteins and sepsis has also been studied, sepsis decreased hepatic apoM mRNA and plasma apoM level in LPS-treated rat,29 and the plasma concentrations of apoM decrease dramatically in patients with sepsis and SIRS, the degree of decrease reflecting the severity of the disease.30 Lipid metabolism emerged as the main altered function in septic patients secondary to hospital-acquired pneumonia, with HDL as a central node in the network analysis, interacting with down-regulated proteins, such as apoC1,31 which was consistent with our findings (Figure 2). However, there are few studies on the relationship between HDL-associated lipoproteins and G-sepsis, mainly using LPS-induced animal models. According to recent reports, LPS decreases apoM expression,32 and apoM possesses protective properties against LPS-induced organ injuries in LPS-treated mice.33 In humans with G-sepsis and in a mouse model of sepsis with LPS administration, these were observed an approximately 50% decrease in serum apoM levels.34 ApoC1 contains structural elements in both N-terminal and C-terminal helix to bind LPS and to enhance the proinflammatory response toward LPS via a mechanism similar to LPS-binding protein (LBP),35 increases the inflammatory response to G-sepsis and protects against fatal sepsis. There have been hardly any studies comparing HDL-associated apolipoproteins in patients with G-sepsis with G+sepsis.
Therefore, we investigated changes in lipoprotein metabolism associated with HDL in G-sepsis in this study. We found that compared with G+sepsis, serum HDL-C, SR-BI, apoM and apoC levels were lower in patients with G+sepsis. Furthermore, serum SR-BI was positively correlated with apoM, apoC and HDL-C in G-sepsis patients (Figure 2). Serum SR-BI, apoM, apoC and HDL-C had good diagnostic efficacy in distinguishing G-sepsis from control subjects, SIRS subjects, G+sepsis subjects (Figure 3), and had a good correlation with APACHE II score and SOFA score, namely the severity of sepsis (Figure 5), especially the changes of SR-BI and apoC levels, which reach 2–3 times within 2 days, which could give prominent indication of disease progress (Figure 4). The levels of HDL-C, SR-BI, apoM and apoC in G-sepsis death were much more decreased than those in the survival, according to logistic regression analysis, serum SR-BI and apoM were risk factors affecting the prognosis of patients with G-sepsis, further analysis showed that the combination of apoM and SR-BI had better sensitivity and specificity (Figure 6). None of the classic inflammatory factors, such as the levels of serum PCT, LAC, IL-6 and CRP, could be compared with those. These results suggested that HDL-associated lipoproteins were closely related to the early diagnosis, progression and prognosis of G-sepsis.
Patients have low utilization of exogenous nutrients when sepsis occurs, most rely on decomposition of their own proteins and mobilization of fat for energy, while lipids and metabolites will combine with endotoxins and early inflammatory factors. In G-sepsis patients, endotoxin is the main inducement, which triggers SIRS through inflammatory cascade. In the inflammatory state, changes in HDL structure can be observed, leading to changes in HDL functions, such as eliminating pathogens.36 The apoC-LPS complex was recognized by the SR-BI receptor on the liver surface and then transferred to the liver for clearance. ApoM promoted the anti-inflammatory effects of HDL by binding to cell surface SR-BI receptors. Studies have shown that apoM+/HDL had better anti-inflammatory effect than apoM/HDL, which may be because the interaction between apoM and SR-BI enhanced the anti-inflammatory effect of HDL and the LPS-scavenging ability of SR-BI. ApoM repaired the vascular barrier by binding to S1P receptor and stimulated production of endothelial nitric oxide synthase (eNOS) to protect blood vessels.37 HDL-associated apolipoproteins were positively correlated with HDL receptor SR-BI, SR-BI has been shown to modulate the susceptibility to LPS-induced tissue injury and the ability of S1P (sphingosine 1 phosphate) to interact with its receptor, linking SR-BI to regulating inflammation.38,39 HDL-associated apolipoproteins can bind to LPS, so HDL-associated apolipoproteins may promote to bind LPS by combing with SR-BI,40 thus scavenging G-bacterial toxicity. Patients with more severe sepsis had higher LPS, and HDL-associated apolipoproteins were bound to be more, indicating that patients with severe sepsis had lower levels of serum HDL-associated apolipoproteins than patients with mild sepsis over the same period of time. Recent studies have shown that the down-regulation of plasma HDL in patients with sepsis may promote inflammatory response, thereby activating SOCS1 signaling pathway, regulating the severity of sepsis and affecting prognosis.41 Therefore, low HDL-associated apolipoproteins can predict the severity and prognosis of acute sepsis.42 Our bioinformatic analysis also found that the lipid metabolism signaling pathways in G-sepsis included cytokine–cytokine receptor interaction, toll-like receptor signaling pathway etc., which are worthy of further study.
Conclusion
In summary, we demonstrated that HDL-associated apolipoproteins and their receptor SR-BI were associated with the type of bacterial infection. Serum SR-BI and apoM levels could be used as potential biomarkers for early diagnosis and progression of G-sepsis. The combination of serum apoM and SR-BI could improve the sensitivity and specificity of prognosis assessment of G-sepsis. We highlight not only their potential roles in sepsis pathogenesis but also the possibility of using HDL-associated lipoproteins as diagnostic and prognostic biomarkers of sepsis. Of course, we have only observed such clinical phenomena, and the mechanism by which HDL-associated lipoprotein protects septic tissue damage needs further investigation.
Acknowledgments
We acknowledge CTD, GeneCards, and DISEASES databases for proving their platforms and contributors for uploading their meaning datasets.
Funding
This study was supported by grant from the Natural Science Foundation of Hunan Province (2018JJ6102).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Fleischmann C, Scherag A, Adhikari NKJ, et al. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–272. doi:10.1164/rccm.201504-0781OC
3. De Backer D, Dorman T. Surviving sepsis guidelines: a continuous move toward better care of patients with sepsis. JAMA. 2017;317(8):807–808. doi:10.1001/jama.2017.0059
4. Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88(Suppl 2):S69–74. doi:10.1016/S0378-3782(12)70019-1
5. Bertani B, Ruiz N, Slauch JM. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus. 2018;8(1). doi:10.1128/ecosalplus.ESP-0001-2018
6. Vardas K, Ilia S, Sertedaki A, et al. Increased glucocorticoid receptor expression in sepsis is related to heat shock proteins, cytokines, and cortisol and is associated with increased mortality. Intensive Care Med Exp. 2017;5(1):10. doi:10.1186/s40635-017-0123-8
7. Endo S, Suzuki Y, Takahashi G, et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J Infect Chemother. 2012;18(6):891–897. doi:10.1007/s10156-012-0435-2
8. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–420. doi:10.1038/nri.2017.36
9. Irahara T, Sato N, Otake K, et al. Alterations in energy substrate metabolism in mice with different degrees of sepsis. J Surg Res. 2018;227:44–51. doi:10.1016/j.jss.2018.01.021
10. Zou G, He J, Ren B, et al. The delta high-density lipoprotein cholesterol ratio: a novel parameter for gram-negative sepsis. Springerplus. 2016;5(1):1044. doi:10.1186/s40064-016-2685-4
11. Zou G, Huang L, Ren B. Clinical significance of high-density lipoprotein cholesterol and apolipoprotein A1 in patients with Gram-negative bacilli infection. Int J Lab Med. 2006;27(3):280–281.
12. Zou G, Huang L, Ren B. Clinical significance of the mensurations of plasma lipids in Gram-negative bacteremia patients. J Mod Lab Med. 2005;20(6):69–70.
13. Davis AP, Grondin CJ, Johnson RJ, et al. Comparative Toxicogenomics Database (CTD): update 2021. Nucleic Acids Res. 2020;49(D1):D1138–D1143. doi:10.1093/nar/gkaa891
14. Stelzer G, Rosen R, Plaschkes I, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinform. 2016;54:
15. Pletscher-Frankild S, Pallejà A, Tsafou K, et al. DISEASES: text mining and data integration of disease-gene associations. Methods. 2015;74:83–89. doi:10.1016/j.ymeth.2014.11.020
16. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi:10.1093/nar/gky1131
17. Doncheva NT, Morris JH, Gorodkin J, Jensen LJ. Cytoscape StringApp: network analysis and visualization of proteomics data. J Proteome Res. 2019;18(2):623–632. doi:10.1021/acs.jproteome.8b00702
18. Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16(5):284–287. doi:10.1089/omi.2011.0118
19. Liu Z, Meng Z, Li Y, et al. Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with Sepsis. Scand J Trauma Resusc Emerg Med. 2019;27(1):51. doi:10.1186/s13049-019-0609-3
20. Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14(7):417–427. doi:10.1038/s41581-018-0005-7
21. Golucci APBS, Marson FAL, Ribeiro AF, et al. Lipid profile associated with the systemic inflammatory response syndrome and sepsis in critically ill patients. Nutrition. 2018;55–56:7–14. doi:10.1016/j.nut.2018.04.007
22. Guirgis FW, Leeuwenburgh C, Moldawer L, et al. Lipid and lipoprotein predictors of functional outcomes and long-term mortality after surgical sepsis. Ann Intensive Care. 2021;11(1):82. doi:10.1186/s13613-021-00865-x
23. Khaliq W, Großmann P, Neugebauer S, et al. Lipid metabolic signatures deviate in sepsis survivors compared to non-survivors. Comput Struct Biotechnol J. 2020;18:3678–3691. doi:10.1016/j.csbj.2020.11.009
24. Guirgis FW, Dodani S, Leeuwenburgh C, et al. HDL inflammatory index correlates with and predicts severity of organ failure in patients with sepsis and septic shock. PLoS One. 2018;13(9):e0203813. doi:10.1371/journal.pone.0203813
25. Nguyen M, Pallot G, Jalil A, et al. Intra-abdominal lipopolysaccharide clearance and inactivation in peritonitis: key roles for lipoproteins and the phospholipid transfer protein. Front Immunol. 2021;12:622935. doi:10.3389/fimmu.2021.622935
26. Ruiz M, Frej C, Holmér A, et al. High-density lipoprotein-associated Apolipoprotein M limits endothelial inflammation by delivering Sphingosine-1-Phosphate to the Sphingosine-1-Phosphate Receptor 1. Arterioscler Thromb Vasc Biol. 2017;37(1):118–129. doi:10.1161/ATVBAHA.116.308435
27. Luo G, Xu N. Apolipoprotein M: research progress and clinical perspective. Adv Exp Med Biol. 2020;1276:85–103.
28. Cheng G, Zheng L. Regulation of the apolipoprotein M signaling pathway: a review. J Recept Signal Transduct Res. 2021;1–8. doi:10.1080/10799893.2021.1924203
29. Li Y, Zhou J, Qiu J, et al. Berberine reduces gut-vascular barrier permeability via modulation of ApoM/S1P pathway in a model of polymicrobial sepsis. Life Sci. 2020;261:118460. doi:10.1016/j.lfs.2020.118460
30. Kumaraswamy SB, Linder A, Åkesson P, et al. Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Crit Care. 2012;16(2):R60. doi:10.1186/cc11305
31. Sharma NK, Ferreira BL, Tashima AK, et al. Lipid metabolism impairment in patients with sepsis secondary to hospital acquired pneumonia, a proteomic analysis. Clin Proteomics. 2019;16:29. doi:10.1186/s12014-019-9252-2
32. Wang L, Tang X, Li S. Propofol promotes migration, alleviates inflammation, and apoptosis of lipopolysaccharide-induced human pulmonary microvascular endothelial cells by activating PI3K/AKT signaling pathway via upregulating APOM expression. Drug Dev Res. 2021. doi:10.1002/ddr.21869
33. Kurano M, Tsuneyama K, Morimoto Y, et al. Apolipoprotein M protects lipopolysaccharide-treated mice from death and organ injury. Thromb Haemost. 2018;118:1021–1035. doi:10.1055/s-0038-1641750
34. Feingold KR, Shigenaga JK, Chui LG, et al. Infection and inflammation decrease apolipoprotein M expression. Atherosclerosis. 2008;199(1):19–26. doi:10.1016/j.atherosclerosis.2007.10.007
35. Berbée JF, Coomans CP, Westerterp M, et al. Apolipoprotein CI enhances the biological response to LPS via the CD14/TLR4 pathway by LPS-binding elements in both its N- and C-terminal helix. J Lipid Res. 2010;51(7):1943–1952. doi:10.1194/jlr.M006809
36. Su X, Zhang G, Cheng Y, Wang B. New insights into the emerging effects of inflammatory response on HDL particles structure and function. Mol Biol Rep. 2021;48(7):5723–5733. doi:10.1007/s11033-021-06553-0
37. Du W, Hu M. Apolipoprotein and sepsis. Chin J Clin Lab Manage. 2014;2(4):37–40.
38. Baranova IN, Bocharov AV, Vishnyakova TG, et al. Class B scavenger receptors BI and BII protect against LPS-Induced acute lung injury in mice by mediating LPS. Infect Immun. 2021;89(10):e0030121. doi:10.1128/IAI.00301-21
39. Lee MH, Appleton KM, El-Shewy HM, et al. S1P in HDL promotes interaction between SR-BI and S1PR1 and activates S1PR1-mediated biological functions: calcium flux and S1PR1 internalization. J Lipid Res. 2017;58:325–338. doi:10.1194/jlr.M070706
40. Yao S, Luo G, Liu H, et al. Apolipoprotein M promotes the anti-inflammatory effect of high-density lipoprotein by binding to scavenger receptor BI. Ann Transl Med. 2020;8(24):1676. doi:10.21037/atm-20-7008
41. Li H, Liu W, Su W, et al. Changes in plasma HDL and its subcomponents HDL2b and HDL3 regulate inflammatory response by modulating SOCS1 signaling to affect severity degree and prognosis of sepsis. Infect Genet Evol. 2021;91:104804. doi:10.1016/j.meegid.2021.104804
42. Barker G, Weiner JR, Guirgis FW, Reddy S. HDL and persistent inflammation immunosuppression and catabolism syndrome. Curr Opin Lipidol. 2021;32(5):315–322. doi:10.1097/MOL.0000000000000782
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.