Back to Journals » Drug Design, Development and Therapy » Volume 18
Harmonising IV Oxycodone with Paediatric Perioperative Medications: A Compatibility Study Through Y-Type Connectors
Authors Youssef SH , Garg A, Song Y , Wylie NE, Garg S
Received 13 October 2023
Accepted for publication 11 March 2024
Published 22 March 2024 Volume 2024:18 Pages 899—908
DOI https://doi.org/10.2147/DDDT.S444581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Souha H Youssef,1 Alka Garg,2 Yunmei Song,1 Nicole E Wylie,3 Sanjay Garg1
1Clinical and Health Sciences, University of South Australia, Adelaide, SA, Australia; 2SA Pharmacy, Women’s and Children’s Hospital, Adelaide, SA, Australia; 3Department of Paediatric Anaesthesia, Women’s and Children’s Hospital, Adelaide, SA, Australia
Correspondence: Sanjay Garg, University of South Australia, Adelaide, SA, 5000, Australia, Tel +618 8302 1575, Email [email protected]
Purpose: Co-administering multiple intravenous (IV) agents via Y-connectors is a common practice in hospitalised and fasting surgical patients. However, there is a lack of reliable data confirming the physical compatibility of some combinations including IV oxycodone, a drug that is gaining increasing popularity in the perioperative period. Concern regarding physical drug incompatibilities precludes concurrent coadministration with other common drugs through a single lumen. This can result in the cessation of infusions to allow the administration of other medications, resulting in exacerbation of acute pain. This study aims to evaluate the physical compatibility of IV oxycodone with some commonly co-administered drugs and IV fluids.
Methods: Mixtures of oxycodone (1mg.mL− 1) and the tested drugs and IV fluids were prepared in a ratio of 1:1. The mixtures were examined at 0 and 60 minutes from mixing and assessed using the European Conference Consensus Standards. This involved visual inspection (precipitation, turbidity, colour change, gas formation), spectrophotometry, and pH change. The tested drugs included ketamine, tramadol, clonidine, vancomycin, piperacillin/tazobactam, dexmedetomidine, cefotaxime, gentamicin, and paracetamol. In addition, the commonly used IV fluids tested included glucose 5% + sodium chloride 0.9% + 60 mmol potassium chloride, plasmalyte + dextrose 5%;plasmalyte + dextrose 5% + 55 mmol potassium chloride, plasmalyte + dextrose 5% + 55mmol potassium acetate, plasmalyte + dextrose 5% + 55mmol potassium dihydrogen phosphate, Hartmann’s solution, Standard pediatric Total Parenteral Nutrition (TPN) 20/100 and TPN 25/150.
Results: IV oxycodone (1 mg.mL− 1) showed no visual changes; no spectrophotometric absorption variability at 350, 410, or 550nm; and no pH changes of > 0.5 at 0 or 60 minutes with any of the tested drugs or fluids in the concentrations tested.
Conclusion: According to European Consensus Conference Standards, IV Oxycodone at 1 mg.mL− 1 is physically compatible in a ratio of 1:1 v/v with all investigated drugs and fluids tested for at least 60 minutes.
Keywords: analgesia, physical compatibility, co-administration, intravenous fluid, oxycodone, Y-connector
Graphical Abstract:
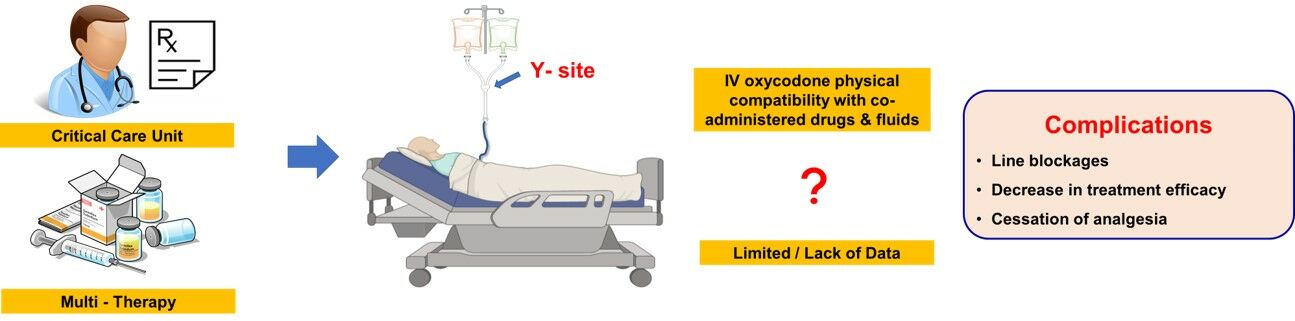
Introduction
Intravenous (IV) drug incompatibility has been defined as the physical or chemical reactions that occur in vitro between two or more drugs when the solutions are combined in the same syringe, tubing, or bottle.1 The increasing diversity and frequency of IV therapies in the perioperative period has led to a robust body of literature around administration errors, with studies quoting error rates of 10.1% to 69.7%2,3 of which up to 25.5% have been classed as ‘serious’ errors.2 Physical drug incompatibility has been identified as one of the four most significant and common sources of IV therapeutics administration errors.2 Efforts to avoid this error have resulted in the practice of non-administration of two agents through the same line at the same time unless physical compatibility has been demonstrated.
Insufficiently managed postoperative pain may predispose individuals to chronic postsurgical pain, impede rehabilitation, hinder recovery efforts, prolong hospitalisation, necessitate readmission, lower quality of life, and reduce patient satisfaction. Providing continuous IV analgesia was found to be favourable compared to other methods in managing pain, especially during the immediate post-operative period, demonstrating faster onset relief and more predictable pharmacokinetics.4
IV oxycodone is available in wards as a continuous infusion or for use in a bolus-dose technique. A 2019 meta-analysis of opioids for post-operative pain demonstrated oxycodone to have better analgesic efficacy than fentanyl, and comparable analgesic efficacy to morphine but with fewer adverse events.5 As such, its use in Patient Controlled Analgesia (PCA), Nurse Controlled Analgesia (NCA), and continuous infusion techniques is becoming widely accepted in Australasia.6
However, there is limited data on IV oxycodone’s physical compatibility with other drugs and fluids commonly used in the perioperative period.
Some studies reported the stability of oxycodone with ketamine in polypropylene syringes and polyvinyl chloride bags7 and with a range of drugs.8 However, not all prescribed combinations have had their compatibility parameters established. This lack of data, and the clear and significant clinical implications of drug incompatibility errors,2 means that oxycodone infusions may be ceased whilst other agents are administered, risking inadequate analgesia and associated risk of pain-related complications and prolonged recovery; or that additional IV access may need to be sited with the risk of infection and thrombophlebitis, especially in fasting perioperative patients.9,10
This study provides physical compatibility data for IV oxycodone with other commonly administered IV drugs and fluids. The compatibility criteria used were the European Consensus Conference Standards11 due to their widespread acceptance in the pharmacy, medication, and nursing literature. The tested drugs were ketamine, clonidine, tramadol, vancomycin, piperacillin/tazobactam, dexmedetomidine, cefotaxime, gentamicin, and paracetamol. The fluids tested included commonly used combinations of glucose 5%, sodium chloride 0.9%, potassium chloride, plasmalyte plus dextrose 5%, potassium acetate, potassium dihydrogen phosphate, Hartmann’s solution, and Total Parenteral Nutrition (TPN) solutions.
The most concentrated clinically relevant preparations and concentrations of these drugs with IV oxycodone (1mg.mL−1) were tested, if any drug was to demonstrate physical incompatibility, increasing dilutions of the drug would be tested until either no changes were observed, or the concentrations became so dilute as to be clinically irrelevant.
Materials and Methods
Materials
The medications and IV fluids used in this study were kindly provided by Women & Children Hospital Pharmacy (Adelaide, South Australia, Australia) and include Oxycodone HCl for injection (Kalceks - Medsurge, Melbourne, Australia), Ketamine HCl for injection (Baxter Pharmaceuticals, Ahmedabad, India), Clonidine (Medicianz Healthcare Pty Limited, Melbourne, Australia), Tramadol HCl for injection (Sandoz, New South Wales, Australia), Vancomycin HCl; 500 mg powder for injection (Alphapharm (Mylan Australia), Queensland, Australia), Dexmedetomidine HCl for injection (InterPharma Pty Ltd, New South Wales, Australia), Piperacillin 4 g (as sodium salt) / Tazobactam 500 mg (as sodium salt) (Piptaz-AFT 4 g/0.5g) (AFT Pharmaceuticals, New South Wales, Australia), Cefotaxime sodium powder for injection (Pfizer, New South Wales, Australia), Gentamicin for injection (Pfizer, New South Wales, Australia), and Paracetamol for injection (B. Braun, New South Wales, Australia). Additionally, the IV fluids included plasmalyte + 5% dextrose (Baxter, New South Wales, Australia), glucose 5% + sodium chloride 0.9% (Baxter, New South Wales, Australia), potassium chloride (10 mmol) (Pfizer, New South Wales, Australia), potassium acetate (25 mmol) (Pfizer, New South Wales, Australia), potassium dihydrogen phosphate (13.6%) (Phebra, New South Wales, Australia), Hartmann's (compound sodium lactate) solution (Baxter, New South Wales, Australia), standard paediatric TPN (20/100) and (25/150) (Baxter, New South Wales, Australia), and sodium chloride injection BP 0.9% (Fresenius Kabi, New South Wales, Australia).
Methods
Oxycodone was tested at a concentration of 1 mg.mL−1 which is generally used in ICU ward settings. Other tested drugs were prepared at the highest concentrations used clinically, made up with the standard diluent of sodium chloride injection BP 0.9% based on local IV administration guidelines.5 The tested drugs are detailed in Table 1 and IV fluids in Table 2.
 |
Table 1 Drugs Used in Compatibility Testing and Their Tested Concentrations |
 |
Table 2 Fluids Used in Compatibility Testing and Their Tested Concentrations |
Physical compatibility was evaluated by preparing mixtures of oxycodone and each co-administered drug or IV fluid at a ratio of 1:1, in transparent tubes at room temperature and unprotected from light to simulate Y-site administration conditions in hospitals. The tested mixtures were prepared in duplicates and examined at times 0 (immediately after mixing) and 60 minutes later. The mixtures were first inspected by the unaided eye against white and black backgrounds under natural light for colour change and gas liberation (represented as effervescence), observations were recorded by analyst S.H.Y.
For subvisual examination, spectrophotometric measurements (Evolution 201 UV-visible spectrophotometer – INSIGHT TM 2 software) at 350, 410, and 550 nm were taken to determine possible undetected turbidity and/or colour change by the unaided eye. Changes in absorbance values above 0.04, 0.04, and 0.01 nm at 350, 410, and 550 nm, respectively, were considered physically incompatible.12,13 For this research, a full spectral scan (200–800 nm) was performed. Any changes in terms of extra peaks or absorbance intensity were to be compared to the original spectrum and assessed for incompatibility. Finally, the pH was measured directly after mixing, the mixture was left at room temperature unprotected from light for 60 minutes to mimic ward conditions and the pH was measured again, changes in the values were noted. Combinations were considered physically compatible when no visual changes were noted (absence of colour change, turbidity, precipitate, and effervescence), the difference in absorbance values at 350, 410, and 550 nm did not exceed acceptable limits and pH changes did not exceed 0.5. Figure 1 shows a schematic diagram for the followed assessment protocol for physical compatibility.14
 |
Figure 1 The methodology and assessment protocol for the determination of physical compatibility of the drug mixtures (Adapted and translated from Juan EP, Palau MM, Cerdá SA, Rubert MA, Nicolau BR. Compatibilité physique de médicaments administrés dans l’unité de soins intensifs. Pharmactuel. 2015;48(3):146–152 with permission from Pharmactuel.14 |
This was an experimental in-vitro study conducted at the University of South Australia, Clinical and Health Sciences academic unit.
Results
The tested mixtures of oxycodone and the selected co-administered drugs and IV fluids mixed at the ratio of 1:1 were assessed for their physical compatibility. The mixtures showed no visual precipitation, turbidity, colour change, or gas liberation in any of the combinations when examined against both white and black backgrounds (Table 3).
 |
Table 3 Results of Visual Inspection of the Studied Drugs and Fluids in Combination with 1 mg.mL−1 Oxycodone at a Ratio of 1:1 for 60 Minutes |
pH changes between values measured at t0 and t60 did not exceed 0.5 units and thus were not significant in any of the tested combinations (Table 4). The spectra of the mixtures immediately after mixing and after 60 minutes were overlapping with no extra or missing peaks. Spectrophotometric absorbance changes at the defined wavelengths were within accepted limits (Table 5).
 |
Table 4 pH Changes in the Studied Drugs and Fluids Combinations with 1mg.mL−1 Oxycodone at a Ratio of 1:1 for 60 Minutes |
 |
Table 5 Absorbance Changes in the Studied Drugs and Fluids Combinations with 1mg.mL−1 Oxycodone at a Ratio of 1:1 for 60 Minutes at Wavelengths 350, 410, and 550 nm |
Discussion
Every year, approximately 7.7 million Australians will receive an intravenous cannula (IVC), a number which includes up to 70% of hospitalised patients.9 Current best practices in the management of intravenous access include minimising the number of cannulae and optimising the type of IVC to increase patient comfort, facilitate device utility, and minimise rates of line-associated infections, phlebitis, dislodgement, and other complications.15
Multiple medications and fluids are routinely administered via these single-site IVCs, often via y-connectors that minimise the duration of, but do not exclude, contact between infusions.16 They are commonly administered in perioperative patients who may be fasting for prolonged periods,17 and in others in whom the oral route may be inappropriate or inaccessible.
As previously mentioned, IV drug incompatibility involves physical or chemical reactions resulting from mixing administered fluids.1 Physical reactions can cause visible changes including precipitation, changes in colour, consistency, or opalescence, or gas production.1,16,18,19 This may impede patient safety and/or therapeutic efficacy.16,19 As a result, physical compatibility studies focusing on these aspects form a pressing part of ongoing research efforts,16,20–22 but data remains lacking, particularly for newer agents such as IV oxycodone.
Concern surrounding drug incompatibility errors has resulted in the practice of ceasing ward-based continuous IV drug and fluid infusions (notably analgesia) for periods ranging from minutes to several hours when the administration of additional therapeutic agents such as antibiotics and electrolyte-specific crystalloid fluids is required. Avoidance of drug incompatibility error forms the basis of many state and institutional guidelines23,24 and drug administration tools.25 This practice may lead to significant pauses in the provision of IV analgesia to fasting perioperative patients experiencing acute pain, leading to complications,26 prolonged rehabilitation,26,27 and risking the development of chronic pain.26,28 Alternatively, it may require the placement of additional IV access and the attendant risks.15
While there remains significant global effort towards antibiotic stewardship, IV antibiotics remain therapeutically crucial, especially in those patients unable to tolerate oral routes, or who are early in their antibiotic course, and these medications see common multi-dose IV use in surgical wards.29,30 Compatibility with the IV preparation of oxycodone has not been established for many of these agents.
Perioperative patients may also require multimodal management of acute surgical pain, including IV analgesics.22,26,31 Opioid-based techniques may include PCA or NCA analgesia, or continuous IV infusions, with the latter two treatment modalities of particular ubiquity in pediatric hospital settings.31 IV oxycodone is increasingly accepted as an agent of choice in these settings,5,6 due to a favorable side effect profile, good analgesic efficacy, and ease of conversion to oral dosages.6
Significant advances in IV fluid therapy since the 1950s have resulted in a proliferation of alternatives to plain 0.9% Sodium chloride for IV crystalloid therapy.6,32,33 This is particularly relevant to pediatric patients.6,33 Compatibility with the IV preparation of oxycodone has not been established for many of these fluids, and many of these drugs.22
Since the early 21st century, physical compatibility assessments have become ever more widely accepted in the pharmaceutical, medical, and nursing literature,16,21 along with the standardisation of infusion solution concentrations.34 For transparent preparations, such as IV oxycodone, these include assessment of visual changes such as turbidity, precipitation, gas formation, and colour change,11,16,20,21 as well as pH changes and spectrophotometry.11,16,20–22 There are several reported methods for the determination of turbidity in compatibility assays of co-administered IV fluids, including spectrophotometric determination,35,36 turbidimeter,37 and light obscuration analysis35 amongst other methods.35 Moreover, visual inspection was applied to almost all reported compatibility assays. As there is no standardised procedure, spectrophotometric measurements were used to confirm precipitation and/or colour changes.35 This formed the basis of our assessment criteria for the physical compatibility of oxycodone with the selected drugs and fluids.
Our study demonstrated physical compatibility over a period of 60 minutes, of all tested drugs and fluids at a ratio 1:1, v:v with IV oxycodone (1 mg.mL−1). The drug contact is short when considering Y-connectors; with the slowest infusion rate (10 mL.h−1) the contact time was predicted to be 10 minutes.21 To ensure comprehensive testing, we selected a duration of 60 minutes, encompassing all potential scenarios. Although a previous study showed incompatibility of oxycodone (50 and 3 mg.mL−1) with 5% dextrose and water for injection in polycarbonate syringes after 7 days,8 our findings of compatibility could be attributed to the lower dosing and contact time applied in the proposed experimental setting. This data fills a gap in the current literature surrounding the avoidance of drug incompatibility errors during the administration of IV oxycodone in clinical settings where other common IV agents must be administered, particularly in the context of limited IV access sites. Of importance is also that this physical compatibility was demonstrated at the most concentrated clinically used preparations in our institution of all agents tested. It is safe to extend the results to lower concentrations of all drugs and IV fluid additives tested.
It is important to note that this study while conforming to European Consensus Guidelines for assessing the physical compatibility of two agents (and also meeting commonly used indicators of compatibility as identified widely in the perioperative and critical care literature), does not answer or attempt to confer surety in regards to drug bioavailability, stability of in vivo pharmacokinetics or pharmacodynamics of either of the two co-infused agents in any single test. Similarly, no comment in regards to the clinical safety of drugs (for example, vancomycin, with its associated risk of “red man syndrome” on rapid infusion30) if “carried inward” to the patient by a bolus of oxycodone.
Further research may be required to establish this aspect of clinical safety of use, however, a degree of confidence in the decreased likelihood of a physical drug compatibility error is provided by this research when clinicians, pharmacists, and nurses are contemplating concurrent administration of IV oxycodone with other infused agents, such as fluids, other common analgesics, and antibiotics, as tested here.
Conclusion
This study addresses a critical issue in intensive care wards, assessing the physical compatibility of IV oxycodone with commonly co-administered drugs and IV fluids. The results provide much needed data that may contribute to clinical decision-making processes in regards to the safe administration of the evaluated combinations through Y-connectors. The information can guide healthcare professionals in optimising pain management strategies while minimising the risk of infusion-related complications, ultimately improving the quality of care for patients.
Abbreviations
IV, Intravenous; IVC, Intravenous cannula; NCA, Nurse Controlled Analgesia; PCA, Patient Controlled Analgesia; TPN, Total Parenteral Nutrition.
Acknowledgments
We gratefully acknowledge Dr. Michael Ward for his management of all scheduled drugs in this study conducted in the University of South Australia. The abstract of this paper was presented at the Medicines Management 2022, 46th Society of Hospital Pharmacists of Australia (SHPA) National Conference as a poster presentation with interim findings. The poster’s abstract was published in the book of abstracts available: https://mm2022.shpa.org.au/wp-content/uploads/225.pdf
Funding
The authors acknowledge the financial support provided the Society of Paediatric Anaesthesia in New Zealand and Australia (SPANZA) (Grant number PG 7111759) and the Women’s and Children’s Hospital Department of Paediatric Anaesthesia Special Purposes fund.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Marsilio NR, Silva D, Bueno D. [Drug incompatibilities in the adult intensive care unit of a university hospital] Incompatibilidades medicamentosas em centro de tratamento intensivo adulto de um hospital universitário. Rev Bras Ter Intensiva. 2016;28(2):147–153. doi:10.5935/0103-507x.20160029
2. Johanna IW, Marilyn IR, Amanda W, Dave P. Errors in the administration of intravenous medications in hospital and the role of correct procedures and nurse experience. BMJ Qual Saf. 2011;20(12):1027. doi:10.1136/bmjqs-2011-000089
3. Adam S, Michela C, Janine C, Michelle R, Tom S, Emma W. Incidence and prevalence of intravenous medication errors in the UK: a systematic review. Eur J Hosp Pharm. 2020;27(1):3. doi:10.1136/ejhpharm-2018-001624
4. Pergolizzi J, Seow-Choen JV, Wexner F, Zampogna SD, Raffa G. Perspectives on intravenous oxycodone for control of postoperative pain. Pain Pract. 2016;16(7):924–934. doi:10.1111/papr.12345
5. Raff M, Belbachir A, El-Tallawy S, et al. Intravenous oxycodone versus other intravenous strong opioids for acute postoperative pain control: a systematic review of randomized controlled trials. Pain Ther. 2019;8(1):19–39. doi:10.1007/s40122-019-0122-4
6. Halliwell R. Patient-controlled analgesia. In: Acute Pain Management: Scientific Evidence.
7. Daouphars M, Hervouët C-H, Bohn P, et al. Physicochemical stability of oxycodone-ketamine solutions in polypropylene syringe and polyvinyl chloride bag for patient-controlled analgesia use. Eur J Hosp Pharm. 2018;25(4):214–217. doi:10.1136/ejhpharm-2016-000965
8. Pleasance S, Hines S. Compatibility of an injectable high strength oxycodone formulation with typical diluents, syringes, tubings, infusion bags and drugs for potential co-administration. EJHP Pract. 2009;15:1.
9. Management of Peripheral Intravenous Catheters Clinical Care Standard. Australian commission on safety and quality in health care; 2023. Available from: https://www.safetyandquality.gov.au/standards/clinical-care-standards/management-peripheral-intravenous-catheters-clinical-care-standard.
10. Guanche-Sicilia A, Sánchez-Gómez MB, Castro-Peraza ME, Rodríguez-Gómez J, Gómez-Salgado J, Duarte-Clíments G. Prevention and treatment of phlebitis secondary to the insertion of a peripheral venous catheter: a scoping review from a nursing perspective. Healthcare. 2021;9(5). doi:10.3390/healthcare9050611
11. Bardin C, Astier A, Vulto A, et al. Guidelines for the practical stability studies of anticancer drugs: a European consensus conference. Eur J Hosp Pharm Sci Pract. 2012;19(3):278. doi:10.1136/ejhpharm-2012-000112
12. Bardin C, Astier A, Vulto A, et al. Guidelines for the practical stability studies of anticancer drugs: a European consensus conference. Ann Pharm Fr. 2011;69(4):221–231. doi:10.1136/ejhpharm-2012-000112
13. Kleinlein M, Marschler S, Neininger MP, Hoeckel M, Bertsche T. Y‐site administration of electrolyte solutions and injectable Acetaminophen—a physical compatibility study with combinations frequently used in pediatric intensive care and anesthesia. Pediatr Anesthesia. 2023;33(1):69–78. doi:10.1111/pan.14569
14. Juan EP, Palau MM, Cerdá SA, Rubert MA, Nicolau BR. Compatibilité physique de médicaments administrés dans l’unité de soins intensifs. Pharmactuel. 2015;48(3):146–152.
15. Evidence sources: management of peripheral intravenous catheters clinical care standard Australian Commission on safety and quality in health care. Available from: https://www.safetyandquality.gov.au/sites/default/files/2021-05/evidence_sources_-_management_of_peripheral_intravenous_catheters_clinical_care_standard.pdf.
16. Lao GC, Reyes MR, Turet JR, Dot MP, Muner DS, Cabezas CL. Compatibility of drugs administered as Y-site infusion in intensive care units: a systematic review. Med Intensiva. 2020;44(2):80–87. doi:10.1016/j.medin.2018.08.004
17. Glassford NJ, French CJ, Bailey M, Martensson J, Eastwood GM, Bellomo R. Changes in intravenous fluid use patterns in Australia and New Zealand: evidence of research translating into practice. Crit Care Resuscitation. 2016;18(2):78–88. doi:10.1016/S1441-2772(23)01009-8
18. Trissel LA. Handbook on injectable drugs. In: Handbook on Injectable Drugs. ASHP; 2007:1720.
19. Paes GO, Moreira SO, Moreira MB, Martins TG. Drug incompatibility in the ICU: review of implications in nursing practice. Rev Eletrôn Enferm. 2017;19:a20.
20. D’Huart E, Vigneron J, Demoré B. Physical compatibility of intravenous drugs commonly used in intensive care units: an observational study and physical compatibility laboratory tests on anti-infective drugs. Pharm Technol Hosp Pharm. 2019;4(1):29–40. doi:10.1515/pthp-2019-0005
21. Kanji S, Lam J, Johanson C, et al. Systematic review of physical and chemical compatibility of commonly used medications administered by continuous infusion in intensive care units. Crit Care Med. 2010;38(9):1890–1898. doi:10.1097/CCM.0b013e3181e8adcc
22. Kim YS, Lee CH, Kim AR, et al. Microbiological and physicochemical stability of fentanyl, oxycodone, hydromorphone, ketorolac, ramosetron, and ondansetron for intravenous patient-controlled analgesia: an in vitro study. Pain Physician. 2021;24(6):E829.
23. South Australian Neonatal Medication Guidelines: intravenous medication compatibility chart. Department of Health, Government of South Australia. Available from: https://www.sahealth.sa.gov.au/wps/wcm/connect/593fd13a-4f41-4bf9-af01-ae686f1e59ed/IV+Compatibility+in+Neonates_Neo_v3_0.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-593fd13a-4f41-4bf9-af01-ae686f1e59ed-ocQIQkC.
24. Critical care intravenous drug administration guide. St George’s Healthcare. Available from: www.gicu.sgul.ac.uk/teaching/resources/pharmacology-and-toxicology/files/itu_IV_guide_-_2008_update_v2.pdf.
25. Lexicomp trissels IV compatibility databases. Wolters Kluwer; 2023. Available from: https://www.wolterskluwer.com/en/solutions/lexicomp/resources/lexicomp-user-academy-old/trissels-iv-compatibility-databases.
26. Garimella V, Cellini C. Postoperative pain control. Clin Colon Rectal Surg. 2013;26(3):191–196. doi:10.1055/s-0033-1351138
27. Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth. 2001;87(1):62–72. doi:10.1093/bja/87.1.62
28. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi:10.1016/s0140-6736(06)68700-x
29. McCarthy K, Avent M. Oral or intravenous antibiotics? Austr Prescr. 2020;43(2):45. doi:10.18773/austprescr.2020.008
30. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139–e152.
31. Gai N, Naser B, Hanley J, Peliowski A, Hayes J, Aoyama K. A practical guide to acute pain management in children. J Anesth. 2020;34(3):421–433. doi:10.1007/s00540-020-02767-x
32. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823–832. doi:10.1542/peds.19.5.823
33. Bruck E, Aceto T Jr, Lowe CU. Intravenous fluid therapy for infants and children. Physiologic principles and a practical regimen with examples of application. Pediatrics. 1960;25:496–516. doi:10.1542/peds.25.3.496
34. Nemec K, Kopelent-Frank H, Greif R. Standardization of infusion solutions to reduce the risk of incompatibility. Am J Health Syst Pharm. 2008;65(17):1648–1654. doi:10.2146/ajhp070471
35. Staven V, Wang S, Grønlie I, Tho I. Development and evaluation of a test program for Y-site compatibility testing of total parenteral nutrition and intravenous drugs. Nutr J. 2016;15(1):29. doi:10.1186/s12937-016-0149-x
36. Kufel WD, Miller CD, Johnson PR, Reid K, Zahra JJ, Seabury RW. Y-site incompatibility between premix concentrations of vancomycin and piperacillin-tazobactam: do current compatibility testing methodologies tell the whole story? Hosp Pharm. 2017;52(2):132–137. doi:10.1310/hpj5202-132
37. Trissel LA, Martinez JF. Visual, turbidimetric, and particle-content assessment of compatibility of vinorelbine tartrate with selected drugs during simulated Y-site injection. Am J Hosp Pharm. 1994;51(4):495–499.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
