Back to Journals » Journal of Hepatocellular Carcinoma » Volume 2
Hapten-enhanced overall survival time in advanced hepatocellular carcinoma by ultro-minimum incision personalized intratumoral chemoimmunotherapy
Authors Gao F, Jing P, Liu J, Lu Y, Zhang P, Han W, Liu G, Ru N, Cui G, Sun C, Che Y, Zhang H, Hu Q, Wang H, Wu Y, Guan C, Fu Q, Ma Z, Yu B
Received 11 January 2015
Accepted for publication 18 February 2015
Published 9 June 2015 Volume 2015:2 Pages 57—68
DOI https://doi.org/10.2147/JHC.S80756
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmed Kaseb
Feng Gao,2 Peng Jing,2 Jian Liu,2 Yuanfei Lu,1 Peicheng Zhang,2 Wei Han,1 Guoliang Liu,1 Ning Ru,2 Guanghui Cui,2 Chenglin Sun,1 Yebing Che,1 Huaming Zhang,1 Qnglong Hu,3 Huan-you Wang,4 Yingli Wu,2 Changjiang Guan,2 Qiang Fu,1 Zhenlu Ma,2 Baofa Yu1–3
1Jinan Baofa Cancer Hospital, Jinan, People's Republic of China; 2TaiMei Baofa Cancer Hospital, Dongping, People's Republic of China; 3Beijing Baofa Cancer Hospital, Beijing, People's Republic of China; 4Department of Pathology, UCSD School of Medicine, La Jolla, CA, USA
Purpose: To compare the therapeutic effects of ultra-minimum incision personalized intratumoral chemoimmunotherapy (UMIPIC) with intratumoral chemotherapy (ITCT) in the treatment of advanced hepatocellular carcinomas and to analyze the effect of hapten as an immune booster.
Materials and methods: Patients with advanced hepatocellular carcinomas were treated with UMIPIC or ITCT with the same therapeutic procedure; the UMIPIC method had a proprietary regimen including an oxidant, a cytotoxic drug, and hapten, while ITCT delivered the same drug excluding hapten. Of 339 patients in total, 119 of the UMIPIC patients (n=214) had response data and 214 had survival data, and of the ITCT patients (n=125), 61 had response data and 125 had survival data. Tumor response was assessed with a computed tomography scan 6–8 weeks after the initial treatment; the survival rate was evaluated by follow-up visits. Tumor size was classified as small (<5 cm), large (5–10 cm), or very large (>10 cm); tumor sizes with liver function categorized using Child–Pugh class (A and B) were analyzed by correlation with overall survival.
Results: The response rates (complete response + partial response + stable disease) were 78.68% and 81.52% in the UMIPIC and ITCT groups, respectively, with no statistically significant difference; however, the median overall survival was 7 months for UMIPIC (test) and 4 months for ITCT (control), respectively (P<0.01). The 6-month and 1-year survival rates for UMIPIC and ITCT were 58.88% vs 32.3% and 30.37% vs 13.6%, respectively (P<0.01). Single and multiple UMIPIC revealed significant improvement in overall survival compared to that of ITCT. Child–Pugh class A patients had a longer duration of survival compared to Child–Pugh class B patients in UMIPIC therapy.
Conclusion: Hapten had enhanced therapeutic effect with improvement in the survival duration in UMIPIC compared to ITCT. After reexamination, the response rate was not different due to inflammation caused by hapten. Hapten has been found to play an important role in immunotherapy to improve patient survival.
Keywords: HCC, intratumoral chemotherapy, ITCT, immune booster
Introduction
Hepatocellular carcinoma (HCC) is an aggressive cancer1,2 – an estimated 30,640 new cases and 21,670 cancer deaths will occur from HCC in the US in 2013.3 The current treatments for advanced HCC, including transcatheter arterial chemoembolization (TACE),4,5 adoptive immunotherapy,6 interferon therapy,7 percutaneous ethanol injection, and radiofrequency ablation with a molecular target drug such as sorafenib,8 used either alone or combination, have shown limited benefits on survival rates.9 Current therapeutic approaches of TACE (oil–water drug emulsion) are often used in clinical practice for HCC therapy; it is considered to be a localized therapy, with fewer side effects compared to systematic chemotherapy.
The concept of percutaneous intratumoral drug delivery has been known for several decades as a type of localized therapy.10 Some successful examples have clearly shown the clinical feasibility of such treatment options, with significant reduction in both toxicity and tumor growth; however, most research into intratumoral drug delivery with a single drug has found limited clinical impact on the survival time. Combining different drugs in an aqueous solution for intratumoral therapy with or without hapten as an immunological booster is necessary for the application of intratumoral drug delivery. Hapten-like non-deleterious dinitrophenyl (DNP) has been used in an in vitro melanoma autologous vaccine, while in vivo vaccine therapy has shown significant improvement of survival rates.11,12 Our published data suggest that UMIPIC offers an ideal percutaneous intratumoral approach for chemical debulking of advanced lung cancer; the hapten plays an important role in prolonging patients’ survival time.13
In the last decade, we have started clinical research into therapeutic combinations in an aqueous solution of single chemotherapeutic drugs and oxidant with hapten (UMIPIC) or without hapten (ITCT) in advanced HCC patients using percutaneous intratumoral injection; the data have not been previously reported, since clinical research into the combination of double chemotherapeutic drugs and oxidant with or without hapten in HCC treatments is still ongoing. These data have now been collected and analyzed. The primary objective of this cohort study was to assess the feasibility, safety, and efficacy of UMIPIC vs injection of ITCT. The role of hapten on patients’ survival time and efficacy of hapten was also evaluated.
Materials and methods
Patient selection and data collection
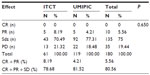
Patients were informed of the study procedure details and agreed to participate by signing the informed consent; the ethics committee at the author’s hospital (ref no TMBFZLYY001) approved the study. Patient data were confirmed by imaging and pathological and cytological diagnoses in primary HCC patients with local advanced and/or metastatic cancer from November 1999 to September 2006 were analyzed. Patients were randomized to receive UMIPIC or ITCT treatment. Data were collected with case report forms (CRFs) completed by hospital physicians. Collected data included clinical characteristics, follow-up time, and response and survival data. For each patient, the first follow-up visit was scheduled 6–8 weeks after treatment initiation and then monthly. The median follow-up duration was 16 months. Records were updated at each follow-up visit. At the end of follow-up, a total of 339 patients (male to female ratio =285:54) with complete survival data were included from the 450 patients initially enrolled; UMIPIC patients (n=214) had 119 cases with response data, 214 cases with survival data, 41 cases with single treatment, and 173 cases with multiple treatments; ITCT patients (n=125) had 61 cases with response data, 125 cases with survival data, 33 cases with single treatment, and 92 cases with multiple treatments. Most of the patients were staged as stage III according to the tumor node metastasis (TNM) staging system, with tumors >5 cm in diameter. The baseline characteristics of the patients were well balanced between the two groups, with no statistically significant difference (P>0.05; Table 1).
UMIPIC and ITCT preparation
UMIPIC and ITCT were carried out with the same therapeutic procedure. The 25-gauge spinal needles and the inflators (inflation device, 30 atm/bar) were purchased from Merit-Medical (South Jordan, UT, USA). UMIPIC and ITCT solutions were freshly prepared before each injection. UMIPIC contains a clinically approved regimen (each regimen contained an oxidant, a cytotoxic drug Ara-C, and hapten) for percutaneous intratumoral delivery; ITCT contains the same regimen, with the oxidant and the cytotoxic drug Ara-C, but without hapten.
Treatment delivery
All patients either had a pretreatment ultrasound or computed tomography (CT) scan of the liver at baseline. Routine examination of cardiopulmonary function was also performed prior to treatment. The laboratory blood tests included hepatitis B and C virus antigen/antibodies, serum alpha-fetoprotein, serum albumin, total serum bilirubin, and alanine aminotransferase. Patients with intestinal obstruction and any heavy infections were not allowed to receive this therapy until their symptoms disappeared. Prior to UMIPIC or ITCT treatment, the patients were asked to fast without water intake for 14 hours in order to avoid side effects such as vomiting. In order to control pain that may occur during the treatment, 50 mg of morphine was injected intramuscularly at least 30 minutes pretreatment. The skin was cleaned and local anesthesia performed at the injection site.
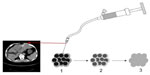
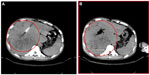
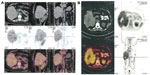
After the spinal needle was inserted into the tumor under CT guidance, the core was taken out of the needle (which was connected to the inflator used as a high-pressure syringe), and then the injection performed (Figure 1). UMIPIC or ITCT was delivered by a spinal needle inserted into the tumor and connected with the inflator for injection under pressure (at the level of atmospheric pressure or a little higher) to obtain fully forced distribution of the regimens in the tumor. Ultrasound or CT (Picker IQ; Phillips Healthcare, Bothell, WA, USA) guidance was used for scanning and monitoring of the density changes in the area of interest in the liver tumor. Special attention was paid to monitoring the CT value changes in the margins of surrounding tumor to ensure full distribution of drugs to the edge of the tumor (Figure 2). Since the combination solution is composed of water-soluble drugs with higher pressure (with the inflator) for injection into the tumor mass and forced distribution in the tumor, it is quite different from oil–water (drug) emulsions, which are sticky and hard to distribute intratumorally into tumor masses. The combination of drugs in UMIPIC and ITCT could penetrate into the full matrix of the tumor, even into tumor cells; therapeutic coagulation then occurred with sustained release in the tumor for an extended period of time with the help of the oxidant.11,13 The procedure took approximately 30–45 minutes; however, if the tumor was difficult to penetrate, a repeat CT was needed for monitoring and deciding whether another injection was required. The volume of the injection was calculated based on the diameter of the tumor (Dt) ×2 for tumors 1–5 cm in size and (Dt) ×1.5 for tumors ≥6 cm in size. Good practice, the key for each therapy, should be based on these calculations to deliver enough dosage into the tumors.
The size of the tumor (tumor mass) is classified by reexamining CT scanning for each week post-injection; if necessary, treatment was repeated simultaneously. Three treatments in total including the initial treatment was taken as one cycle of UMIPIC or ITCT (multiple treatment); some patients received an initial treatment with UMIPIC or ITCT without continuing treatment (single treatment). If the tumor size did not stabilize or reduce in size after 6–8 weeks of initial treatment when the tumor was reexamined, additional injections were added to improve efficacy.
Patients were closely monitored on the first day post-treatment; significant systematic or local adverse effects were evaluated.
Clinical pharmacokinetics
In an HCC patient with two tumor masses, 99Tcm labeled to Ara-C was successful with a 99.9% labeling rate measured; 0.5 mCi of 99Tcm-Ara-C in cytotoxic Ara-C and oxidant (A, UMIPIC) and the same dose of 99Tcm-Ara-C in normal saline (B, ITCT) were injected into two tumors in the same liver and observed for 99Tcm isotope activity and imaging at different time points under SPECT GEStarcom400, a single photon emission computed tomography machine with gamma emission; sustained release of 99Tcm in the two tumors was assessed.
Assessment
The response to treatment was evaluated by the solid tumor effect evaluation criterion of EORTC (European Organization for Research and Treatment of Cancer) and RECIST (Response Evaluation Criteria in Solid Tumors) by the US National Cancer Institute (NCI) in October 1999.14 All CRFs were filled in by treating physicians from hospitals.
Statistical analysis
Statistical analysis was performed at Binzhou Union Medical College. The primary objective was to evaluate the overall survival (OS), which was defined as the duration from the first treatment date to the death date and estimated according to Kaplan–Meier analysis. The secondary endpoint was response rate at 4–6 weeks post-treatment, defined as the proportion of patients with complete response (CR), partial response (PR), or stable disease (Sdz) according to RECIST (v1.0). The response and survival rates were analyzed and statistical differences between groups were based on the chi square test. Tumor size was classified as small (<5 cm), large (5–10 cm), or very large (>10 cm); tumor sizes with liver function categorized using Child–Pugh class A and B (Table 1) were analyzed by correlation with OS. Statistical analysis was conducted using SPSS (v17.0; SPSS Inc, Chicago, IL, USA); P-values <0.05 were considered to indicate statistical significance.
Results
Efficacy evaluation
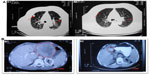
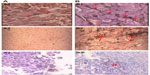
The response rates (CR + PR + Sdz/total) were 78.68% and 81.52% in the UMIPIC and ITCT groups, respectively, and revealed no significant difference (Table 2) due to a slight increase in mean tumor size observed in the UMIPIC group, which was likely due to an inflammatory response induced by therapeutic coagulation or the interaction of malignant cells with the extremely high concentration cytotoxic drug from the local administration.10,11 It has been shown in animals that the inflammation in the tumors is induced by intratumoral chemoimmunotherapy and lung cancer patients were treated using the same UMIPIC therapy, with similarly efficacy;13 however, most of the tumors were found to be in a stable condition (Figure 2B) and some of them had CR (Figure 3B) with tumor necrosis (Figure 4B).
The mean and median OS (censored observations) were 10.55 vs 7 months and 7.42 vs 4 months in the UMIPIC and ITCT groups, respectively (Table 3). This represents a statistically significant difference between the two groups (P<0.01), with Kaplan–Meier curves for both groups depicted in Figure 2. The 6-month and 1-year survival rates of the UMIPIC and ITCT groups were 58.88% vs 32.8% and 30.37% vs 13.6% (Table 3 and Figure 2A) with a statistically significant improvement (P<0.01) in the UMIPIC and ITCT groups; these results strongly suggest that the hapten played a part in the immunological response against potential tumor cells in the body to prolong the patients’ survival time.
  | Table 3 Comparison of overall survival time between UMIPIC and ITCT |
Data on single treatment showed that the mean (6.04 months) and median (4 months) survival time with UMIPIC were higher than that of single treatment with ITCT (4.21 months and 3 months); this denotes a statistically significant difference (P<0.01; Table 4); additionally, the 6-month (36.58%) and 1-year survival rates (24.39%) for single treatments using UMIPIC were higher than the 6-month (18.18%) and 1-year survival rates (3.03%) for single treatment using ITCT; these results show a statistically significant improvement in OS (P<0.01; Table 4 and Figure 2B).
Data on multiple treatments revealed that the mean (11.37 months) and median survival times (7 months) were higher than that for single treatment (10.08 months and 4 months), with a statistically significant difference (P<0.01; Table 5); in addition, the 6-month (64.16%) and 1-year survival rates (38.04%) for multiple treatments with UMIPIC were higher than that of the 6-month (38.04%) and 1-year survival rates (17.37%) for multiple treatment with ITCT; these findings revealed statistically significant improvement in OS (P<0.01; Table 5 and Figure 2C).
Both single and multiple treatment of UMIPIC revealed that the hapten with UMIPIC therapy had a role in the immunological response against the tumor cells, which prolonged the patients’ survival time compared to ITCT.
A pharmacokinetic study for sustained release was observed in another clinical study between the UMIPIC and ITCT. At 12 hours, drug retention of 82% vs 16% was found in each tumor between UMIPIC and ITCT, respectively; at 24 hours after the injection, drug retention of 60% vs 0% was found in each tumor between UPIPIC and ITCT, respectively (Figure 5A). Moreover, in the presence of the inflator, it is particularly important to note that the advantages of this approach, including high-sustaining and homogeneous drug diffusion in the tumor, could present a satisfying clinical outcome.
Tumor size was classified as small (<5 cm), large (5–10 cm), or very large (>10 cm), tumor sizes with liver function categorized using Child–Pugh class A and B (Table 1) were analyzed by correlation with OS (Tables 6 and 7). This revealed that the median time and rate of 6-month and 1-year survival rates for the UMIPIC group was significantly better than for the ITCT group for all tumor sizes, including small, large, and very large tumors; there was no significant difference between the large and very large tumors with UMIPIC therapy (Table 6). It was also revealed that the median time and rate of 6-month and 1-year survival rates in Child–Pugh class A patients’ liver function after UMIPIC therapy was significantly better than that of Child–Pugh class B patients after ITCT; there was no significant difference between Child–Pugh class A and B patients after ITCT treatment; nor was there any significant difference for Child–Pugh class B patients between UMIPIC and ITCT treatment.
  | Table 6 Tumor size correlation with overall survival time and rate |
  | Table 7 Child A and B inflection on the patient’s survivals |
Common complications included temporary mild fever (not over 38°C) for a few hours, minor pain at the injection area, and minimal hemorrhage around the tumor, and needle track. No other significant systemic or local adverse effects were observed. Common chemotherapy side effects such as myeloid suppression, neutropenia, thrombocytopenia, gastrointestinal toxicity, and apparent loss of hair and/or appetite were not seen.
Discussion
HCC is believed to be a potentially ideal tumor for targeting by immune-based therapies;15 the immunotherapy approach may be a crucial addition to current treatment. To date, the immunotherapeutic strategies for HCC have included the administration of immune stimulator cytokines,16 gene therapy with cytokines and costimulatory molecules,17 immunotherapy with dendritic cells loaded with specific tumor antigens,18 and stimulation with immunogenic vaccines or antibodies.19 Effective combinations of immunotherapy and chemotherapy or TACE remain to be explored. Rescigno et al showed that cytotoxic drugs are not always detrimental to the immune system; they can actually enhance antitumor immune response by increasing tumor antigen presentation and depleting tumor-promoting regulatory T cells, as well as through other mechanisms.20 Moreover, chemoimmunotherapy has demonstrated synergistic efficacy in the treatment of HCC,21 lymphoma,22 and leukemia.23
TACE is now considered the standard of care worldwide for patients with primary HCC but is limited in that it may not be suitable for HCC patients with larger tumors and extrahepatic metastases.11,24 TACE can shut down hepatic arterial flow toward tumors with the help of an oil–drug emulsion; when the drug moves from the oil to the tumor and kills the cancer cells, the drug also moves to the rest of the body, which is why TACE produces side effects including vomiting, hair loss, and leukocytopenia in most HCC cases following treatment with TACE. We developed a unique approach of UMIPIC that creates a drug depot in the tumor through therapeutic coagulation with drug-sustained release for a prolonged time and avoiding circulation of the drug to the whole body. UMIPIC also integrates therapeutic coagulation with chemotherapy drug, both of which are synergized with hapten, thus UMIPIC produced functioning tumor in situ autologous vaccine is another possibility; another advantage over TACE, which does not have this function. Clinically, UMIPIC is much easier to perform than TACE; it is similar in difficulty to a biopsy and does not need a catheter or oil–drug emulsion.11,13 In general terms, UMIPIC can overcome the shortcomings of systematic chemotherapy or TACE and extend patient survival based mainly on the following principles.
First, upon initiation of UMIPIC treatment, the oxidant can instantly and effectively change the extracellular matrix and alter morphological and biochemical components of tumor cells, such as collagen and other high-molecular-weight substances, resulting in therapeutic coagulation in the tumor, which leads to its transformation into a soft, semisolid tumor mass, stopping tumor metabolism and causing fibrosis (Figure 5B). Coagulation can now shut down blood flow like TACE and entrap the injected drugs at higher concentrations within the coagulated tumors, and the water-soluble drug can penetrate into the whole matrix and tumor, even into tumor cells, which are not located in the blood vessels like the oil–water emulsion in TACE. This improves drug utilization by extending the duration of the drug in the tumor and decreasing systemic drug exposure through sustained drug release. Meanwhile, the drug Ara-C can continue to kill the tumor cells that were not killed by the initial therapeutic coagulation. Intratumoral therapy, as an obvious and attractive alternative to systemic treatment, was approved for clinical use many years ago.25 Intratumoral delivery of anticancer drugs can significantly increase local accumulation of the drug (up to 10–100 times more than systemic administration).11,13 In the clinical pharmacokinetic study, it was revealed that the drug was retained at 82% in UMIPIC vs 16% in ITCT after 12 hours in each tumor, and 60% in UMIPIC vs 0% in ITCT after 24 hours in each tumor, respectively (Figure 6). Therefore, it is particularly important to note that the advantages of this approach, including highly sustained and homogeneous forced drug diffusion in the tumor, can be obtained by injection with the inflator at the level of atmospheric pressure or higher as needed.
It is believed that the coagulation procedure and chemotherapy drugs sustained in tumors play a powerful role in chemical debulking tumor mass (similar to chemical surgery) (Figure 3) and provide an opportunity for the patient’s own immunotherapy to take place and guard against microtumors (≤108 tumor cells), which immunotherapy could make functionally effective. Also, the instant therapeutic coagulation of tumors can kill Treg cells in a tumor mass following UMIPIC treatment and enhance upregulation of T cell activity.
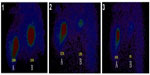
Secondly, as has been previously reported, the ‘abscopal effect’ results in the regression of distant tumors after localized treatment.26 In this study, hapten can induce an immunological (accepted correlation to abscopal-like) antitumor response, and has been documented in an HCC patient with bilateral pulmonary metastases (Figure 4A). This means that UMIPIC-induced immunological response can be effective against microtumors at a different challenged site even those that cannot be seen by imaging or are inaccessible. Ludgate27 noted that pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) may have the desired abscopal effect on metastatic tumor sites by evoking an acute anticancer immune response using an ‘endogenous’ vaccine approach. Similar results have been mentioned in clinical reports.28,29 One patient in clinical observation had a systemic response to distant tumor with UMIPIC. Another patient with bilateral pulmonary metastatic lesions received eleven UMIPIC treatments targeting the primary tumor; he survived more than 5 years from the time of diagnosis. The patient had disease regression in the primary mass as well as distant sites in the lungs, which were not injected (Figure 4A), further supporting the concept of the ‘abscopal effect’ caused by primary tumor debulking with hapten-enhanced systemic immune response.
Thirdly, more than 90% of the tumor cells were killed at the site of injection by the coagulated tumor and cytotoxic drug. Thereafter, multiple autologous tumor-associated antigens are released from the dead tumor cells, which continue to trigger a mild immune response, like a form of self-vaccination. That is, the quantity of the autologous tumor-associated antigens are just enough to reach the threshold required for stimulating immune response. Our earlier animal studies showed that there is a significant boost to systemic immunity after UMIPIC, especially for the number of CD4+ and CD8+ T cells. When multiple autologous tumor-associated antigens were released from the apoptotic or necrotic tumor cells caused by coagulation and the cytotoxic drug, theoretically they could trigger an immune response as a form of self-vaccination. Studies have reported, however, that cell death can be a priming event for T cell response and induce potent immunity.30,31 In the presence of an immunological modulator (ie, small-molecule hapten inlaying the denatured tumor), the lysed tumor cells in the resulting depot are modified with hapten and generate stronger tumor-associated antigens, which are referred to as an autologous tumor in situ vaccine (making the tumor itself more immunogenic). Accordingly, the systemic immunity against patient-specific tumor-associated antigens was boosted by significantly increasing active antigen-presenting cells (including dendritic cells [DCs] and macrophages), which are recognized by T cells and natural killer cells. These fight active (or pathogenic) tumor cells in and around the primary tumor, as active tumor cells are not killed by the initial coagulation, as well as active tumor cells in elsewhere in the patient’s body.
Inflammatory response may involve antitumor immunity – the therapeutic coagulation mass acts as an inflammatory tissue, with cytokine and chemokine release attracting dendritic and other antigen-presenting cells to the antigens released from the dead tumor to drain the lymph nodes, driving an adaptive acute immunity to further eradicate cancer cells at distant sites. This study revealed that UMIPIC can induce higher inflammatory response in local tumors, with no significant difference in the response of the UMIPIC and ITCT groups at the time of reexamination. However, for long-term follow-up, the UMIPIC group exhibited significant improvement in OS. We believe that UMIPIC-induced inflammatory response with hapten may play an important role in the tumor’s autologous in situ vaccine-like function; for patients with large tumors this immunological function may have weak significance in terms of clinical benefit, so that multiple treatments are needed to debulk the tumor mass to the level that immunotherapy can take place (Figure 7). In our study, multiple treatments have demonstrated clinical efficacy and safety with compelling clinical evidence (Table 4). This may contribute to long-term immunological memory induced by constitutively released antigens, leading to a more effective antitumor response. Furthermore, as the marker of the activated T cells, elevation of the costimulator may be associated with clinical benefits and better OS.32
Patients’ tumor sizes and Child–Pugh class A and B were correlated with OS; small tumors had a better survival improvement than large and very large tumors in UMIPIC treatment. Conversely, this was not the case in ITCT treatment. This encourages patients to be treated as early as possible. Child–Pugh class A patients’ liver function has a better survival improvement than Child–Pugh class B patients in UMIPIC therapy. Child–Pugh class A patients have a better survival improvement in liver function with UMIPIC treatment than ITCT in Child–Pugh class A patients, but there was no difference between Child–Pugh class A and B for ITCT, nor between UMIPIC and ITCT in class B patients. This suggests that improvement of liver function is an important role in the improvement of patients’ survival time.
Compared with traditional chemotherapy, the side effects of UMIPIC involve minimal fever and tolerable local pain and the clinical outcome is an improved quality of life.
In conclusion, UMIPIC provides a new method of decreasing tumor mass while boost the patient’s own immunological power to fight against microtumor cells in a specific and innovative manner, which is one of its advantages over TACE. Another advantage is that it is not limited in terms of tumor size, number, or location in the liver, which is not always the case with TACE. In future, it is possible that UMIPIC may overtake TACE in the treatment of all stages of HCC, even for survival patients in which HCC might be suitable to TACE therapy. UMIPIC can take the place of TACE in patients who are not suited for TACE therapy or when TACE fails. More effective control of all stages of HCC is strongly needed. We hope to continue to investigate UMIPIC therapy with double cytotoxic drugs with hapten under clinical study to improve effectiveness. We believe UMIPIC has provided a new method for the treatment of primary HCC: UMIPIC is safe, easy to operate, and reproducible.
Author contributions
Jian Liu, most of the data collection; Peng Jing, data collection; Yuanfei Lu, participated in writing the manuscript; Wei Han, data collection; Feng Gao, data collection; Peicheng Zhang, data collection; Guoliang Liu, data collection; Ning Ru, data analysis; Guanghui Cui, data analysis and imaging assistance; Chenglin Sun, data analysis and imaging assistance; Changjiang Guan, data collection; Xinhai Xu, data collection; Yebing Che, data collection and data analysis; Huaming Zhang, data collection and data analysis; Huan-You Wang, manuscript correction and histopathology; Yingli Wu, data collection; Zhenlu Ma, data collection; Qiang Fu, data collection and data analysis; Baofa Yu, principal investigator. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work. These hospitals are non-profit organizations in the People’s Republic of China.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | |
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008: Cancer Incidence and Mortality Worldwide: IARC Cancer Base No 10. Lyon, France: International Agency for Research on Cancer; 2010. | |
American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society. Available from: http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/2013-cancer-facts-and-figures.pdf. Accessed February 24, 2015. | |
Rossi L, Zoratto F, Papa A, et al. Current approach in the treatment of hepatocellular carcinoma. World J Gastrointest Oncol. 2010;2(9):348–359. | |
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–442. | |
Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356(9232):802–807. | |
Lo CM, Liu CL, Chan SC, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245(6):831–842. | |
Rampone B, Schiavone B, Martino A, Viviano C, Confuorto G. Current management strategy of hepatocellular carcinoma. World J Gastroenterol. 2009;15(26):3210–3216. | |
Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma: an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23(11):1535–1547. | |
Goldberg EP, Almond BA, Enriquez I. Nano-mesosphere drug carriers for localized cancer chemotherapy. Nanotech. 2006;2:1–4. | |
Qiong J, Yu B. Slow intra-tumor release of drugs on B16 melanoma in mice. J Shandong Univ. 2007;45:988–992. | |
Berd D. M-Vax: an autologous, hapten-modified vaccine for human cancer. Expert Rev Vaccines. 2004;3(5):521–527. | |
Yu B, Lu Y, Gao F, et al. Hapten-enhanced therapeutic effect in advanced stages of lung cancer by ultra-minimum incision personalized intratumoral chemoimmunotherapy. Lung Cancer: Target and Therapy. 2015,6:1–11. | |
Duffaud F, Therasse P. New guidelines to evaluate the response to treatment in solid tumors. Bull Cancer. 2000;87(12):881–886. French. | |
Butterfield LH. Immunotherapeutic strategies for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S232–S241. | |
Shiratori Y, Shiina S, Teratani T, et al. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138(4):299–306. | |
Cao L, Kulmburg P, Veelken H, et al. Cytokine gene transfer in cancer therapy. Stem Cells. 1998;16 Suppl 1:251–260. | |
Melcher A, Todryk S, Bateman A, Chong H, Lemoine NR, Vile RG. Adoptive transfer of immature dendritic cells with autologous or allogeneic tumor cells generates systemic antitumor immunity. Cancer Res. 1999;59(12):2802–2805. | |
Matar P, Alaniz L, Rozados V, et al. Immunotherapy for liver tumors: present status and future prospects. J Biomed Sci. 2009;16:30. | |
Rescigno M, Avogadri F, Curigliano G. Challenges and prospects of immunotherapy as cancer treatment. Biochim Biophys Acta. 2007; 1776(1):108–123. | |
Lau WY, Leung TW, Lai BS, et al. Preoperative systemic chemoimmunotherapy and sequential resection for unresectable hepatocellular carcinoma. Ann Surg. 2001;233(2):236–241. | |
Aguiar Bujanda D, Aguiar Morales J, Bohn Sarmiento U, Saura Grau S, Rodríguez Franco C. Clinical experience with biweekly CHOP plus rituximab chemoimmunotherapy for the treatment of aggressive B-cell non-Hodgkin lymphoma. Clin Transl Oncol. 2009;11(9):604–608. | |
Schulz H, Klein SK, Rehwald U, et al; German CLL Study Group. Phase 2 study of a combined immunochemotherapy using rituximab and fludarabine in patients with chronic lymphocytic leukemia. Blood. 2002;100(9):3115–3120. | |
Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol. 2006;23(2):119–125. | |
Klutz K, Willhauck MJ, Wunderlich N, et al. Sodium iodide symporter (NIS)-mediated radionuclide ((131)I, (188)Re) therapy of liver cancer after transcriptionally targeted intratumoral in vivo NIS gene delivery. Hum Gene Ther. 2011;22(11):1403–1412. | |
Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–870. | |
Ludgate CM. Optimizing cancer treatments to induce an acute immune response: radiation abscopal effects, PAMPs, and DAMPs. Clin Cancer Res. 2012;18(17):4522–4525. | |
Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. | |
Abei M, Okumura T, Fukuda K, et al. A phase I study on combined therapy with proton-beam radiotherapy and in situ tumor vaccination for locally advanced recurrent hepatocellular carcinoma. Radiat Oncol. 2013;8:239. | |
Nowak AK, Lake RA, Robinson BW. Combined chemoimmunotherapy of solid tumours: improving vaccines? Adv Drug Deliv Rev. 2006;58(8):975–990. | |
Lake RA, Robinson BW. Immunotherapy and chemotherapy – a practical partnership. Nat Rev Cancer. 2005;5(5):397–405. | |
Carthon BC, Wolchok JD, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16(10):2861–2871. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.