Back to Journals » International Medical Case Reports Journal » Volume 17
HCV Reactivation in a Patient with Hepatocellular Carcinoma Due to Sorafenib: A Case Report
Authors Cheng J, Pan J, Zhao D, Ma X, Sun Q, Li J
Received 27 October 2023
Accepted for publication 1 February 2024
Published 12 February 2024 Volume 2024:17 Pages 121—124
DOI https://doi.org/10.2147/IMCRJ.S444521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Jun Cheng,* Jinjin Pan,* Dongmei Zhao, Xuejiao Ma, Qiulin Sun, Jiabin Li
Department of Infectious Disease, The First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiabin Li, Tel/Fax +86-551-62922281, Email [email protected]
Abstract: The global prevalence of hepatitis C virus (HCV) infection is approximately 3%, with a post-infection chronicity rate of up to 50%– 85%. HCV reactivation can occur when anti-HCV positive individuals receive antineoplastic therapy. In this study, we report a case of an anti-HCV positive patient with negative HCV RNA after 12 weeks of direct antiviral therapy. Two months later, sorafenib was used to treat hepatocellular carcinoma, and HCV reactivation occurred after 8 months of the treatment. HCV RNA was negative after 12 weeks of antiviral treatment with Sofosbuvir-velpatasvir. We also discussed the mechanism of HCV reactivation caused by sorafenib and the antiviral treatment regimen after HCV reactivation with the relevant literature.
Keywords: hepatocellular carcinoma, sorafenib, HCV-RNA, anti-HCV therapy, reactivation
Introduction
Sorafenib is one of the first molecularly targeted drug used in the first-line treatment of hepatocellular carcinoma (HCC). Several clinical trials have demonstrated the survival benefit of sorafenib in patients with advanced HCC from different regions and with varying causes of hepatopathy.1,2 Previous studies have confirmed that chemotherapy or immunosuppressive therapy may lead to reactivation of hepatitis B virus (HBV) and different degrees of liver injury, which can affect the course of tumor treatment and patient prognosis. In recent years, it has been found that the risk of hepatitis C virus (HCV) reactivation is increased in cancer patients after a series of anticancer treatments such as chemotherapy or immunosuppressive therapy.3,4 However, there are no clear guidelines for the safety and management of antineoplastic therapy in patients coinfected with HCV. In this article, we report a case of HCV reactivation during sorafenib administration in a patient with HCC and review the current literature to discuss the mechanism of occurrence and prevention measures of the disease, with the aim of improving the patient’s prognosis and raising awareness among clinical staff.
Case Presentation
A 52-year-old male patient who had a fever for 7 days visited the Department of Respiratory Medicine of the Second People’s Hospital in Chuzhou on July 11, 2020. The maximum temperature was 38.5 °C, with mild chills before the fever and cough with a few white sticky sputum, and without other accompanying symptoms. Physical examination showed no moist rales in either lung, hepatic palp positive, spider nevus positive, hepatic spleen subcostal not palpable, abdomen distended, shifting dullness negative, and no tenderness and rebound tenderness. Further examination on admission revealed a positive anti-HCV level, HCV-RNA of 3.27×104 IU/mL, and alpha-fetoprotein of 164.8 ng/mL; chest and abdominal CT showed pulmonary infection and a suspicious occupying lesion in the right liver lobe. The patient was diagnosed with pulmonary infection, post-hepatitis C cirrhosis, and hepatic space occupying lesion by combining the patient’s symptoms, signs, and relevant ancillary test results. Anti-infection treatment was given, along with direct antiviral drugs (specific name unknown) for 12 weeks and reviewed after 4 and 12 weeks, the last of which showed HCV-RNA of <15 IU/mL. However, due to recurrent fevers, the patient was admitted to the Department of Tuberculosis of Anhui Provincial Chest Hospital on January 5, 2021. The examination revealed alpha-fetoprotein of 317.2 ng/mL; CT of the chest and abdomen revealed an old tuberculosis and a space-occupying lesion in the right lobe of the liver. Results from PET-CT showed hypodense foci in the upper segment of the right posterior lobe of the liver with a marked increase in FDG metabolism, raising the possibility of a primary malignant lesion. The diagnosis was then pulmonary tuberculosis, post-hepatitis C cirrhosis, and primary HCC. For antitubercular treatment, the patient received cycloserine (250 mg orally in the morning and 500 mg in the evening), clofazimine (100 mg orally once daily), and moxifloxacin (400 mg orally once daily). For anticancer therapy, sorafenib (400 mg orally twice daily) was employed. The patient was admitted to the Department of Hepatobiliary Surgery of the First Affiliated Hospital of Anhui Medical University on April 21, 2021, for surgical treatment of primary HCC (Figure 1). The patient’s status remained stable throughout therapy with sporadic episodes of low-grade fever. A post-admission examination found alpha-fetoprotein of 382.5 ng/mL and HCV-RNA of <15 IU/mL; liver function tests showed ALB of 29.3 g/L, TBIL of 21.7 µmol/L, ALT of 16 U/L, and AST of 125 U/L. The results of the liver-occupying lesions biopsy’s histopathology revealed significantly differentiated HCC. HPC (+), CK7 (partial +), CD34 (abundant sinusoidal +), CD10 (small +), K1-67 (around 15%), and AFP (-) were all detected by immunohistochemistry examination. On April 26, 2021, laparoscopic microwave ablation of tumor on the right lobe of liver was performed under general anaesthesia. With normal liver function and an alpha-fetoprotein level of 24.1 ng/mL, the patient continued to take sorafenib at a dosage of 400 mg twice daily in the postoperative period. On August 21, 2021, the patient was readmitted to the Department of Hepatobiliary Surgery of the First Affiliated Hospital of Anhui Medical University due to a low-grade fever that had persisted for 6 days, with abdominal distension, poor appetite, and an occasional cough without obvious sputum, and no obvious chills or shivers, no abdominal pain, and no diarrhea.
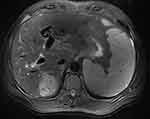 |
Figure 1 Liver MRI image of a patient in the First Affiliated Hospital of Anhui Medical University, Hefei, China. Abbreviation: MRI, magnetic resonance imaging. |
After admission, relevant tests showed HCV-RNA of 7.04×105 IU/mL, HCV genotype of 1b; liver function tests revealed ALB of 34.6 g/L, TBIL of 18.98 µmol/L, ALT of 9 U/L, and AST of 46 U/L; alpha-fetoprotein of 252.4 ng/mL. PET-CT showed subcapsular hypodense foci in the right posterior lobe of the liver with FDG hypometabolism considered altered after tumor treatment, and multiple nodules in the liver and lung with varying degrees of abnormal increase in FDG metabolism considered highly active even after tumor treatment. During these 4 months, the patient did not receive any surgical treatment or transfusions of blood products and had no drug abuse or HIV infection. HCV reactivation was considered taking into account along with the patient’s medical history and pertinent assistant tests, and 12 weeks of antiviral therapy with sofosbuvir-velpatasvir (one tablet orally once daily) was initiated, while sorafenib was continued as anticancer therapy (0.4g orally twice daily). Liver function of the patient was essentially normal on September 26, 2021, and methemoglobin was 451.3 ng/mL. After another antiviral treatment for 4 weeks, the recheck result after 12 weeks was HCV-RNA<15 IU/mL.
Discussion
Some scholars define HCV reactivation as an at least threefold increase in serum ALT level in oncology patients in whom the tumor has not infiltrated the liver, who did not receive hepatotoxic drugs and who had no recent blood transfusions or other systemic infections besides HCV, with or without positive HCV-RNA or ≥1 log10 IU/mL higher than baseline levels.3–5 HCV reactivation can generally be divided into three distinct stages. Firstly, a monoclonal antibody, hormone, or cytotoxic drug-induced immunosuppression which accelerates HCV replication by weakening the immune response to viral infection, with elevated HCV-RNA and usually normal liver biochemical parameters. Secondly, when the administration of cytotoxic chemotherapy or immunosuppressive drugs is suspended in patients with chronic HCV infection, the period of depressed cellular immunity can be followed by an “immunological rebound”. This phenomenon is characterized by restoration of immune function and increased inflammatory activity in the liver, resulting in rapid destruction of the HCV-infected hepatocytes and liver injury. Finally, in the recovery stage, HCV-RNA return to baseline level and liver biochemistry normalized.6
Because monoclonal antibodies can alter T cell activation, which results in cellular immunological dysfunction, as well as B cell depletion and humoral immune dysfunction, they may increase the risk of HCV reactivation.7 By increasing the amounts of mRNA, occludin, and the scavenger receptor group B type I-2 host cell protein needed for HCV infection and by removing RU-486 (an inhibitor of the glucocorticoid signaling pathway), glucocorticoids can boost the efficiency of HCV penetration by up to tenfold.8 A typical side effect of cytotoxic medicines is abnormal liver function. After receiving therapy with cytotoxic medications, HCV reactivation may happen in patients with the virus. For instance, it has been demonstrated that the drugs gemcitabine, vinorelbine, etoposide, cisplatin, fluorouracil, irinotecan, and vinblastine can reactivate HCV.9–11
The patient described in this article is at the initial stage of HCV reactivation, as per the aforementioned description. However, there are few reports of HCV reactivation by targeted drug treatment of solid tumors. Sorafenib, a kinase inhibitor of serine and threonine protein and tyrosine, prevents tumor neovascularization by inhibiting VEGFR and platelet-derived growth factor receptors, and it also directly reduces tumor cell proliferation by blocking cellular signaling pathways mediated by RAF/MEK/ERK. Although there is no evidence that sorafenib therapy for HCC will induce patients’ HCV to reactivate, it is clear from the available literature and cases that the tyrosine kinase inhibitor imatinib can cause HBV to reactivate.12–17 T cells, B cells, NK cells, DC cells, and macrophages all need tyrosine kinase to take part in cell signaling. Tyrosine kinase inhibitors may theoretically modulate the immune system by preventing these cells’ signaling. In vitro studies have found that tyrosine kinase inhibitors suppress the proliferation and activation of T lymphocytes by inhibiting the activity of the Src family kinase LCK.18 Tyrosine kinase inhibitors have also been demonstrated to prevent NK and DC cells from developing and working properly.19 However, the exact mechanism by which sorafenib causes HCV reactivation is yet unknown and requires further study.
In conclusion, HCV activation is a possibility in individuals with HCV infection who are receiving sorafenib therapy.
Ethics and Consent Statements
The patient provided informed consent to publish their case details and any accompanying images. The institutional approval was not required to publish the case details.
Funding
This work was supported by Natural Science Research Project of Higher Education Institutions in Anhui Province (KJ2021A0285).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
2. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomized, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
3. Yazici O, Sendur MA, Aksoy S. Hepatitis C virus reactivation in cancer patients in the era of targeted therapies. World J Gastroenterol. 2014;20(22):6716–6724. doi:10.3748/wjg.v20.i22.6716
4. Torres HA, Hosry J, Mahale P, Economides MP, Jiang Y, Lok AS. Hepatitis C virus reactivation in patients receiving cancer treatment: a prospective observational study. Hepatology. 2018;67(1):36–47. doi:10.1002/hep.29344
5. Costa L, Caso F, Atteno M, et al. Long-term safety of anti-TNF-alpha in PsA patients with concomitant HCV infection: a retrospective observational multicenter study on 15 patients. Clin Rheumatol. 2014;33(2):273–276. doi:10.1007/s10067-013-2378-0
6. Torres HA, Davila M. Reactivation of hepatitis B virus and hepatitis C virus in patients with cancer. Nat Rev Clin Oncol. 2012;9(3):156–166. doi:10.1038/nrclinonc.2012.1
7. Sagnelli E, Pisaturo M, Sagnelli C, Coppola N. Rituximab-based treatment HCV replication, and hepatic flares. Clinical Dev Immunol. 2012;2012:945–950. doi:10.1155/2012/945950
8. Ciesek S, Steinmann E, Iken M, et al. Glucocorticosteroids increase cell entry by hepatitis C virus. Gastroenterology. 2010;138(5):1875–1884. doi:10.1053/j.gastro.2010.02.004
9. Melisko ME, Fox R, Venook A. Reactivation of hepatitis C virus after chemotherapy for colon cancer. Clin Oncol. 2004;16:204–205.
10. Miura Y, Theriault RL, Naito Y, et al. The safety of chemotherapy for breast cancer patients with hepatitis C virus infection. J Cancer. 2013;4(6):519–523. doi:10.7150/jca.6231
11. Shoji H, Hashimoto K, Kodaira M, et al. Hematologic safety of breast cancer chemotherapies in patients with hepatitis B or C virus infection. Oncology. 2012;82(4):228–233. doi:10.1159/000336904
12. Ikeda K, Shiga Y, Takahashi A, et al. Fatal hepatitis B virus reactivation in a chronic myeloid leukemia patient during imatinib mesylate treatment. Leuk Lymphoma. 2006;47(1):155–157. doi:10.1080/14639230500236818
13. Thia TJ, Tan HH, Chuah TH, Chow WC, Lui HF. Imatinib mesylate-related fatal acute hepatic failure in a patient with chronic myeloid leukaemia and chronic hepatitis B infection. Singapore Med J. 2008;49:86–89.
14. Kang BW, Lee SJ, Moon JH, et al. Chronic myeloid leukemia patient manifesting fatal hepatitis B virus reactivation during treatment with imatinib rescued by liver transplantation: case report and literature review. Int J Hematol. 2009;90(3):383–387. doi:10.1007/s12185-009-0386-2
15. Kim SG, Chun JM, Jin R, Kim JY, Won DI, Hwang YJ. Living donor liver transplantation for acute hepatic failure caused by reactivation of hepatitis B virus infection after chemotherapy for hematologic malignancy: case reports. Transplant Proc. 2010;42(3):843–845. doi:10.1016/j.transproceed.2010.02.038
16. Wang YD, Cui GH, You Y, Li M, Xia J, Hu Y. Reactivation of chronic hepatitis B infection related to imatinib mesylate therapy in patients with chronic myeloid leukemia: two cases report and literatures review. Zhonghua Xue Ye Xue Za Zhi. 2012;33(9):743–746. doi:10.3760/cma.j.issn.0253-2727.2012.09.012
17. Lai GM, Yan SL, Chang CS, Tsai CY. Hepatitis B reactivation in chronic myeloid leukemia patients receiving tyrosine kinase inhibitor. Word J Gastroenterol. 2013;19(8):1318–1321. doi:10.3748/wjg.v19.i8.1318
18. Seggewiss R, Lore K, Greiner E, et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2015;105(6):2473–2479. doi:10.1182/blood-2004-07-2527
19. Olivieri J, Coluzzi S, Attolico I, Olivieri A. Tirosin kinase inhibitors in chronic graft versus host disease: from bench to bedside. Sci World J. 2011;11:1908–1931. doi:10.1100/2011/924954
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
