Back to Journals » Chronic Wound Care Management and Research » Volume 3
Hard-to-heal diabetes-related foot ulcers: current challenges and future prospects
Authors Nube V, Frank G, White J, Stubbs S, Nannery S, Pfrunder L, Twigg SM, McLennan SV
Received 2 February 2016
Accepted for publication 24 May 2016
Published 9 November 2016 Volume 2016:3 Pages 133—146
DOI https://doi.org/10.2147/CWCMR.S84990
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Marco Romanelli
Video abstract presented by Vanessa Nube.
Views: 6686
Vanessa Nube,1 Georgina Frank,1 Jessica White,1 Sarah Stubbs,1 Sara Nannery,2 Louise Pfrunder,2 Stephen M Twigg,3 Susan V McLennan4
1Department of Podiatry, Sydney Local Health District, Camperdown, NSW, Australia; 2Diabetes Centre High Risk Foot Service, Royal Prince Alfred Hospital, Camperdown, NSW, Australia; 3Discipline of Medicine, Sydney Medical School, University of Sydney, Camperdown, Sydney, NSW, Australia; 4Department of Endocrinology, Royal Prince Alfred Hospital, Camperdown, Sydney, NSW, Australia
Abstract: Diabetes-related foot ulceration is a frequent cause for hospital admission and the leading cause of nontraumatic lower limb amputation, placing a high burden on the health system, patient, and their families. Considerable advances in treatments and the establishment of specialized services and teams have improved healing rates and reduced unnecessary amputations. However, amputation rates remain high in some areas, with unacceptable variations within countries yet to be resolved. Specific risk factors including infection, ischemia, ulcer size, depth, and duration as well as probing to bone (or osteomyelitis), location of ulcer, sensory loss, deformity (and high plantar pressure), advanced age, number of ulcers present, and renal disease are associated with poor outcome and delayed healing. To assist in prediction of difficult-to-heal ulcers, more than 13 classification systems have been developed. Ulcer depth (or size), infection, and ischemia are the most common risk factors identified. High-quality treatment protocols and guidelines exist to facilitate best practice in the standard of care. Under these conditions, 66%–77% of foot ulcers will heal. The remaining proportion represents a group unlikely to heal and who will live with a non-healing wound or undergo amputation. The authors have applied their experience of managing patients in this discussion of why some ulcers are harder to heal. The article explores the effects of patient non-adherence to treatment, comorbid mental illness, a failure of research to be translated into the everyday practice of many clinicians, and the impact of delayed access to specialized treatment. These factors when combined with the main published risk factors of size, infection, ischemia and pressure are perceived as critical barriers to healing.
Keywords: diabetic foot, healing, infection, delayed treatment, referral
Introduction
Diabetes and its complications represent a major health challenge in both the developed and developing countries, with an estimated 415 million adults affected globally.1 A largely underestimated complication of diabetes is diabetes-related foot ulcer (DFU), which carries a lifetime risk of 15% of all persons with diabetes2 and is responsible for much morbidity and mortality.3 The International Diabetes Federation has studied the impact of this complication and estimates that an amputation due to diabetes occurs every 4 seconds somewhere in the world. For this reason, there has been a large amount of effort directed to coordinated approaches to the prevention, identification, and management of DFUs. The implementation of treatment guidelines such as those published by the International Working Group on the Diabetic Foot (IWGDF),4 The National Institute for Health and Care Excellence,5 Australia’s National Guidelines,6 and many other notable documents has resulted in better organized care, prevention of amputation, and improvement in patient outcomes.7,8 While direct comparisons should be made with caution because of differences in measurement and ascertainment of diabetes incidence across countries, it is evident that there is high variation between and within countries.9–11 This suggests that universal adoption of best practice and equity of access to contemporary foot care for people with diabetic foot complications are still a work in progress and may not be adequately prioritized in some regions.
It is well recognized that DFUs are notoriously hard to heal; however, when treatment is provided according to the evidence-based practice guidelines (EBG) whereby there is identification and management of infection and ischemia, in combination with wound debridement, pressure offloading, and appropriate patient and health professional (HP) education, many patients will achieve healing. Results from large studies and wound registries with a 1-year follow-up period have reported healing rates of 66%–77%, respectively.12,13 While such studies provide an overall benchmark for expected percentage of ulcers healed, they point to over a quarter of DFUs failing to heal. Why this occurs is not certain. The time to healing and risk of amputation for individual patients are known to vary markedly based on patient and ulcer factors such as infection, ischemia, ulcer size, and ulcer duration, as well as more difficult to quantify extrinsic factors such as the standard of foot care provided14,15 and patient adherence to prescribed foot ulcer care. Therefore, we need to identify and explore what makes a wound “hard to heal” and to mitigate the barriers to healing from the perspective of HPs involved in the care of people with DFU.
The purpose of this article is to discuss some of the issues we (HPs and research workers) believe contribute to the poor prognosis of some DFUs and make them hard to heal. These are shown schematically in Figure 1. While the physical risk factors for delayed or non-healing of DFU are largely well described and evidenced, other issues we explore are difficult to quantify and have no proven causal relationship. We include issues relating to the health care provider in terms of translation of evidence into everyday practice and patient behavior, both of which affect the timeliness and quality of treatment. These factors are complex and many are interrelated. Each warrants due consideration if healing outcomes are to be improved.
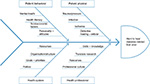  | Figure 1 Ishikawa diagram of suspected issues, which contribute to the problem of the hard-to-heal DFU. Abbreviation: DFU, diabetes-related foot ulcer. |
Defining the hard-to-heal DFU
The measureable risk factors to delayed healing or amputation, which define DFUs that will be hard to heal, have now been extensively studied across patient populations. Analysis of different variables has informed predictive models, and at least 13 grading or classification systems have been proposed for use in research and clinical practice including the University of Texas (UT), PEDIS (perfusion, extent, depth, infection and sensation), S(AD) (size (area, depth)), SAD (sepsis, arteriopathy and denervation), and MAID systems.16–18 The documented factors most strongly associated with poor outcomes are advanced age,19 infection,12,16,20 biofilm presence,21 the presence of ischemia,12,16,18,20,22 wound duration,18,23,24 large wound size (depth and circumference),16,18,24–26 site,27 having multiple ulcers,18,22,24 end-stage renal disease,12 heart failure,12 male sex,12 immobility,12 and depressive symptoms.28
The use of predictive data in grading and classification of wounds is useful in guiding management, aids communication between HPs, and helps describe the case mix for the purpose of reporting and evaluating performance of a particular service. Importantly, use of predictive grading informs conversations with patients and their families regarding the goals of treatment based on ulcer severity and prognosis.
Wound fluid analysis may also have utility in predicting wound healing for individual patients to guide treatments. In particular, increased wound fluid levels of matrix-degrading enzymes called matrix metalloproteinases (MMPs) have been detected in human non-healing wounds including DFUs, and measurement of wound fluid MMP-9 in combination with transforming growth factor β and the MMP inhibitor TIMP-1 can predict future poor healing with high sensitivity and specificity.29 Other possible wound fluid factors that have received less attention in diabetic wounds include pH and endotoxin levels both of which are known to be altered in chronic non-healing wounds.30–32
Health professionals and health system
Translating evidence into clinical practice
The translation of quality research into the clinical environment ensures that HPs are fully informed and treatment is evidence based. The application of evidence-based practice has further potential to improve health outcomes and strengthen health systems by providing more efficient and cost-effective care.33–35
To support the translation of the high volume of studies and often-complex information, EBG citing quality evidence together with expert consensus (where evidence is lacking) have been developed. However, despite evidence that support a prompt referral to a multidisciplinary team with standardized clinical practices when a hard-to-heal DFU is identified,6,36,37 HPs do not consistently implement these processes.38,39 A broad illustration of this is the 2008 report of the Eurodiale Studies, which documents that across 14 treatment centers, a quarter of patients had delayed referral to multidisciplinary teams of >3 months, the majority had no or inadequate offloading, and almost half the patients with severe ischemia had no vascular imaging.39 This suggested widespread lack of adoption of the well-known “International Working Group, Diabetic Foot Consensus Guidelines”4 or those of other peak organizations by established treatment centers within developed countries and also by their referrers.
Understanding the reasons why EBG are not successfully translated into practice could help inform funding and policy decisions with potential to support better health outcomes for people with DFU and reduce associated costs. Key individual HP attributes associated with translation of research into clinical practice are open-mindedness and capacity for critical thinking; however, for many HPs, experiential learning, formal education, and discussions with other HPs will inform their practice more than professional journals.40 At an organizational or system level, historical precedence has been shown to influence how knowledge translation is valued and developed and how HPs are supported during its implementation. Resistance from professional groups to the introduction of EBG and the degree to which recommendations are implemented by clinicians are often raised as a reason that positive change is not forthcoming.41,42 This lack of change may be explained within the general health community by HPs finding the continual introduction of new practice information difficult to implement within a constantly changing environment.43 To address this, a system-based approach that introduces behavior change support is suggested as a viable means of facilitating required changes in practice.42 Factors that impede implementation of EBG include cost of the intervention (including where too expensive or too resource intensive), the research not being applicable to the particular patient population,44 HPs lacking skills and equipment to apply recommendation, or changes being inconsistent with desired practice of the HP.41
To be adopted, evidence and EBG must be known to HPs and health system administrators, and as with any change, the adoption of best practice requires a motivating force, as well as alignment of the goals of the organization and available resources. The utilization of inexpensive, passive approaches, which rely on publication, mail outs, and email, is only minimally effective in ensuring the uptake and utilization of EBG by HPs.44 These approaches fail to capture and engage all the stakeholders who have a role in both the provision of the resources and the planning required for implementation of EBG and ongoing reinforcement of their practice. Ultimately, successful application of EBG depends on adequate funding as well as political and organizational support around developing an environment that is able to fully implement best practice. A range of strategies that identify and target modifiable barriers is important, and effective implementation methods should be employed.41 A system-based approach that includes performance measurement, point-of-care access to practical information on treatment including where to refer, education for HPs, and adequate resources are approaches likely to improve translation of research and achievement of sustainable benefits in terms of patient outcomes.42
Performance monitoring through auditing processes is a recommendation of the IWGDF45 for all foot care services. Monitoring and reporting process and patient outcomes has been shown to be effective in influencing positive change on a large scale and represents a future prospect for improvement of care and patient outcomes.36,46 Data on costs and better prevalence information arising from initiatives such as the UK’s DFU registry and US Wound Registry will provide incentives for investment in these approaches at an organizational level. Training of HPs is also essential. The International Diabetes Federation “Train-the-Foot-Trainer” program, teaching DFU management and how to set up and use data to monitor outcomes, has achieved gains largely through education.47
Some of the most important evidence for improving outcomes for people with DFU comes from the establishment of specialized or dedicated, multidisciplinary foot care teams (MDTs).48,49 This multidisciplinary approach requires the alignment of many disciplines. While this is challenging, many countries have achieved significant improvements in patient outcomes with this approach.
Within our region, reinforcement of best practice through a multifaceted approach and standardized data collection has contributed to reduced rates of lower extremity amputation and hospital admission in Queensland, and development of an Australian Register for DFU is underway.36,50 With the establishment of these services comes the imperative that patients have prompt and equitable access.
Delayed referral to multidisciplinary services
A coordinated MDT is supported in EBG, but universal access to a MDT is not yet a reality. Even in countries where specialized treatment centers exist, practical reasons such as locality, resources, and patient mobility may prevent attendance. Therefore, a proportion of DFUs are managed outside the multidisciplinary team, and under the care of the patient’s primary care doctor, nurse, or podiatrist.
Key circumstances where MDT management is of a necessity include ulcers probing to tendon, joint, or bone, ulcers that fail to reduce in size after 4 weeks, ischemia, and ascending cellulitis.51 At its most critical stage, such as when the DFU is complicated by limb- or life-threatening sepsis, particularly in the presence of critical limb ischemia, hospital admission is essential. However, if EBG were implemented fully, earlier treatment by an MDT in the ambulatory or outpatient setting, such as high-risk foot service (HRFS), would avert the need for hospitalization in most cases.
Treatment delay is a risk factor for amputation and is associated with longer treatment time,15,39 increased wound size,23 and poorer outcomes.52 Conversely, improved outcomes for patients with a DFU are largely attributed to wounds presenting at a stage when they were more “prognostically favorable”, suggesting earlier access to care.8,53 Time to presentation to a HRFS for patients with a DFU has been reinforced as a key performance indicator for our service54 since local data (unpublished) showing increased ulcer severity with delayed referral.
Reasons for treatment delay are often ascribed to patient behavior, but HPs behaviors explain at least some of the delay. In Europe, 27% of all patients had been treated for >3 months before referral to a specialized multidisciplinary foot service, and a primary care physician had treated close to half of these until referral.39 Sanders et al reported a median delay of 7 days (0–279 days) between the first HP consulted for DFU and referral to a podiatrist. Despite this relative brief delay and the small study size, they detected an associated increase in the time to healing.55 Given the data supporting ulcer duration as a risk factor for poorer outcomes,19,22,24,26 it is likely that prompt referral would reverse this effect.
HPs play a significant role in ensuring that delayed treatment is not a barrier to healing by ensuring they conduct routine foot examinations in their patients and are prompt to act when ulcers meet criteria for referral. Education of patients and carers is important, as is instilling in patients the understanding that foot problems are serious and deserve attention.45 One of the most powerful ways to convey this must be for HPs to perform routine foot assessments according to EBG. To help overcome the barrier of time required for busy clinicians to complete foot screening, Woodbury et al56 have recently reported on their simplified 60-second diabetic foot screening tool. This tool was designed as a fast and reliable assessment, particularly for health care workers in low- and middle-income countries, to aid timely identification and referral for patients who are at risk of or currently have a DFU.
It is also vital that specialized services promulgate their existence and measure access and time to treatment of patients, so that they can ensure they are being appropriately utilized. In evaluation of service impact, Ellis et al57 found that only 33% of patients admitted to hospital for DFU had accessed the region’s MDT and Plusch et al58 found that 75% of patients with an acute hospital admission for DFU had been admitted without prior treatment by the HRFS. In the UK, increased admission days for diabetic foot complications were associated with reduced podiatry resourcing, which impacted on early access to treatment.59 This observation, when reported on, motivated health administrators to fund the reinstating of the podiatry workforce, after which the trend reversed.
This delay in referral leads us to seek a solution but demands our recognition of the complex, interrelated, progressive, and location-specific factors that must be addressed to remove the inequity of access to specialist foot care as a mitigating factor in making wounds harder to heal.
Once referred, the healing of a DFU involves the management of the complex physical, biological, and behavioral aspects of this disease.
Patient factors – physical
Defective healing and chronic inflammation
Inflammation is required for normal wound repair, and the process in normal wound healing is tightly regulated both temporally and spatially.60 Any pathological process, many of which are present in DFUs (eg, impaired immune response, bacterial burden, and/or ischemia), can interfere with this physiological process and result in a non-healing wound. The effect of diabetes on wound healing can be seen from the very first moments of injury. The normal three-phase process of inflammation (Lewis–Flare) is partly mediated by stimulation of C nociceptive small nerve fibers, which secrete substances to enable vasodilation following injury. This process is impaired in patients with diabetes and neuropathic foot ulcers61 and is believed to contribute to their vulnerability and poor wound healing.
Chronic and hard-to-heal DFUs are characterized by a chronic inflammatory state, which is manifested by imbalances in 1) proteases and their antiproteases and 2) proinflammatory cytokines and their natural inhibitors.62–65 These imbalances occur because of sustained production of inflammatory mediators and influx of inflammatory cells, which prevent matrix synthesis and remodeling essential for progression to a healed wound.66–68
Having established the role of inflammation in delayed healing, we now need to better understand the mechanism by which healing fails and find evidence for treatments to address this. Despite intensive research, most therapeutic strategies have not been as successful in humans as in animal studies. Why this occurs is likely due to a number of factors including 1) animal models that do not fully replicate the conditions present in DFUs especially where the etiology of the ulcer is different (eg, diabetic neuropathic ulcer vs vascular insufficiency), 2) patient compliance with treatment regimens, 3) variability in wound care, and 4) variability in delivery of therapeutics with problems of retention. There does appear some hope that new therapies directed to molecular defects (eg, addition of stem cells) can impact on hard-to-heal DFUs. Treatments such as the inclusion of growth factors (eg, platelet-derived growth factors)69 and the use of neutraceuticals added either topically or to wound dressings70 may also have some utility in the treatment of hard-to-heal DFUs.
Infection
The defects in the early immune response in people with diabetes also delay wound healing by increasing the risk of infection.71 Typically arising in neuropathic, ischemic, or neuroischemic wounds, diabetic foot infections (DFIs) are the most frequent diabetes-related complication requiring hospitalization72,73 and greatly increase probability of amputation.72 Prompt identification and grading of infections from mild, involving superficial structures, moderate to severe limb and/or life threatening using validated criteria as a key step in the appropriate management of infection is therefore paramount.74 In addition to identification of the presence of infection, it has become clear that bacteria can form organized communities that are encased in a polymeric substance called biofilm. Biofilm exists on the surface of most chronic wounds, and its presence protects bacteria from the effects of most conventional antimicrobial treatments.21,75 The presence of biofilm is difficult to identify, highlighting the need for improved detection technology. Additionally, it is clear that frequent debridement that can physically remove biofilm as well as prevent biofilm formation also improves wound healing.75,76
Management of DFI is well documented, including most notably, the freely available Infectious Diseases Society of America and the IWGDF CPG and Bader and Brooks in 2012.77–79 These highlight the need for assessment of clinical signs of inflammation to identify infection, appropriate methods for assessing severity, debridement, assessment of peripheral arterial disease (PAD), and correct technique in collection of tissue samples for the analysis of bacterial phenotype. Empiric systemic antibiotic therapy can be commenced while awaiting formal culture results to inform ongoing selection of antimicrobial therapy, and this is particularly important for severe infections when failure to treat infection rapidly threatens limb and life.
Current reliance on traditional microbiological sampling, culture, and sensitivity testing, which takes several days, would delay treatment if not for recommendations of empiric prescription. However, concerns arise that widespread use of poorly targeted therapy contributes to antimicrobial resistance and poor outcomes. The evidence is unclear about the efficacy of one treatment over another. Methods using DNA analysis, which can assess the polymicrobial nature of the wound bacteria, have already demonstrated that traditional techniques underdetect important pathogens and do not capture the full diversity of organisms present in wounds and biofilms.76,80 In future, widespread use of methods for detecting infecting organisms based on more accurate and rapid analysis of bacterial DNA may lead to more targeted and effective treatment. In chronic wounds, DNA tests detect a wide variety of genotypically distinct bacteria often present in biofilms.80 How this new information will be translated to more targeted therapeutics is not as yet clear, although a recent study has shown that personalized treatment based on type of bacteria present, identified by DNA analysis, can improve wound healing and at reduced cost.81
All major guidelines recommend referral and management of these patients via a dedicated MDT.82,83 Delay in referral has been identified as a risk factor for lower extremity amputation15 and can be due either to the underrecognition of infection by the primary care physician or the patients’ delay in seeking care, or both. Healing rate is not only affected by bacterial number but also by the type of bacteria present. Gram-positive cocci, most notably Staphylococci, are the most commonly detected organisms.84 The factors that affect the pattern of sepsis (such as the presence of Gram-negative and/or Gram-positive organisms or diversity of the bacterial biofilm) are not yet clear. Metabolic control has been shown to play a role85 as has delayed referral.86 More recently, studies using animal models suggest that insulin therapy may promote antibiotic resistance in two important species commonly implicated in DFI, Staphylococcus aureus and Pseudomonas aeruginosa.87,88
Of particular importance is the prompt treatment of infected DFU complicated by PAD present in a high proportion of DFU.89 Diminished blood flow and neuropathy often result in dampening of the visual cues of infection. These deficits, especially in a patient with sensory neuropathy who also lacks the ability to sense pain or warmth, can delay awareness of an infection by the patient and the HP. DFI in wounds complicated by ischemia will often rapidly result in a contiguous spread to the adjacent bone if they are not managed promptly.90 With osteomyelitis comes a high probability of non-healing and amputation.72
In general, urgent action to overcome barriers and enhance collaboration among the various specialties involved in managing DFI is needed. Given the propensity for neurological and vascular impairment to mask signs of infection, in these conditions, high levels of suspicion for infection are needed to detect early changes.
Ischemia
Ischemia or PAD is present in up to 50% of patients with DFU.91 PAD is an independent baseline predictor of non-healing and also an independent risk factor for both ulcer recurrence and amputation.89 When caring for patients with DFU, reliably identifying PAD and knowing when to refer and how to provide best management will greatly influence the healing potential of a DFU.
The identification of PAD begins with checking for history of intermittent claudication or rest pain and palpation of pedal pulses. However, symptoms may be absent due to peripheral neuropathy,92 and palpation of pulses alone is an unreliable sign for determining PAD.93 Hence, noninvasive bedside assessments that largely exclude PAD should be conducted. Significant PAD may be excluded using the following criteria: ankle brachial index (ABI) is 0.9–1.3, toe brachial index ≥0.75, and triphasic pedal Doppler arterial waveforms94 are present or there is adequate perfusion demonstrated with transcutaneous oximetry. ABIs need to be interpreted with caution due to the prevalence of arterial calcification that can falsely elevate results, reducing the sensitivity of the test in people with diabetes and neuropathy.95,96 While the toe brachial index and ABI closely correlate and ABI is still widely recommended,94,97 the digital arteries are less likely to be calcified, and therefore, toe brachial index detects more people with PAD (increased sensitivity) in people with diabetes.98 As a guide, patients with DFU will generally heal if toe pressure is >55 mmHg92 but should be promptly referred for further vascular imaging and revascularization if toe pressure is <30 mmHg.99 Irrespective of noninvasive bedside vascular assessment results, referral for further vascular imaging is indicated if a DFU fails to improve within 6 weeks of standard care.99
Literature to date does not provide a definitive guide to which patients will benefit most from revascularization or whether open bypass or endovascular procedures are optimal. However, the recent review by the IWGDF indicates that overall ~60% of ulcers when revascularized proceed to heal.99 The 1-year survival rates are 80%–90% for open bypass and 70%–85% following endovascular procedures.91 Despite some variability in results, the increased accessibility to endovascular procedures provides a window of hope for healing of DFUs even if the resultant increased perfusion is temporary. However, many patients will continue to experience slow healing and frequent re-ulceration due to failure of the stent100 and the progressive nature of the PAD. For patients with critical limb ischemia for whom revascularization is unsafe or not appropriate, for example, in severely frail patients or in those whose life expectancy is <6–12 months101 or those who decline intervention, there is evidence that healing can still be achieved. In their prospective study of patients with ischemia and PAD, deemed unsuitable for or who declined revascularization, Elgzyri et al102 showed a 50% rate of healing without major amputation in an average of 27 weeks. This suggests that time and good care without revascularization can be appropriate management for some patients with ischemia.
The presence of PAD is of course due in part to modifiable risk factors. Earlier intensification of preventive measures (including smoking cessation, management of dyslipidemia, hyperglycemia, and hypertension) are all likely to improve outcomes. Additionally, data are emerging to support a role for long-term lipid-lowering treatment and a reduction in risk of amputation.103
Neuropathy, deformity, and offloading
In the presence of peripheral neuropathy, chronic repetitive mechanical stress on areas of high pressure such as those frequently created by foot deformity is a common pathway to DFU.104,105 The alleviation of localized pressure on the ulcer site is integral to successful healing irrespective of the injury being a consequence of chronic mechanical stress and acute physical, thermal, or chemical trauma. Without effective off-loading, other therapies, including advanced treatments, are unlikely to succeed.106
Most evidence to date supports total contact casting and irremovable walking casts for plantar DFUs because they provide effective pressure relief to the plantar aspect of the forefoot and compliance is forced.107,108 This is reinforced by the IWGDF and other peak bodies recommending offloading in an irremovable knee high device with an appropriate foot interface for plantar neuropathic ulcers or other modalities such as felt or felted-foam deflective padding109 and footwear when these treatments cannot be used.51,110
As with other areas of clinical management, there is a significant gap between evidence and practice. Most (77%) of the patients in the Eurodiale89,111 studies had no or inadequate offloading at study entry, and only 35% of patients were treated with some form of casting. Surveys in both the USA and Australia have shown the relative infrequency of the most recommended treatment by HPs; 68% of Australian podiatrists working in HRFS only used non-removable below-knee casts or walkers 11.2% of the time.112 Similarly in the USA, most HP’s reported use of total contact casts in <25% of patients.113 While lack of training and experience may be implicated, this was not considered a major driver in choosing other offloading devices in the Australian survey. In practice, irremovable devices are not prescribed for all patients for a range of reasons including nonacceptance by the patient or unsuitability of the treatment based on wound factors (infection, ischemia, fluctuating edema, depth, and location), patient behaviors that render an irremovable device unsafe, instability of gait, vision impairment, or the need to drive or a physical environment not conducive to wearing a cast. A lack of reimbursement for devices was suggested as a barrier in the USA and Australia.113,114
A lack of transferability of research to the clinic population may exist due to the nature of clinical trials, whereby researchers may need to exclude the very patients who clinicians have difficulty treating. The use of practical trials, which increase the applicability of research outcomes to real world, has been proposed to address this issue.44
Therefore, the authors agree with Armstrong et al107 that “thoughtful patient selection and diligent monitoring” are important. When irremovable devices are not deemed suitable, HPs must seek alternatives, often creatively and extrapolating from available evidence and experience. There are also reports of good healing outcomes achieved with devices such as felt deflection and healing shoes.109,110,115 Emerging reports support surgical offloading to prevent recurrence where foot deformity is the cause of ulceration, and other treatments to heal or prevent recurrence have failed.116,117 This is reflected in the latest international guidelines from the IWGDF, which recommend surgical procedures to achieve pressure offloading.110
Future research in the area of pressure offloading will need to focus on individualized treatments, using patients who are representative of the cohort, testing commonly used modalities that have potential clinical effect, and using patient-focused outcomes such as acceptability in addition to healing outcomes.
Patient factor – behavioral
Adherence to treatment
It is widely acknowledged by HPs that poor adherence to treatment is common in patients with DFU and that this makes it harder to heal a wound. People with DFU are of course a heterogeneous group, and there are a multitude of reasons why the rigorous treatment regimens recommended are not fully complied with. Patient non-adherence can take many forms, including the failure to keep appointments, follow recommended dressing and offloading instructions, make alterations to lifestyle including weight-bearing activity, and follow other aspects of treatment. There are two concepts to consider when exploring non-adherence to treatment regimens: unintentional and intentional, and in each case, it is important to determine the cause of non-adherence before commencing efforts to address it.118,119
Unintentional non-adherence applies when patients have an inadequate understanding of the disease or treatment regimen to competently complete the given tasks, whether due to poor literacy skills, lack of affordability, poor comprehension, reduced cognitive function, not acknowledging the seriousness of the condition due to lack of pain or other causative factors. Cognitive function must be considered since increased prevalence has been documented in patients with DFU.120,121
As the day-to-day care of the wound rests in the hands of the patient, reviewing our behavior, as clinicians, in order to engage, empower, and provide an optimal patient-centered experience for our patients has been shown to influence healing.118,119 Simple education and knowledge exchange strategies can be effective when managing people who unintentionally do not follow advice, and it is the responsibility of the clinician to ensure the patient is equipped with adequate information and that the type of communication is optimized and individualized. Advice and instructions need to be:
- Clear and unambiguous;
- Use nontechnical, everyday language;
- Limited to three or four major points during each discussion;
- Include written materials to support information;
- And involve the patient’s family members and friends.118,119
However, education and information alone will not always address the cause of non-adherence, particularly in the case of intentional non-adherence. Intentional non-adherence is a complex and at times incredibly frustrating encounter for clinicians. Intentional non-adherence is a deliberate and purposeful choice of patients to modify or reject treatment regimens for reasons important to themselves.118,119 As with all human behavior, progress can be cyclic, with individuals oscillating between making positive progress in their behavior change to “regressing” back to previous negative behaviors. Determining their motivation for doing so is the key to improving adherence. Motivating factors can include:
- Not taking their condition seriously enough;
- Patients feeling the side effects of the treatment outweigh the benefits; and/or
- The patient may not believe that the treatment is working.118,119
Most behavior change interventions are targeted toward intentional non-adherence, as it is widely accepted that motivation is a dynamic state that can be influenced.122 However, there is little evidence of sufficient efficacy to conclude that one method of behavior change intervention has a clear advantage over the others.119 As such, multifaceted approaches should be used until greater evidence is established. Importantly, motivation and patient adherence fluctuate in response to clinicians’ counseling style and communication methods.123,124 Therefore, a shift in our traditional role as an authoritative HP to a more collaborative “health coach”, focusing on interactive communication and partnership with our patients, is one way forward.125 This can be achieved by applying the skills of:
- Open-ended questions
- Reflective listening/making affirmations
- Summarizing/using reflections.
These skills will help to determine what the patient’s motivations and goals are so that we can bring them toward a common goal with the clinician.124,126 The acknowledgment and consideration of the patient’s preferences and perspectives are essential in gaining the patient’s buy in.127
Another important and emerging consideration, when exploring ways of engaging and motivating patients, is the use of Information Communication Technology (ICT). Interest in ICT is growing primarily because of its potential to improve facilitation of patient–provider communication, patient self-management, and the coordination of care across settings. Technology can be used to supplement care by providing both educational and motivational support.128,129 Types of ICT that have been trialed and reviewed include mobile phones (for communication, education, and monitoring), Internet-based education programs, and Internet-based self-management programs.128,130 Along with the potential benefits, it is important to also note the challenges associated with the use and access to emerging technologies, namely, the presence of the “digital divide”. In Australia, data suggest that a lack of access to computers, mobile phones, and the Internet, particularly in lower socioeconomic households, has the potential to exclude some people from information communicated through ICT.131 Technological advancements will never replace the crucial face-to-face role clinicians play in DFU management, but there are certainly indications of their benefit when applied in conjunction with traditional care delivery.
In some instances, we do not have the skills, resources, or support to bring about behavior change interventions, or occasionally, the need for change is expedited and there is not enough time to address the underlying non-adherence issues. This is particularly the case for patients with mental illness and other mental health comorbidities, as discussed in the next section. In these situations, HPs can seek to make it as easy as possible for patients to follow the aspects of care that are of greatest importance and enlist the support of carers and family members and refer if indicated for treatment of mental health problems.
Just because behavior change and adherence initiatives are complex,119 it does not mean we should give up. To achieve patient-centered care, we must strive to strike a balance between treatment that aligns with best clinical evidence and treatment that aligns with the patient’s wishes.
Appointment nonattendance
Not keeping scheduled appointments is an important, readily identifiable form of non-adherence to care, and it is a well-recognized predictor of poor health outcomes in chronic diseases.132 In diabetes and foot disease, appointment nonattendance has been documented as a predictor of repetitive foot ulceration.133,134 Individual factors such as reduced mobility, lack of prioritizing appointments in a busy workday, and reduced motivation, as well as clinic organizational factors such as proximity, parking access, and timeliness of appointment provision, may contribute to reduced appointment attendance in diabetes.135 These factors reflect the dynamic and complex nature of health care delivery and imply the need to customize care including in people with foot ulceration in diabetes, to support a patient in their clinic attendance.136,137 More studies are needed to examine to what degree appointment nonattendance may be linked to foot ulcer development and healing rate in diabetes and related predictive factors; for example, a recent publication from the UK did not find that clinic access based on geographical factors affected foot ulceration development or amputation.138
Comorbid mental health problems: depression and stress
Mental illness, in particular depression, is strongly associated with chronic physical diseases including diabetes.139 Depressive symptoms and reduced quality of life in people are frequent companions to DFU, with depression diagnosed in ~30% of people with DFU.140,141 Patients with DFU in the presence of neuropathy have an increased risk of depression, which is associated with delayed healing.141 With severe depression, comes a twofold increase in incidence of DFU and greater mortality risk.142,143 Anecdotally, at least, healing of DFU in people with comorbid mental illness is harder.
The causal effect of depression on self-care behaviors, healing outcomes, and mortality is not clear, and there is likely a bidirectional relationship between DFU and depression. However, there is a link between depression, non-adherence, and worse healing outcomes for DFU, due to reduced adherence to prescribed treatments144 potentially explained by the effect of depression on planning capacity, motivation, communication, and adherence to treatment. To become overwhelmed with the frequency of different appointments, conflicting medical advice and complexity of dressing, and antibiotic regimens, pressure offloading and diabetes medication management are common and understandable in the context of having both a physical and a mental health problem. Further to this, there is the propensity of neuropathic patients to treat foot problems as a low in priority in the absence of pain.
Healing may also be impacted by the effect of stress on health, reportedly due to the disruption of the neuroendocrine immune equilibrium.145 The event of having a foot ulcer and the experience of treatment are plausible stressors given the threat of amputation, restrictions on mobility, and restrictions on activities of daily living.146 How patients cope with this stress may also have a major influence on the healing process and overall well-being of the patient.
Management of DFU often focuses on physical interventions; however, the integration of specialists’ services offered by multidisciplinary teams allows for a more holistic approach. The challenge is to integrate the silos that exist, particularly between the physical health and mental health areas, and remove system barriers and financial disincentives in order to realize more integrated and coordinated treatment. In Australia, both mental health and diabetes are highlighted as national health priorities; yet, there is limited focus on their comorbidity with the majority of research and guidelines focusing on only the single disease states. Better identification of depression by those treating DFU and primary care and application of a more coordinated approach among HPs in order to achieve the individual patient’s goals for both physical and mental wellness are likely to provide better outcomes.
Collaborative care models offer promise, and there is evidence of successful programs integrating physical and mental health management for some noncommunicable diseases.147 In our local area, chronic care co-coordinators provide an additional layer of support to help patients who are struggling with the complexities of their treatment due to the burden of additional physical or mental problems. Anecdotally, this appeared to help these patients to follow treatment plans. Katon et al148 represented examples where management of depression has improved depression outcomes for patients without increasing the net cost of treatment. While these programs have not necessarily shown a change in physical health outcomes, there can be little argument that improving the well-being of patients with physical and mental health burden and high mortality risk is a valuable and progressive approach to caring for people with DFU.
A summary of recommendations from these discussions is provided in Table 1.
  | Table 1 Summary of recommendations to address the issues related to hard-to-heal DFUs Abbreviations: DFUs, diabetes-related foot ulcers; EBG, evidence-based practice guidelines. |
Conclusion
Research into effective prevention and treatment of diabetic foot complications is important and ongoing work. Already, there are gains in healing outcomes in many areas, but there is still considerable variation in outcomes and ~25% of foot ulcers do not heal readily. Grading systems and data on predicting outcomes are of value in planning and implementing treatment and in communication and performance monitoring. It is clear that the main risk factors for non-healing, such as size, duration, infection, and ischemia, need to be mitigated if we are to continue to improve on the healing outcomes for people with DFUs. To achieve this, focus on achieving better adherence to treatment guidelines and translating evidence into practice is needed and addressing patients’ mental health and supporting adherence to treatment will be important. Future prospects for improved care include the coordinated implementation and monitoring of services, collaborative care models integrating management of comorbid mental health, better strategies to manage patient adherence through patient-centered care, methods for early identification of hard-to-heal wounds, and therapies to address factors such as infection, ischemia, inflammation, and pressure.
Acknowledgment
The authors wish to acknowledge Ms Thyra Bolton for her expert advice.
Disclosure
The authors report no conflicts of interest in this work.
References
IDF Diabetes Atlas – 7th Edition. [webpage on the Internet]. Brussels: IDF Online Resources. Available from: www.idf.org/diabetesatlas. Accessed April 15, 2016 | ||
Reiber GE. The epidemiology of diabetic foot problems. Diabet Med. 1996;13(suppl 1):S6–S11. | ||
Jeffcoate WJ, Chipchase SY, Ince P, Game FL. Assessing the outcome of the management of diabetic foot ulcers using ulcer-related and person-related measures. Diabetes Care. 2006;29(8):1784–1787. | ||
Brussels: International Working Group on the Diabetic Foot (IWGDF) Guidelines. [homepage on the Internet]. Available from: http://iwgdf.org/. Accessed April 15, 2016. | ||
National Institute for Health and Care Excellence Guideline: Diabetic Foot Problems: Prevention and Management. [webpage on the Internet]. Available from: https://www.nice.org.uk/guidance/ng19/chapter/1-recommendations. Accessed November 16, 2015. | ||
NHMRC [webpage on the Internet]. National Evidence-Based Guideline on Prevention, Identification and Management of Foot Complications in Diabetes. Melbourne: National Health and Medical Research Council Australia Melbourne [Updated May 2011]. Available from: http://www.nhmrc.gov.au/guidelines/. Accessed January 8,2016. | ||
Baba M, Davis WA, Norman PE, Davis TM. Temporal changes in the prevalence and associates of diabetes-related lower extremity amputations in patients with type 2 diabetes: the Fremantle Diabetes Study. Cardiovasc Diabetol. 2015;14:152. | ||
Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Healing diabetic neuropathic foot ulcers: are we getting better? Diabet Med. 2005;22(2):172–176. | ||
Wrobel JS, Mayfield JA, Reiber GE. Geographic variation of lower extremity amputation in individuals with and without diabetes in the Medicare population. Diabetes Care. 2003;26(11):3042–3047. | ||
Margolis DJ, Jeffcoate W. Epidemiology of foot ulceration and amputation: can global variation be explained? Med Clin North Am. 2013;97(5):791–805. | ||
Moxey PW, Gogalniceanu P, Hinchliffe RJ, et al. Lower extremity amputations- a review of global variability of incidence. Diabet Med. 2011;28(10):114–153. | ||
Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747–755. | ||
Fife CE, Horn SD, Smout RJ, Barrett RS, Thomson T. A predictive model for diabetic foot ulcer outcome: a wound healing index. Adv Wound Care. 2016;5(7):279–287. | ||
Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg. 1996;183(1):61–64. | ||
Jeffcoate WJ, van Houtum WH. Amputation as a marker of the quality of foot care in diabetes. Diabetologia. 2004;47(12):2051–2058. | ||
Armstrong DG, Lavery LA, Harkless LB. Validation of diabetic wound classification system: the contribution of depth, infection and ischaemia to risk of amputation. Diabetes Care. 1998;21:855–859. | ||
Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev. 2004;20(suppl 1):S90–S95. | ||
Beckert S, Pietsch AM, Küper M, et al. M.A.I.D: a prognostic score estimating probability of healing in chronic lower extremity wounds. Ann Surg. 2009;448(4):677–681. | ||
Horn SD, Barrett RS, Fife CE, Thomson B. A predictive model for pressure ulcer outcome: the wound healing index. Adv Skin Wound Care. 2015;28(12):560–572. | ||
Treece KA, Macfarlane RM, Pound N, Game FL, Jeffcoate WJ. Validation of a system of foot ulcer classification in diabetes mellitus. Diabet Med. 2004;21(9):987–991. | ||
Metcalf DG, Bowler PG. Biofilm delays wound healing: a review of the evidence. Int J Burns Trauma. 2013;1(1):5–12. | ||
Beckert S, Witte M, Wicke C, Konigsrainer A, Coerper S. A new wound-based severity score for diabetic foot ulcers: a prospective analysis of 1,000 patients. Diabetic Care. 2006;29(5):988–992. | ||
Ince P, Game FL, Jeffcoate WJ. Rate of healing of neuropathic ulcers of the foot in diabetes and its relationship to ulcer duration and ulcer area. Diabetes Care. 2007;30(3):660–663. | ||
Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: the association of wound size, wound duration, and wound grade on healing. Diabetes Care. 2002;25(10):1835–1839. | ||
Oyibo SO, Jude EB, Tarawneh I, et al. The effect of ulcer size and site, patients age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabet Med. 2001;18(2):133–138. | ||
Ince P, Kendrick D, Game F, Jeffcoate W. The association between baseline characteristics and the outcome of foot lesions in a UK population with diabetes. Diabet Med. 2007;24(9):977–981. | ||
Holman N, Young B, Stephens H, Jeffcoate W. Pilot study to assess measures to be used in the prospective audit of the management of foot ulcers in people with diabetes. Diabet Med. 2014;32:78–84. | ||
Monami M, Longo R, Desideri CM. The diabetic person beyond a foot ulcer. J Am Podiatr Med Assoc. 2008;98(2):130–163. | ||
Liu Y, Min D, Bolton T, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care. 2009;32(1):117–119. | ||
Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res. 2007;298(9):413–420. | ||
Shukla VK, Shukla D, Tiwary SK, Agrawal S, Rastogi A. Evaluation of pH measurement as a method of wound assessment. J Wound Care. 2007;16(7):291–294. | ||
Yuan R, Geng S, Chen K, Diao N, Chu HW, Li L. Low-grade inflammatory polarization of monocytes impairs wound healing. J Pathol. 2016;238(4):571–583. | ||
Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82(4):581–629. | ||
Philip K. Allied health: untapped potential in the Australian health system. Aust Health Rev. 2015;39(3):244–247. | ||
World Health Organisation Report of Knowledge for Better Health; 2004. [webpage on the Internet]. Available from: http://www.who.int/rpc/meetings/en/world_report_on_knowledge_for_better_health2.pdf. Accessed November 15, 2015. | ||
Lazzarini PA, O’Rourke SR, Russell AW, Derhy PH, Kamp MC. Standardising practices improves clinical diabetic foot management: the Queensland Diabetic Foot Innovation Project, 2006–09. Aust Health Rev. 2012;36(1):8–15. | ||
Wraight PR, Lawrence SM, CAmpbell DA, Coleman PG. Creation of a multidisciplinary, evidence based, clinical guideline for the assessment, investigations and management of acute diabetes related foot complications. Diabet Med. 2005;22(2):127–136. | ||
Fife CE, Carter MJ, Walker D. Why is it so hard to do the right thing in wound care? Wound Repair Regen. 2010;18(2):154–158. | ||
Prompers L, Huijberts M, Apelqvist J, et al. Delivery of care to diabetic patients with foot ulcers in daily practice: results of the Eurodiale Study, a prospective cohort study. Diabet Med. 2008;25(6):700–707. | ||
Estabrooks CA. Translating research into practice: implications for organizations and administrators. Can J Nurs Res. 2003;35(3):53–68. | ||
Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7:50. | ||
Gross PA, Greenfield S, Cretin S, et al. Optimal methods for guideline implementation: conclusions from Leeds Castle meeting. Med Care. 2001;39(8):85–92. | ||
Rushmer R, Kelly D, Lough M, Wilkinson JE, Davies HT. Introducing the learning practice–II. Becoming a learning practice. J Eval Clin Pract. 2004;10(3):387–398. | ||
Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health. 2007;28:413–433. | ||
Bakker K, Apelqvist J, Schaper NC. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28(suppl 1):225–231. | ||
Doggen K, Van Acker K, Beele H, et al. Implementation of a quality improvement initiative in Belgian diabetic foot clinics: feasibility and initial results. Diabetes Metab Res Rev. 2014;30(5):435–443. | ||
Abbas ZG, Lutale JK, Bakker K, Baker N, Archibald LK. The ‘Step by Step’ Diabetic Foot Project in Tanzania: a model for improving patient outcomes in less-developed countries. Int Wound J. 2011;8(2):169–175. | ||
Canavan RJ, Unwin NC, Kelly WF, Connolly VM. Diabetes and non-diabetes related lower extremity amputation incidence before and after the introduction of better organized diabetes foot-care: continuous longitudinal monitoring using standard method. Diabetes Care. 2008;31(3):459–463. | ||
Krishnan S, Nash F, Baker N, Fowler D, Rayman G. Reduction in amputations over 11 years in a defined UK population: benefits of multidisciplinary teamwork and continuous prospective audit. Diabetes Care. 2008;31:99–101. | ||
Lazzarini PA, O’Rourke SR, Russell AW, Derhy PH, Kamp MC. Reduced incidence of foot-related hospitalisation and amputation amongst persons with diabetes in Queensland, Australia. PLoS One. 2015;10(6):e0130609. | ||
Bergin SM, Gurr JM, Allard BP, et al; Australian Diabetes Foot Network. Australian Diabetes Foot Network: management of diabetes-related foot ulceration – a clinical update. Med J Aust. 2012;197(4):226–229. | ||
Mills JL, Beckett WC, Taylor SM. The diabetic foot: consequences of delayed treatment and referral. South Med J. 1991;84(8):970–974. | ||
Phillips CB, Patel MS, Cabaron Y. Diabetes among aboriginal people in central Australia: a high prevalence based on health service attendance. Med J Aust. 1990;153(6):314–318. | ||
Nube V, Veldhoen D, Frank G, Bolton TM, Twigg S. Developing meaningful performance indicators for a diabetes high-risk foot service: is it hot or not? Wound Repair Regen. 2014;22(4):221–225. | ||
Sanders AP, Stoeldraaijers LG, Pero MW, Hermkes PJ, Carolina RC, Elders PJ. Patient and professional delay in the referral trajectory of patients with diabetic foot ulcers. Diabetes Res Clin Pract. 2013;102(2):105–111. | ||
Woodbury MG, Sibbald RG, Ostrow B, Persaud R, Lowe JM. Tool for rapid & easy identification of high risk diabetic foot: validation & clinical pilot of the simplified 60 second diabetic foot screening tool. PLoS One. 2015;10(6):e0125578. | ||
Ellis E, Ballance K, Lunt H, Lewis D. Diabetes outpatient care before and after admission for diabetic foot complications. J Wound Care. 2010;19(4):150–152. | ||
Plusch D, Penkala S, Dickson HG, Malone M. Primary care referral to multidisciplinary high risk foot services – too few, too late. J Foot Ankle Res. 2015;8:62. | ||
Gooday C, Murchison R, Dhatariya K. An analysis of clinical activity, admission rates, length of hospital stay, and economic impact after a temporary loss of 50% of the non-operative podiatrists from a tertiary specialist foot clinic in the United Kingdom. Diabet Foot Ankle. 2013;4:21957. | ||
Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. | ||
Parkhouse N, Le Quesne PM. Impaired neurogenic vascular response in patients with diabetes and neuropathic foot lesions. N Engl J Med. 1988;318(20):1306–1309. | ||
Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. | ||
Robson MC, Mustoe TA, Hunt TK. The future of recombinant growth factors in wound healing. Am J Surg. 1998;176:80S–82S. | ||
Martin P. Wound healing – aiming for perfect skin regeneration. Science. 1997;276:75–81. | ||
Fahey TJ, Sadaty A, Jones WG, Barber A, Smoller B, Shires GT. Diabetes impairs the late inflammatory response to wound healing. J Surg Res. 1991;50(4):308–313. | ||
Pilcher BK, Wang M, Qin XJ, Parks WC, Senior RM, Welgus HG. Role of matrix metalloproteinases and their inhibition in cutaneous wound healing and allergic contact hypersensitivity. Ann N Y Acad Sci. 1999;878:12–24. | ||
Madlener M, Parks WC, Werner S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res. 1998;242:201–210. | ||
Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen. 1999;7:423–432. | ||
Henshaw FR, Boughton P, Lo L, McLennan SV, Twigg SM. Topically applied connective tissue growth factor/CCN2 improves diabetic preclinical cutaneous wound healing: potential role for CTGF in human diabetic foot ulcer healing. J Diabetes Res. 2015;2015:236238. | ||
McCarty SM, Percival SL. Proteases and delayed wound healing. Adv Wound Care. 2013;2(8):438–447. | ||
Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26(3–4):259–265. | ||
Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006;29(6):1288–1293. | ||
Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years?(*). Int Wound J. 2012;9(suppl 2):1–19. | ||
Lipsky BA, Peters EJ, Berendt AR, et al; International Working Group on Diabetic Foot. Specific guidelines for the treatment of diabetic foot infections 2011. Diabetes Metab Res Rev. 2012;28(suppl 1):234–235. | ||
Alavi A, Sibbald RG, Mayer D, et al. Diabetic foot ulcers: part II. Management. J Am Acad Dermatol. 2014;70(1): 21.e1–24. | ||
Davis SC, Martinez L, Kirsner R. The diabetic foot: the importance of biofilms and wound bed preparation. Curr Diab Rep. 2006;6(6):439–445. | ||
Lipsky BA, Berendt AR, Cornia PB, et al; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132–e173. | ||
Bader MS, Brooks A. Medical management of diabetic foot infections. Postgrad Med. 2012;124(2):102–113. | ||
Lipsky BA, Aragon-Sanchez J, Diggle M, et al; International Working Group on the Diabetic Foot. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32(suppl 1):45–74. | ||
Dowd SE, Callaway TR, Wolcott RD, et al. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol. 2008;8:125. | ||
Wolcott R. Economic aspects of biofilm-based wound care in diabetic foot ulcers. J Wound Care. 2015;24(5):189–190, 192–184. | ||
Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders: a clinical practice guideline (2006 Revision). J Foot Ankle Surg. 2006;45(5):S1–S66. | ||
Wounds-International [webpage on the Internet]. International Best Practice Guidelines: Wound Managment in Diabetic Foot Ulcers; 2013. Available from: http://www.woundsinternational.com/best-practices/view/bpg-wound-management-in-diabetic-foot-ulcers. Accessed June 10, 2016. | ||
Lipsky BA, Peters EJ, Senneville E, et al. Expert opinion on the management of infections in the diabetic foot. Diabetes Metab Res Rev. 2012;28(suppl 1):163–178. | ||
Morain D, Cohen L. Wound healing in diabetes mellitus. Clin Plast Surg. 1990;17:493–498. | ||
Macfarlane RM, Jeffcoate WJ. Factors contributing to the presentation of diabetic foot ulcers. Diabet Med. 1997;14(10):867–870. | ||
Watters C, DeLeon K, Trivedi U, et al. Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice. Med Microbiol Immunol. 2013;202(2):131–141. | ||
Watters C, Everett JA, Haley C, Clinton A, Rumbaugh KP. Insulin treatment modulates the host immune system to enhance Pseudomonas aeruginosa wound biofilms. Infect Immun. 2014;82(1):92–100. | ||
Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50(1):18–25. | ||
Lipsky BA, Berendt AR, Deery HG, et al; Infectious Diseases Society of America. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39(7):885–910. | ||
Hinchliffe RJ, Brownrigg JR, Apelqvist J, et al. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab Res Rev. 2016;32(suppl 1):37–44. | ||
Schaper NC, Andros G, Apelqvist J, et al. Diagnosis and treatment of peripheral arterial disease in diabetic patients with a foot ulcer. A progress report of the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2012;28(suppl 1):218–224. | ||
Brearley S, Shearman CP, Simms MH. Peripheral pulse palpation: an unreliable physical sign. Ann R Coll Surg Engl. 1992;74(3):169–171. | ||
The International Working Group on the Diabetic Foot (IWGDF). [webpage on the Internet]. Guidance on PAD [Updated 2015]. Available from: http://iwgdf.org/guidelines/guidance-on-PAD-2015/. Accessed November 15, 2015. | ||
Brownrigg JR, Hinchliffe RJ, Apelqvist J, et al; International Working Group on the Diabetic Foot. Effectiveness of bedside investigations to diagnose peripheral artery disease among people with diabetes mellitus: a systematic review. Diabetes Metab Res Rev. 2016;32(suppl 1):119–127. | ||
Xu D, Zou L, Xing Y, et al. Diagnostic value of ankle-brachial index in peripheral arterial disease: a meta-analysis. Can J Cardiol. 2013;29(4):492–498. | ||
Stoekenbroek RM, Ubbink DT, Reekers JA, Koelemay MJ. Hide and seek: does the toe-brachial index allow for earlier recognition of peripheral arterial disease in diabetic patients? Eur J Vasc Endovasc Surg. 2015;49(2):192–198. | ||
Bundo M, Urrea M, Munoz L, Llussa J, Fores R, Toran P. Correlacion entre los indices dedo-brazo y tobillo-brazo en pacientes con diabetes mellitus tipo 2. [Correlation between toe-brachial index and ankle-brachial index in patients with diabetes mellitus type 2]. Med Clin. 2013;140(9):390–394. | ||
Hinchliffe RJ, Brownrigg JR, Andros G, et al; International Working Group on the Diabetic Foot. Effectiveness of revascularisation of the ulcerated foot in patients with diabetes and peripheral artery disease: a systematic review. Diabetes Metab Res Rev. 2016;32(suppl 1):136–144. | ||
Forsythe RO, Brownrigg J, Hinchliffe RJ. Peripheral arterial disease and revascularization of the diabetic foot. Diabetes Obes Metab. 2015;17(5):435–444. | ||
Schaper NC, Andros G, Apelqvist J, et al; International Working Group on Diabetic foot. Specific guidelines for the diagnosis and treatment of peripheral arterial disease in a patient with diabetes and ulceration of the foot 2011. Diabetes Metab Res Rev. 2012;28(suppl 1):236–237. | ||
Elgzyri T, Larsson J, Thorne J, Eriksson KF, Apelqvist J. Outcome of ischemic foot ulcer in diabetic patients who had no invasive vascular intervention. Eur J Vasc Endovasc Surg. 2013;46(1):110–117. | ||
Rajamani K, Coleman PG, Li LP, et al; FIELD study investigators. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet. 2009;373(9677):1780–1788. | ||
Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. J Am Podiatr Med Assoc. 2010;100(5):360–368. | ||
Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13(5):513–521. | ||
Snyder RJ, Frykberg RG, Rogers LC, et al. The management of diabetic foot ulcers through optimal off-loading: building consensus guidelines and practical recommendations to improve outcomes. J Am Podiatr Med Assoc. 2014;104(6):555–567. | ||
Armstrong DG, Lavery LA, Wu S, Boulton AJ. Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds: a randomized controlled trial. Diabetes Care. 2005;28(3):551–554. | ||
Lewis J, Lipp A. Pressure-relieving interventions for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2013;1:CD002302. | ||
Nube V, Molyneaux L, Bolton T, Clingan T, Palmer E, Yue DK. Use of felt deflective padding in the management of hallux and forefoot ulcers in patients with diabetes. Foot. 2006;16(1):38–43. | ||
Bus SA, Armstrong DG, van Deursen RJ, et al; International Working Group on the Diabetic Foot. IWGDF Guidance on footwear and offloading. Interventions to prevent and heal foot ulcers in patients with diabetes. Diabetes Metab Res Rev. 2015;32(suppl S1):25–26. | ||
Akhtar S, Schaper N, Apelqvist J, Jude E. A review of the Eurodiale studies: what lessons for diabetic foot care? Curr Diab Rep. 2011;11(4):302–309. | ||
Raspovic A, Landorf KB. A survey of offloading practices for diabetes-related plantar neuropathic foot ulcers. J Foot Ankle Res. 2014;7:35. | ||
Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care. 2008;31(11):2118–2119. | ||
Lazzarini PA, Gurr JM, Rogers JR, Schox A, Bergin SM. Diabetes foot disease: the Cinderella of Australian diabetes management? J Foot Ankle Res. 2012;5(1):24. | ||
Birke JA, Pavich MA, Patout CA Jr, Horswell R. Comparison of forefoot ulcer healing using alternative off-loading methods in patients with diabetes mellitus. Adv Skin Wound Care. 2002;15(5):210–215. | ||
Frigg A, Pagenstert G, Schaifer D, Valderrabano V, Hintermann B. Recurrence and prevention of diabetic foot ulcers after total contact casting. Foot Ankle Int. 2007;28(1):64–69. | ||
Richter M, Zech S. Four-stage regimen for operative treatment of a diabetic foot ulcer with deformity - results of 300 patients. Foot Ankle Surg. 2012;18:247–254. | ||
Cameron C. Patient Compliance: recognition of factors involved and suggestions for promoting compliance with therapeutic regimens. J Adv Nurs. 1996;24(2):224–250. | ||
Price PE. Education, psychology and ‘compliance’. Diabetes Metab Res Rev. 2008;24(1):S101–S105. | ||
Marseglia A, Xu W, Rizzuto D, et al. Cogntive functioning among patients with diabetic foot. J Diabetes Complications. 2014;28(6):863–868. | ||
Natovich R, Harman-Boehm I, Cukierman-Yaffe T, et al. Cognitive Dysfunction: Part and Parcel of the Diabetic Foot. Diabetes. Vol. 64.1701. Alexandia, VA: Americal Diabetes Association; 2015. | ||
Hall K, Gibbie T, Lubman DI. Motivational interviewing techniques. Facilitating behaviour change in the general practice setting. Aust Fam Physician. 2012;41(9):660–667. | ||
Australian Commission of Safety and Quality in Health Care [webpage on the Internet]. Sydney: Patient-centred care: Improving quality and safety through partnerships with patients and consumers; 2011. Available from: http://www.safetyandquality.gov.au/wp-content/uploads/2012/03/PCC_Paper_August.pdf. Accessed November 15, 2015. | ||
Gabbay RA, Kaul S, Ulbrecht JS, Scheffler NM, Armstrong DG. Motivational interviewing by podiatric physcians: a method for improving patient self-care of the diabetic foot. J Am Podiatr Med Assoc. 2011;101(11):78–84. | ||
Lindner H, Menzies D, Kelly J, Taylor S, Shearer M. Coaching for behaviour change in chronic disease: a review of the literature and the implications for coaching as a self-management intervention. Aust J Prim Health. 2003;9(2/3):177–185. | ||
Kubina N, Kelly J. Navigating Self-Management: A Practical Approach to Implementation for Australian Health Care Agencies. Nunawading: Whitehorse Division of General Practice; 2007. | ||
Arbuthnott A, Sharpe D. The effect of physician-patient collaboration on patient adherence in non-psychiatric medicine. Patient Educ Couns. 2009;77(1):60–67. | ||
Hunt CW. Technology and diabetes self-management: an integrative review. World J Diabetes. 2015;6(2):225–233. | ||
Wiseman JT, Fernandes-Taylor S, Barnes ML, Tomsejova A, Saunders RS, Kent KC. Conceptualizing smartphone use in outpatient wound assessment: patients’ and caregivers’ willingness to use technology. J Surg Res. 2015;198(1):245–251. | ||
Wang L, Pedersen PC, Strong DM, Tulu B, Agu E, Ignotz R. Smartphone-based wound assessment system for patients with diabetes. IEEE Trans Biomed Eng. 2015;62(2):477–488. | ||
O’Mara B, Babacan H, Borland H. Sending the Right Message: ICT Access and Use for Communicating Messages of Health and Wellbeing to CALD Communities. Melbourne: Victoria University; 2010. | ||
Currie CJ, Peyrot M, Morgan CL, et al. The impact of treatment non-compliance on mortality in people with type 1 diabetes. J Diabetes Complications. 2013;27(3):219–223. | ||
Connor H, Mahdi OZ. Repetitive ulceration in neuropathic patients. Diabetes Metab Res Rev. 2004;20(suppl 1):S23–S28. | ||
Nube V, Molyneaux L, Constantino M, et al. Diabetic foot ulceration: why are some patients in a “revolving door”? Diabetic Foot J. 2008;11(4):187–193. | ||
Kawahara R, Amemiya T, Yoshino M, Miyamae M, Sasamoto K, Omori Y. Dropout of young non-insulin-dependent diabetics from diabetic care. Diabetes Res Clin Pract. 1994;24(3):181–185. | ||
Goudswaard AN, Stolk RP, de Valk HW, Rutten GE. Improving glycaemic control in patients with Type 2 diabetes mellitus without insulin therapy. Diabet Med. 2003;20(7):540–544. | ||
Hung SL, Fu SN, Lau PS, Wong SY. A qualitative study on why did the poorly-educated Chinese elderly fail to attend nurse-led case manager clinic and how to facilitate their attendance. Int J Equity Health. 2015;14:10. | ||
Schwennesen N, Henriksen JE, Willaing I. Patient explanations for non-attendance at type 2 diabetes self-management education: a qualitative study. Scand J Caring Sci. 2015;30(1):187–192. | ||
Cole-King A, Harding KG. Psychological factors and delayed healing in chronic wounds. Psychosom Med. 2001;63(2):216–220. | ||
Hoban C, Sareen J, Henrikson CA, Kuzyk L, Embil JM, Trepman E. Mental health issues associated with foot complications of diabetes mellitus. J Foot Ankle Surg. 2015;21(1):49–55. | ||
Winkley K, Sallis H, Kariyawasam D, et al. Five-year follow-up of a cohort of people with their first diabetic foot ulcer: the persistent effect of depression on mortality. Diabetologia. 2012;55(2):303–310. | ||
Schaper NC. Lessons from Eurodiale. Diabetes Metab Res Rev. 2012;28(suppl 1):21–26. | ||
Williams LH. Depression and incident diabetic foot ulcers: a prospective cohort study. Am J Med. 2010;123(8):748–754. | ||
DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for non-compliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. | ||
Vileikyte L. Stress and wound healing. Clin Dermatol. 2007;25(1):49–55. | ||
Brod M. Quality of life issues in patients with diabetes and lower extremity ulcers: patients and care givers. Qual Life Res. 1998;7(4):365–372. | ||
Ngo VK, Rubenstien A, Ganju V, et al. Grand challenges: integrating mental health care into the non-communicable disease agenda. PLoS Med. 2013;10(5):e1001443. | ||
Katon WJ, Russo JE, Von Koff M, Lin EH, Ludman E, Ciechanowski PS. Long-term effects on medical costs of improving depression outcomes in patients with depression and diabetes. Diabetes Care. 2008;31(6):1155–1159. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
