Back to Journals » Clinical Interventions in Aging » Volume 18
Gut Microbiota and Aging: Traditional Chinese Medicine and Modern Medicine
Authors Li J, Li D, Chen Y, Chen W, Xu J, Gao L
Received 28 March 2023
Accepted for publication 8 June 2023
Published 17 June 2023 Volume 2023:18 Pages 963—986
DOI https://doi.org/10.2147/CIA.S414714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Zhi-Ying Wu
Jinfan Li,1,2,* Dong Li,3,* Yajie Chen,4,* Wenbin Chen,2,5,6 Jin Xu,2,5,6 Ling Gao2,5,6
1Department of First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, 250000, People’s Republic of China; 2Key Laboratory of Endocrine Glucose & Lipids Metabolism and Brain Aging, Ministry of Education, Department of Endocrinology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, People’s Republic of China; 3Department of Diabetes, Licheng District Hospital of Traditional Chinese Medicine, Jinan, Shandong, 250100, People’s Republic of China; 4Department of Rehabilitation and Health Care, Jinan Vocational College of Nursing, Jinan, Shandong, 250100, People’s Republic of China; 5Shandong Key Laboratory of Endocrinology and Lipid Metabolism, Jinan, Shandong, 250021, People’s Republic of China; 6Shandong Institute of Endocrine and Metabolic Diseases, Jinan, Shandong, 250021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ling Gao, Key Laboratory of Endocrine Glucose & Lipids Metabolism and Brain Aging, Ministry of Education, Department of Endocrinology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, People’s Republic of China, Email [email protected]
Abstract: The changing composition of gut microbiota, much like aging, accompanies people throughout their lives, and the inextricable relationship between both has recently attracted extensive attention as well. Modern medical research has revealed that a series of changes in gut microbiota are involved in the aging process of organisms, which may be because gut microbiota modulates aging-related changes related to innate immunity and cognitive function. At present, there is no definite and effective method to delay aging. However, Nobel laureate Tu Youyou’s research on artemisinin has inspired researchers to study the importance of Traditional Chinese Medicine (TCM). TCM, as an ancient alternative medicine, has unique advantages in preventive health care and in treating diseases as it already has formed an independent understanding of the aging system. TCM practitioners believe that the mechanism of aging is mainly deficiency, and pathological states such as blood stasis, qi stagnation and phlegm coagulation can exacerbate the process of aging, which involves a series of organs, including the brain, kidney, heart, liver and spleen. Our current understanding of aging has led us to realise that TCM can indeed make some beneficial changes, such as the improvement of cognitive impairment. However, due to the multi-component and multi-target nature of TCM, the exploration of its mechanism of action has become extremely complex. While analysing the relationship between gut microbiota and aging, this review explores the similarities and differences in treatment methods and mechanisms between TCM and Modern Medicine, in order to explore a new approach that combines TCM and Modern Medicine to regulate gut microbiota, improve immunity and delay aging.
Keywords: gut microbiota, aging, traditional Chinese medicine, microbiota-gut-brain axis
Introduction
“For many millions of people worldwide, traditional medicine is the first port of call to treat many diseases”, said Dr. Tedros Adhanom Ghebreyesus, WHO director-general.1 In fact, Traditional Chinese Medicine (TCM) has a long history of more than 2000 years in China. From the leading medicine in agricultural civilisation to a current supplementary treatment, the transformation of its role has not affected its applicability and effectiveness. As an ancient medicine, TCM embodies people’s most simple feelings and wishes for life, including that of prolonging life and preventing diseases. Its low drug toxicity, low susceptibility to drug resistance and drug dependence, as well as its multi-component and multi-target effects, and its achievements in regulating the composition of gut microbiota and delaying aging in recent years, have attracted the attention of Modern Medicine.
 |
Figure 1 Symbiotic ecosystem. Healthy and stable gut microbiota and host constitute a harmonious symbiotic ecosystem. |
Prolonging life expectancy is a medical breakthrough expected by most citizens as people are eager to have more time to finish their unfinished businesses. Immortality and aging have always been a paradox in the western concept of aging, but it is still valid in the East.2 We have indeed made some positive changes, but we still face many problems in prolonging life while ensuring the quality of life and finding ways to make people live healthier and more independent in their later years. Global total life expectancy between 2000 and 2015 increased by 5 years, while the healthy life expectancy was only 4.6 years.3 Moreover, about 20% of life has to endure disease entanglement in the later years.3,4 Ironically, fear of aging is also an important factor that hinders healthy aging. In other words, people’s healthy life span is always a bit slower than their growth. Some studies have pointed out that after the age of 65, the time of poor health is 9 years for men and 11 years for women on average.5,6
More importantly, although Modern Medicine has delved deeply into the study of aging and various mechanisms such as free radical damage theory and oxidative stress theory have been constantly mentioned, there has been no significant progress in the prevention and treatment as well as the development of new drugs for related diseases and weakened states of the body (organs) that accompany the aging process. For example, in the treatment of cognitive impairment (CI) in the process of aging, although the pathogenesis of patients is different, the current standardised treatment drugs are limited by focusing on cholinesterase inhibitors and excitatory amino acid receptor antagonists, and the newly developed targeted drugs also scarcely achieve satisfactory efficacy.7 However, the challenges encountered in Modern Medicine seem to be possible to be solved through TCM. The multi-component and multi-target effects of TCM, combined with its low toxicity and low drug resistance, have attracted widespread attention from researchers.
However, special technical means or media must be relied on in the exploration of the internal-mechanism laws in the numerous and complicated ingredients of TCM. At present, most studies use gut microbiota in exploring the biotransformation process of TCM. Moreover, in recent years, studies on the regulation of gut microbiota by TCM have been widely reported, and the use of Chinese herbal medicine to improve gut microbiota composition has become a safer and more reliable choice after the intake of probiotics and other modern medical models.8–10
Gut microbiota refers to a symbiotic ecosystem composed of microbial communities on the gut mucosa surface, including bacteria, fungi, archaea and phages.11 In recent years, the relationship between host gut microbiota and aging has become a hot topic in biomedical research. More and more evidence shows that the balanced symbiosis mode of the host gut microbiota is closely related to the host’s health.12–14 This is because the gut microbiota itself can act as a barrier to external pathogens, stimulate the host to produce antibacterial compounds and facilitate the health maintenance function of the host by consuming nutrient sources and occupying attachment sites. The imbalance of composition and function of gut microbiota is a vital link point from digestive-system diseases that are represented by local gastrointestinal diseases to the nervous system, metabolic system and circulatory system.15,16 More specifically, in addition to causing digestive-system diseases, the imbalance and dysfunction of gut microbiota have been recognised as the core cause of cognitive impairment, diabetes and kidney diseases.2,13,15,16 Recent studies have pointed out that the gut microbiota is also involved in mediating tumour immune regulation, and metagenomic analysis has revealed significant differences in gut microbiota composition of cancer patients and healthy individuals.17 In addition, the gut microbiota has also been proven to be a therapeutic target for age-related diseases such as sarcopenia.18 These exciting new studies have linked gut microbiota with aging. These factors, on the one hand, determine how the gut microbiota affects the progress of aging and how that, in turn, leads to diseases related to gut microbiota imbalance;19–21 on the other hand, these also reveal the importance of gut microbiota as key biomarkers and therapeutic targets in age-related diseases.
This review aims to summarise the latest progress in research on the relationship between gut microbiota and host aging. In addition, the integration of TCM intervention with gut microbiota and aging provides us with a new model to elucidate the relationship between them from the perspective of TCM. In this way, we hope to determine more clearly the relationship between gut microbiota and host aging in order to provide a sufficiently persuasive documentation on the effectiveness of traditional alternative medicine.
Brief Summarisation of TCM
TCM is the crystallisation of the wisdom of the Chinese people who have inherited and carried it forward through the long history and tradition of China. TCM has been in clinical practice for around two thousand years and has, thus, accumulated a very rich experience. The main treatment methods of TCM include herbal medicine, acupuncture and other physical therapies.22 TCM has a strong support base in China and the surrounding East Asian countries. On one hand, it is rather effective; for example, the participation rate of TCM in the treatment of COVID-19 in all parts of China has exceeded 90%.23 Moreover, its excellent curative effects have been verified by a multi-centre, randomised and controlled prospective clinical trial.24 On the other hand, the concept of health preservation advocated under its unique macro-theoretical system of “holistic concepts” and “preventive treatment of disease” is deeply rooted in people’s hearts as well.
The holistic concept, in short, emphasises the integrity of the human body and the intimate relationship between human beings and their natural environment and society. It considers that the integrity of the human body comprises the integrity of the spirit and body and the coordinated operation of various organs. Here, the preventive treatment of disease refers to promoting a healthy qi through health care and other conditioning means to achieve the effect of disease prevention.
However, in Modern Medicine, the acceptance of TCM is minimal as its connotation of science lies in verifying any conjecture and hypothesis through experiments. However, the herbal medicine compatibility principle of “King, Vassal, Assistant, and Delivery Servant” in TCM and the scientific effectiveness of traditional skills, such as Qigong, are difficult to be verified under modern scientific methods.25,26 Thus, the field of Chinese herbal medicine is a bridge between TCM and western medicine. Nonetheless, as many modern drugs are derivatives of herbal medicines and natural products, Modern Medicine also has a strong interest in deriving new drugs based on Chinese herbal medicine.22,27,28 Around 341 AD, Dr. Ge Hong described the application of TCM in treating malaria in his book Zhou hou bei ji fang. This inspired Professor Tu Youyou to use ether to separate artemisinin, an effective substance used in the treatment of malaria, from Artemisia annua and he won the Nobel Prize in Physiology and Medicine in 2015.29 Chinese herbal medicine contains multiple active ingredients and has various biological effects on various pathways and targets. Among them, complex compounds including polysaccharides, peptides, glycoproteins and lipids, as well as their metabolic derivatives, such as glycosides, amines, fatty acids, flavonoids, terpenoids, phenols and alkaloids, interact closely with each other to regulate a series of biological processes of the host, such as immune and inflammatory responses.30,31 For example, astragalosides extracted from Astragalus membranaceus have been validated to exhibit anti-aging function by restoring the activity of MnSOD and GSH/GSSG ratio, as well as enhancing thymic index and spleen cell proliferation.32 Similarly, parishin from Gastrodia elata exerts anti-aging effects by regulating Sir2/Uth1/TOR signals to prolong yeast lifespan.33 The compound rhein showed beneficial effects on diabetic nephropathy, which is related to reduced levels of TGF-β1.34 Buyang Huanwu decoction can significantly inhibit fat accumulation in the body of type-2 diabetic rats and reduce cholesterol and triglyceride levels.35 Fuzheng Huayu is widely administered to ameliorate chronic liver diseases and functions through the modulation of multiple signalling pathways in a number of organs.36
Gut Microbiota in Health and Disease
Almost all living creatures and all environments on earth have their own microbial communities.37 For instance, the abundance and functional status of the different microbial species in the human gastrointestinal tract are closely related to the host’s health. The gut microbiota maintains the host’s health in several ways by regulating the gut endocrine function, providing bioenergy, synthesising vitamins and various metabolic compounds, constructing the immune system by colonising the gut mucosa, and producing different antibacterial substances to resist pathogens, thereby influencing brain-gut communication and the psychological and neural functions of the host.38–41 Therefore, the gut microbiota is essential to maintain the host’s health and normal gut physiology in them.
Further, the gut microbiota is generally responsible for metabolising food intake into bioactive components.42 These microorganisms can metabolise a series of indigestible carbohydrates into short-chain fatty acids (SCFA), which are represented by acetic acid, propionic acid and butyric acid, and directly participate in the metabolism of bile acid (BA) and lipopolysaccharide (LPS).43 Moreover, any interference in the biosynthesis of any of these is the cause of many pathological conditions.42,44 Because of that, changes in the gut microbiota population and its quantity will profoundly affect the physiological and pathological functions of the host. For instance, many diseases, especially chronic diseases, focus on exploring the potential causal relationship with gut microbiota, including chronic diseases of the elderly like chronic inflammation, cardiovascular and cerebrovascular diseases and psychiatric diseases.15,42 All this will be widely discussed in this review.
Gut Microbiota and Age-Related Changes
As mentioned earlier, the human gut harbours thousands of bacteria. These bacteria affect the function of the gut and participate in the overall health regulation of the body, such as digestion and the absorption of nutrients as well as the synthesis of vitamins, amino acids and other substances. They are also involved in the regular operation of the host’s nervous system and immune functions.45–48 Indeed, the composition of gut microbiota is affected by individual factors, genetics and various components of lifestyle, such as diet, exercise, sleep quality, consumed drugs and even mental health.49–51 Therefore, we can regard the gut microbiota and host as a symbiotic ecosystem; they interact and influence each other, which is consistent with the holistic concept of TCM (Figure 1).
Although many factors affect the composition of the gut microbiota, the researchers found that in the intestines of healthy adults, the microbiota is mainly composed of bacteria—of which more than 90% are Bacteroidetes and Firmicutes—and this distribution trend remains relatively balanced in the whole adult segment.52
Interestingly, remaining relatively stable does not imply immutability. It is believed that a person’s age significantly impacts their gut health.53,54 The researchers indicated that the gut microbiota loses microbiota diversity with the increase in age, which is characterised by a decrease in the amount of glycolytic and proteolytic bacteria and an increase in sub-dominant species.55,56 (Figure 2) In the elderly, the abundance of Bacteroides, bifidobacteria and thick-walled bacteria is reduced, leading to chronic low-grade inflammation and declining immune system function. This results in many diseases related to aging, including gastrointestinal diseases, type-2 diabetes, metabolic syndrome, atherosclerotic diseases, neurodegenerative diseases, cancer and cachexia.56–58 In addition, there is a concept that bacterial cells in the gut do not age themselves; however, with age, people may begin to experience comorbidities associated with the gut and gut bacteria.59 Therefore, it is a significant challenge to clarify the relative contribution of age, drugs, diet and comorbidity to microbial dysbiosis.60 Indeed, research on the elderly adjusted for diet, lifestyle and medication shows that age is a critical factor in the increase or decrease of the microbial species in the gut,61 which can be explained by the change in gut physiology with age as the microbiota is shaped in large part by physiology.62 The changes in the microbial composition are also accompanied by the changes in the metabolic spectrum of the microbiota, such as short-chain fatty acids, secondary bile acids and mucin. These substances are crucial in regulating physiological functions such as host immunity and metabolism.63–65
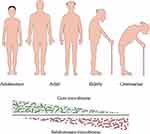 |
Figure 2 Variation trend of the gut dominant population at different ages. With the increase of age, the dominant microbial population will undergo succession. |
It is to be noted that faecal microbiota transplantation (FMT) is essential to explain the relationship between gut microbiota and aging. This microbial transplantation technique transfers the gut microbiota of one individual to the gut of another by oral administration, enema and colonoscopy. Incredibly, about 1700 years ago, Chinese physician Ge Hong recorded cases of patients with food poisoning and severe diarrhoea who were treated by oral human faecal suspension, which is the oldest existing record of FMT. In modern era, on the other hand, Smith et al used turquoise killifish to show that when middle-aged fish were treated with antibiotics and colonised by microorganisms transferred from young fish, they lived longer and were more active in later life than the control fish. It was also observed that middle-aged fish grafted with immature fish microorganisms maintained a more diverse microbial community throughout their adulthood and shared essential microorganisms with the young fish.66 Moreover, Omar et al transplanted the faecal microbiota of young mice into old mice and observed that the disorder of gut microbiota of old mice had been significantly improved, followed by cognitive impairment.67 In another FMT study in mice, older mice transplanted with the microbiota of young mice also exhibited reduction in age-related cognitive-behavioural impairments.68 These findings revealed the close relationship between gut microbiota and aging and brought the dream of rejuvenation closer to reality.
As pointed out earlier, the gut microbiota of an organism is in a constant state of flux throughout its lifespan. Recent studies have confirmed that the placental microbiota can colonise the foetus,69–71 which overturns the notion that the placenta and uterus are sterile. Studies using germ-free animals have shown that microbiota plays a causal role in ensuring blood-brain barrier development.72 Further, after the birth, the gut microbiota is characterised by a high abundance of Enterobacteriaceae, Bifidobacteriaceae and Clostridiaceae members.73–75 With the growth and development of infants, the quantity of strict anaerobes gradually increases before they turn a year old, and the overall diversity of microbiota reaches the adult level around the age of one to three.76,77 However, the composition and function of microbiota would still be significantly different from those of healthy adults.78 It is also worth noting that the function of gut microbiota at this stage is reflected in supporting development, such as its participation in vitamin synthesis and improving anti-inflammatory pathways.69 Adolescents have higher relative abundances of Bifidobacterium and Clostridium but lower relative abundances of Prevotella and Sutterella at the genus level.79 These differences are similar to those reported in other studies of child microbiota.80 Then the core microbiota changes dynamically.81 For these reasons, the age-related change in microbiota composition and diversity is closely related to the health outcomes of the elderly, especially in terms of vulnerability.59,82 A recent clinical study has shown that the variability of gut microbiota composition in elderly people over the age of 60 is much higher than in adults aged 20–60.60 The reason for this variability is the loss of beneficial bacteria and the transformation of the core microbiota. A deep, large-scale study showed that the core microbiota of elderly people is mainly composed of Bacteroidetes, with a high proportion of Firmicutes, and a decrease in the proportion of Bifidobacteria.81 Overall, the general characteristic of the gut microbiota during the aging process is the loss of existing dominant core microbiota such as Prevotella, Fecal bacilli, and Bifidobacteria, and their replacement by the subdominant microbiota such as Bacteroides, Akkermansia, Christensencellaceae and Butyricimonas.83–85
In addition, the situation of extreme age groups (such as centenarians) is also very different. Biagi et al showed that the gut microbial composition of the elderly, aged 70 or above, is significantly different from that of the centenarians, which is mainly reflected in the rearrangement of the Firmicutes population and enrichment in proteobacteria.55,86 Such impaired microbiota host homeostasis may be associated with the increased inflammatory status in the centenarians.
In addition to these conventional aging changes, the relationship between changes in gut microbiota and age-related diseases is also particularly important. Musculopenia is defined as the loss of skeletal muscle mass and strength, which is often closely related to aging. A cohort study proved that the relative abundance of Prevotella and Fecal bacilli in patients with sarcopenia was significantly reduced, while Enterobacteriaceae population was increased.87 In animal experiments, compared with the control group, the muscle mass and strength of mice treated with antibiotics decreased, but when FMT was used to restore the original intestinal microbiota composition.88 Research on the relationship between weakness and cachexia shows that the abundance of beneficial bacteria such as Bifidobacteria and Lactobacilli decreases, while the abundance of Enterobacteriaceae increases.87
The existing studies mentioned above indicate that the composition of gut microbiota changes with age, characterised by a decrease in microbial diversity and significant individual differences. Therefore, maintaining an appropriate composition of gut microbiota is particularly important in the later decades of human life. Although we have made substantial progress in understanding the gut microbiota, very little is known about how changes in gut microbiota composition affect the physiological and pathological changes of hosts and when these age-related changes begin. Through a study that established three independent cohorts and included over 9000 individuals for a four-year follow-up period,83 we may understand that the composition of the gut microbiota changes based on the host’s health status during the aging process. This also indicates that gut microbiota is an important biomarker of aging, which has a positive monitoring and recognition role in determining whether an individual is in a “healthy aging” state. This finding could inspire people to try to treat and prevent age-related diseases by adjusting the core gut microbiota.
Gut Microbiota and TCM
It is widely known that TCM interprets physiology and pathology from a macro perspective, so there is no such micro-concept of gut microorganisms in ancient TCM. However, this does not mean that TCM has nothing to do with the gut microbiota, as one of the characteristics of TCM is oral administration. TCM mainly prepares water extract by immersing components in boiling/hot water. The section would contain a mixture of multiple chemical elements, which is usually called “decoction”.30 Therefore, drugs inevitably interact with the gut microbiota. In addition, in the classic TCM treatise, Huangdi Neijing—which dates back more than 2000 years—there is a discussion that mentions, “The operation of anal function and the normal excretion of faeces depend on the coordinated operation of various organs”. It emphasises that the difficulty of defecation and the state of faeces are inseparable from whether the body is in a healthy and operational state. This is probably the earliest macro-discussion on the impact of intestinal microbiota on host physiology and pathology.
Research shows that TCM can regulate the structure of gut microbiota and the metabolism of gut microbiota to achieve a therapeutic effect.89 It is no exaggeration to say that any decoction is related to the gut microbiota. Moreover, the interaction between TCM and gut microbiota can be attributed to two aspects: One is that the active components of TCM regulate the composition and metabolism of gut microbiota, and the other is that the gut microbiota decomposes and metabolises the active ingredients of TCM.89,90 These interactions can produce a series of metabolites, which can affect the physiological and pathological functions of the body.
Take the effects of TCM on ameliorating diabetic kidney disease (DKD) as an example.91–93 Comprehensive studies show that almost all bacterial species seem to be affected by TCM, out of which Bacteroides, Firmicutes, Proteobacteria and Actinomycetes are reported the most. Existing studies show that a variety of TCM components and decoction affect the abundance and diversity of gut microorganisms and have a specific antibacterial effect. This phenomenon may also affect the occurrence or development of some diseases (Table 1). For example, Ephedra sinica can affect the abundance of Blautia, Roseburia, Clostridium and Akkermansia, further affecting the output of LPS and SCFA.94 As for the impact of pathogenic bacteria, the study said that E. sinica could also effectively respond to Neisseria gonorrhoeae, Streptococcus mutans and, especially, the novel SARS-Coronavirus-2.95 Moreover, Berberine can affect a variety of microorganisms, including Bifidobacterium, Bacteroides, Anaerofilum, Sutterella, Bilophila, Blautia, Allobaculum, Phascolarctobacterium, Escherichia coli, Clostridium, etc., as well as the Bacteroidetes/Firmicutes ratio, all of which further affect the output of LPS, SCFA and BA.96 The findings of this research were similar to the study on Sophora flavescens.97 In addition, berberine has significant effects on glucose and lipid metabolism and insulin resistance in diabetic mice.98,99 We call it “decoction” in terms of prescriptions. For instance, Xiexin decoction would be a good case. The application of Xiexin decoction could increase the abundance of Alloprevotella, Barnesiella, Papillibacter, UGC-001 and Prevotellaceae NK3B31 group in Sprague-Dawley rats,100 while also improving type-2 diabetes.101 In addition, Huanglian Jiedu decoction, Daesiho-Tang, Gegen Qinlian decoction and Qushi Huayu decoction all play their role in disease resistance while improving the gut microbiota composition of the users.102–105
 |
Table 1 The Relationship Between TCM Components and Decoction, Gut Microbiota/Bacteria Affected, and Potential Diseases |
These studies have strongly confirmed the relationship between TCM and gut microbiota. Meanwhile, the metabolites produced by the interaction between TCM and microorganisms are significant for maintaining the gut barrier and even the balance of physiological functions. Take the example of the three primary metabolites mentioned in Table 1: SCFA, LPS and BA. SCFA is the primary gut microbial metabolite, which comes from dietary fibre and other indigestible carbohydrates—mainly acetic acid, propionic acid and butyric acid. It has the function of regulating metabolism.111 Studies have also shown that SCFA can promote the secretion of glucose-like peptide-1 (GLP-1) and peptide tyrosine-tyrosine (PYY), which can be regulated by the microbiota-gut-brain axis to enhance satiety and inhibit appetite. In addition, GLP-1 can also increase the secretion of insulin, acetate and propionate, which can also regulate glucose metabolism and inhibit appetite.111–114 Therefore, there is a theory that TCM can regulate the output of SCFA to control blood glucose and reduce body weight.37 Further, LPS is a potent virulence factor from the outer membrane of Gram-negative bacteria that can enter the blood circulation and cause metabolic endotoxemia.
It is observed that LPS binds to TLR and pattern recognition receptors on the cell membrane, triggers various signal pathways, induces immune and inflammatory responses and triggers insulin resistance.37,110 Notably, LPS can activate the classical pathway TLR4/NF-κB, promoting the occurrence of chronic low-grade inflammation.115
According to the functional characteristics of LPS, the Scutellaria-Coptis herb combination and berberine were found to inhibit the production of LPS-induced inflammatory mediators by weakening the LPS/TLR4/NF-κB pathway.96,115,116 On the other hand, BA is synthesised in the liver and stored in the gallbladder. They are released into the duodenum to digest nutrients and are reabsorbed into gut cells, mainly in the ileum.115 As BA is a vital signal molecule regulating glucose and lipid metabolism. It can stimulate gut endocrine cells to secrete GLP-1, promote insulin secretion, protect pancreatic islet cell function, increase insulin sensitivity and maintain blood-glucose homeostasis.117,118 In addition, BA can interact with gut microbiota and inhibit bacterial colonisation and growth in the gut.119
In general, TCM has a significant effect on increasing the number of beneficial gut microorganisms, producing beneficial SCFA, reducing pathogenic LPS and accelerating the excretion of BA. Still, the more specific mechanism needs to be confirmed by further research.
Aging and TCM
We know that aging is a complex molecular process driven by various molecular pathways and biochemical events affected by the interaction of multiple genetic and environmental factors.120 The classical theories around the mechanism of aging include mitochondrial mutation, oxidative damage, carbonyl toxicity and free radical theory.121 In fact, aging involves a large number of genes and proteins and changes in many endogenous metabolites.120,122 In recent years, there have been many breakthroughs in the research on the use of metabolomics for these changes.
People are increasingly aware that coping with aging and its vicious spiral will be an effective way to combat aging-related diseases.123 Precisely because of this, the anti-aging effect of TCM with the holistic concept and nutritional health is receiving more and more attention.
In modern research, caloric restriction (CR) seems to be the safest and most effective intervention to prolong the lifespan of model organisms. Still, its requirement of long-term dietary control limits its popularity.120 In addition, the side effects, specific targets and multiple drug resistance of western medicine also make it difficult in practical clinical application. On the contrary, studies on TCM have found that traditional herbal extracts have the advantages of a multi-target mechanism and fewer adverse reactions. For example, Rhodiola extract can prolong the life span of worms and flies without negative effects on their reproduction or metabolic rate.124 In addition, some active ingredients and prescriptions of TCM can play a unique role in anti-aging by improving telomerase activity or inhibiting telomere shortening. A telomere is a unique nucleotide sequence at the end of the chromosome, which has been proven to be related to the replication of the life of normal somatic cells.125,126 Regulating the expression of sirtuins (SIRTs) is another direction of anti-aging research of TCM. SIRTs are a highly conserved protein family that plays an important role in regulating cell functions, such as gene repair, cell cycle, metabolism and oxidative stress.127,128 Resveratrol has been reported as an effective regulator of SIRT1.129,130 Moreover, polysaccharide from Cornus officinalis regulates the expression of downstream genes by regulating SIRT1, inhibits or delays the apoptosis of lens epithelial cells and achieves the purpose of slowing down the progression of age-related cataracts.120 Further, icariin has been reported to increase the expression of SIRT6 and inhibit NF-κB expression and inflammatory response, thereby slowing down cell aging.120
The longevity of the body is controlled by nutrition and energy-sensing signal transduction pathways, in which the targets of rapamycin (TOR) and AMP-activated protein kinase (AMPK) are essential nutrition and energy-sensing signals.120,131 mTOR is a serine/threonine protein kinase that is highly conserved in evolution and can mediate stress response. mTOR signal is a crucial regulator of aging and can prolong the life span of yeast, worms, flies and mice.132–135 Rapamycin, a western medicine, is an inhibitor of mTOR and has a considerable efficacy in treating age-related diseases, but the side effects of immunosuppression in its case are inevitable.136 In terms of TCM, as a valuable traditional Chinese herbal medicine, ginseng has been considered to “nourish healthy qi and prolong life” since ancient times. Ginsenoside Rb1 extracted from it has been proven to show anti-aging activity by reducing the expression of mTOR protein.120,137 Similarly, 6-gingerol extracted from ginger has also been shown to reduce the protein level of mTOR and, thus, has anti-aging activities.120,138 In addition, it has also been shown to have anti-tumour and anti-inflammatory properties.139
AMPK is a negative regulator of the mTOR pathway. Its overexpression can delay the aging of mammalian skeletal muscle and prolong the life span of Caenorhabditis elegans.140,141 Studies have shown that AMPK can sense the changes in AMP/ATP and maintain a balance between cell carbon utilisation efficiency and ATP production.120 It has also been observed that curcumin extracted from ginger can inhibit the proliferation and induce apoptosis of tumour cells.142 In addition, curcumin was also found to inhibit NF-κB signal-mediated inflammatory processes.143 As a widely-used traditional Chinese herbal medicine, Panax notoginseng has many roles in treating cardiovascular disease, pain, inflammation and trauma.144 The saponins extracted from Panax notoginseng can increase the level of p-AMPK protein in a dose-dependent manner, suggesting that its anti-aging function may be related to AMPK activation.120
In addition, TCM and its active components can protect the integrity of the DNA double-strand and prevent gene mutation by resisting DNA damage. Studies on puerarin and tannic acid have confirmed this conclusion as well.120 TCM has also been proven to play an anti-aging role in scavenging free radicals, anti-lipid peroxidation and enhancing the regulation of the antioxidant defence system.
Important Influencing Factors of Gut Microbiota and Aging in Modern Medical Research
Many common factors that affect gut microbiota and aging include gender, eating habits, lifestyle, diseases and the use of drugs. With the deepening of the research on this topic, people increasingly find that immune response and inflammation, especially chronic low-level inflammation, are the “two carriages” connecting gut microbiota and aging. In addition, the role of the microbiota-gut-brain axis cannot be ignored.
Gender and Sex Hormones
Gender and sex hormones have a specific impact on the distribution of gut microbiota and the health and life expectancy of organisms. In terms of microorganisms, the ratio of bacteria to human cells is 1.3 for men and 2.2 for women.145 A study of 1135 people showed that the gut microbial diversity in women was better than that of men, and the abundance of Akkermansia was the most prominent. Taking menopause as a reference, the Bacteroidetes/Firmicutes ratio is higher in premenopausal women, and there are relatively more Lachnospira and Roseburia.146 Moreover, on one hand, endogenous or exogenous oestrogen can be metabolised by gut microbiota, and the metabolites formed by decomposition can impact the host;147 while, on the other hand, oestrogen can trigger inflammatory reactions through the immune pathway, which can directly affect the gut microbiota, cause the change in gut permeability and force the gut microbiota to migrate to the lamina propria of the gut epithelium.148 In addition, the gut microbiome with β-glucuronidase activity can deconjugate the conjugated circulating oestrogens, thereby recirculating oestrogen and affecting organs and systems.149
This remains the case for androgens as well. Studies have shown that the gut microbiota of male and female patients with high testosterone or oestradiol levels are more diverse.150 At the same time, patients with polycystic ovary syndrome (PCOS) are more likely to get gut microbiota disorders than healthy people. Androgen excess leads to an increase in the abundance of Bacteroides, Escherichia/Shigella, and Streptococcus and a decrease in the abundance of Ackermann and Ruminococcaceae.151
In terms of aging, women live longer than men,152 but women often have a more extended period of weakness, the so-called “male-female health survival paradox”.153 There are many possible mechanisms to explain the problem of life expectancy caused by gender differences, such as sex-chromosome-related mechanisms and hormonal effects.154 The former emphasise the decisiveness of gene sets; for example, the male XY genotype is more likely to suffer from X gene recessive diseases. The latter emphasises the outstanding contribution of various hormones in the life cycle.
Effect of Diet
Diet is one of the critical factors in regulating gut microbiota composition, and it is also an essential factor in ensuring the health and delaying aging.155 Different eating habits significantly affect the composition of gut microorganisms.156 When changing from one eating habit to another, the gut microbiota perceive and respond within 24 hours.157 Of course, different eating habits affect the composition of gut microorganisms and bodily health. The mice fed with a high-fat diet have the changes of reducing the level of Bacteroidetes and increasing the levels of Proteobacteria and Firmicute.158 The high-sugar and high-fat diet is also the main factor in the pathogenesis of metabolic disorders and related pathological conditions. The Mediterranean diet, mainly composed of cereals, nuts, vegetables and fruits, has attracted much attention because it has been proven to be beneficial for health. This diet can effectively reduce the incidence rate of cardiovascular diseases, mental diseases and cancer and affect the gut microbiome—increasing the abundance of Bacteroides and Clostridium and decreasing the abundance of Proteobacteria and Firmicutes.159–161 Another diet characterised by high fat and low carbohydrates has been proven to inhibit apoptotic proteins, improve mitochondrial activity and increase the relative abundance of Akkermansia, Sutterella and Erysipelotrichaceae in mice gut microbiota.162,163 However, it is essential to note that an increase in age should also be reinforced with changes in lifestyle and eating habits to cope with the physiological changes in taste and smell thresholds, such as reduced physical activity, chewing dysfunction and so on. Therefore, the diet of the elderly may incorporate less fibre and protein, which hurts the diversity of microbiota and the abundance of bacteria that degrade fibre and produce SCFA. These problems should be considered as fully as possible.
Meanwhile, a recent study using metabolomics has showed that fasting and calories are the decisive factors in improving the survival rate of organisms, rather than the widely discussed diet itself,164 which is also worthy of in-depth research.
Immune System
With aging, the function of the immune system becomes increasingly incompetent, resulting in an aging state of immune remodelling. On one hand, it makes the body more vulnerable to bacterial and viral infections.165 On the other hand, it also causes autoimmune disorders, demonstrating the characteristics of immune aging such as the secretion of a phenotype related to inflammatory aging, the decline of bactericidal activity of monocytes and neutrophils and delayed clearance of apoptotic cells.5,166 In acquired immunity, thymic atrophy results in reduced production of immature T cells, which may affect the responsiveness of the elderly to new pathogens such as the COVID-19 virus. At the same time, aging also increases the frequency of regulatory CD28-veCD57+ve T cells, which enhance immune suppression and may lead to an increased incidence rate of cancer in the elderly.167 It is observed that the ability of B cells to produce antibodies is impaired, accompanied by the loss of B-cell diversity, which may be the reason for the poor effect of vaccination in the elderly.168
Let us turn our attention back to the gut, where immune cell culture depends on the presence of microbiota. During childhood, when the composition of gut microbiota is not stable, the gut microbiota contributes to the development of Peyer’s patches, mesenteric lymph nodes and isolated lymph follicles;169 this microbial stimulation can also promote the maturation and recruitment of B and T cells.170 As we know, the gut microbiota also includes potentially pathogenic bacteria. If they are too many, they may cause damage to the immune system, resulting in gastrointestinal inflammation and inflammatory bowel disease. This situation mainly occurs when the gut barrier function of the elderly is destroyed.171 It is worth noting that these pathogens or microorganisms may be beneficial in moderate quantities and may cause diseases only when they grow excessively. The growth of such opportunistic pathogens or microorganisms is related to autoimmune diseases outside the gut.14
The fundamental mechanism of the interaction between gut microbiota and the host involves the release of metabolites such as SCFA, BA and the direct activation of innate immune cells. SCFA is the energy source of epithelial cells. It plays a role in immune cells by activating G protein-coupled receptor (GPCR) and by inhibiting histone deacetylase (especially butyrate). Moreover, it has highly significant immunomodulatory properties and has been proven to induce the differentiation of regulatory T cells (Treg), inhibits the polarisation of T helper cells 17 (Th17) and induces the secretion of interleukin-10 (IL-10) through gut macrophages.172–174
Recent studies have also revealed its positive role in enhancing regulatory B-cell differentiation.175 Indeed, gut microorganisms can decompose bile acids into secondary bile acids, such as deoxycholic acid (DCA) and lithocholic acid (LCA).172 Secondary bile acids can then promote the production of tolerant dendritic cells and Treg, inhibit Th17 polarisation and induce the production of type-2 macrophages.176,177
In addition, the animal model system clearly shows that the presence of gut microbiota has a significant impact on immunity. Drosophila melanogaster is an excellent model. Studies have confirmed that the changes in the composition of the gut microbiota of Drosophila melanogaster are related to age-related gut-barrier dysfunctions.178 At the same time, it has also been found to have the ability to activate systemic immunity and eventually lead to its death.179 In addition, in the absence of adaptive immune function, the primary immune defence was confirmed to be provided by an innate immune response.180 Two main innate immune pathways play a role in gut stem cells: One produces reactive oxygen species (ROS) by activating dual oxidase (Duox) in response to bacterial uracil and the other responses to bacterial-derived peptidoglycan by activating the immune deficiency pathway, resulting in increased antimicrobial peptide (AMP) expression. The increased ROS or AMP can immediately initiate an immune response against invading pathogens or intestinal ecological disorders. This innate immune response caused by the ecological imbalance in the gut can lead to gut dysplasia and increased host mortality.180–182
Inflammatory Reaction
Similar to immune function, inflammatory response runs through the whole process of biological aging. The causes of inflammation are multiple, including oxidative stress, DNA damage, aging cell accumulation and immunity. Inflammatory aging and immune aging can be understood as highly intertwined processes, as inflammatory aging induces immune aging and vice versa.183,184 Moreover, inflammatory aging is characterised by elevated circulating levels of proinflammatory mediators, such as cytokines IL-6, IL-1 β, tumour necrosis factor TNF-α and inflammatory mediators such as prostaglandin E2 and anti-inflammatory mediators.185 In longitudinal studies of several elderly cohorts, many of these inflammatory mediators have been identified as predictors of the risk of all-cause death and indicators of the poor prognosis of age-related diseases.186–188
Chronic low-grade inflammation is a sign of inflammatory aging. Unlike acute inflammation, chronic low-grade inflammation is characterised by maintenance of a low-level and continuous inflammatory state even in the absence of acute infection and clinical diagnostic diseases.189
More and more evidence shows that chronic low-level inflammation is a risk factor leading to the decline of tissue repair and production capacity, which is related to many aging-related diseases.189–191 In addition, aging cells help maintain the state of chronic low-grade inflammation. Aging cells often secrete inflammatory mixtures of cytokines, growth factors and matrix metalloproteinases to form the so-called senescence-associated secretory phenotype (SASP).192,193 Further, the central signalling pathway produced by SASP may be shared among different types of aging cells with the transcription factor NF-κΒ confluence. As NF-κΒ is the primary regulator of immune cell inflammation, it mediates the activation of NLRP3 inflammatory bodies and proinflammatory cytokines, such as IL-1 β and the release of IL-18, which promotes the activation of inflammation. NF-κΒ also plays a vital role in the occurrence of SASP,194,195 and in causing secondary senescence of adjacent cells.196–198 For example, transplanting aging cells into the knee joint will lead to the osteoarthritis-like phenotype of mice, suggesting that the tissue accelerates aging.197,198 In contrast, the osteoarthritis of mice is significantly relieved after the local removal of aging cells.199
Studies using germ-free animal models provide direct evidence of the importance of the gut microbiota in macrophage and neutrophil activities.200 Since there is no contact with microorganisms after birth, germ-free animals are valuable in understanding the relationship between microorganisms and their host. A study of young, germ-free mice reported that when these mice lived with older mice, the cytokine levels and macrophage dysfunction of the former increased.201 In another study, the transfer of gut microbiota from elderly conventional mice to young germ-free mice promoted T cell activation and gut inflammation.202 These findings suggest a relationship between age-related microbiota disorders and age-related changes in the inflammatory response.
The Gut Barrier
The gut barrier consists of an overlying mucous layer, epithelial cells and lamina propria.5 Despite the exposure to many external antigens in our foods, a healthy gut barrier always balances tolerance and immunity. This barrier effect can be achieved through the complex interaction between gut physics and immune defence, and this physical defence is achieved through the inner gut wall and mucus coverage.203 Goblet cells secrete mucin to form a protective barrier to capture pathogens and prevent invasive colonisation.204 Coordination between these defences can regulate the production of critical immune modulators necessary for gut immune homeostasis, such as secretory IgA, which plays a significant role in gut immunity.205
Gut mucosa is an essential regulator of host immunity.203 Gut mucosa is rich in antimicrobial peptides that regulate gut-barrier function and mediate immune defence against bacterial invasion.206 In addition, intestinal epithelial cells themselves can regulate the proliferation and functional differentiation of innate and adaptive immune cells, which more effectively maintains the integrity of immunity and gut homeostasis.203
With an increase in age, the imbalance of gut microbiota, the thinning of the mucin layer and the addition of endothelial cell gap after the gradual aging of the body lead to the increase in the permeability of the mucosal barrier. The phenomenon of allowing microorganisms, toxins and antigens to enter the circulation is called “leaky gut”.5,203 Moreover, this state of leaky gut caused by age-related microbiota changes is considered to be an essential inducement of chronic low-grade inflammation. Therefore, leaky gut is also considered to be involved in the occurrence and development of many elderly chronic diseases. Take chronic kidney disease (CKD) as an example. On one hand, uremic toxins accumulate in the circulation when renal function declines, making the colon the main excretion route. This leads to the corresponding increase of urea content in the gut cavity, affecting the pH value in the gut cavity and the integrity of the gut barrier and allowing the leakage of harmful microbiological products into the circulation. These leaks, such as lipopolysaccharide and peptidoglycan, are induced by innate immune receptors of immune cells and renal parenchymal cells, leading to systemic and renal inflammatory response, which aggravates renal insufficiency. On the other hand, in the gut tract, due to uremic status, dietary restriction and reduced gut transport, the selective pressure on the microbial community affect the balance between glycolytic bacteria and proteolytic bacteria, making them change to the direction conducive to the growth of pathogenic bacteria. This change reduces the production of beneficial SCFA, leading to production of more protein-end products, such as p-Cresol, indole, trimethylamine, etc., further aggravating the immune and inflammatory responses of the body.13,14,207
In conclusion, maintaining the integrity of the gut barrier plays a positive role in preventing inflammation and immune injury. The available evidence suggests that prebiotics and probiotics can correct malnutrition in the elderly, change the gut microbiota and restore microbial homeostasis, thereby reducing gut permeability and maintaining gut-barrier functions.5,208,209
The Microbiota-Gut-Brain Axis and Age-Related Diseases
“The stability of the internal environment is the condition for the free and independent life”.210 This famous saying can be used as the basis for our understanding of the homeostasis of the internal environment. As early as the ancient Greek period, philosophers such as Hippocrates, Plato and Aristotle began to hypothesise that there is an internal relationship between the brain and the other parts of the body. However, it was not until the 1840s that William Beaumont proved experimentally that emotional state would affect digestion speed. It was then that the initial theoretical prototype of the gut-brain axis was established.211 With the emergence of brain imaging technology in the 1980s, people realised that this axis attributes to a two-way regulation. Studies have shown that gut dilation can lead to an activation of the critical pathways in the brain. The activation of these pathways is more evident in diseases like the irritable bowel syndrome (IBS).212,213 Then, in the past decades, with the in-depth development of microbiology research, it was found that tens of thousands of microbiotas in the gut occupy a critical regulatory position in the gut-brain axis.
Unlike the earlier studies on gut-brain communication, which mainly focused on digestive function and satiety,214,215 recent studies have increasingly focused on the high-order cognitive and psychological effects of gut-brain communication.216–218 These studies can better understand some pathophysiological phenomena caused by abnormal gut-brain interaction, such as gut inflammation, acute or chronic stress responses and changes in the behavioural states—many of which are inextricably linked to aging.219–224
Social Behavior
Indeed, social behaviour refers to various forms of interaction between animals, such as learning, cooperation, protection and mating. It is the fundamental behaviour of all species.80 During the aging period, human social activities and interests are reduced; when compared with young people, the elderly tends to retain smaller social networks.225–227 Experiments using germ-free mice showed that compared with ordinary mice, the older mice spent less time interacting with new homologous mice.228–231 These results are also confirmed by the research on the application of antibiotics to rodents. The study shows that antibiotics can significantly reduce gut microbial diversity, and this kind of experimental animal shows some social behaviour defects.232,233
At present, some studies have confirmed that bacterial components such as peptidoglycan can enter the brain from the gut and affect the social behaviour of the host through signal pathway transmission.234 Another study on mice taking Lactobacillus reuteri shows that this increases circulating levels and oxytocin expression in mice and directly or indirectly increase the performance of their social behaviour.228,235 However, we have to admit that the exact mechanism of microbiota-mediated social behaviour regulation is unclear and likely to involve the synergism of multiple biological pathways.80 We speculate that future research should focus on converting these animal studies into human social behaviour disorders, and alleviating social behaviour disorders in the elderly may be one of the critical topics.
Cognition
Like the lack of social behaviour described in the previous section, the elderly can exhibit general deficits in cognition, such as the theory of mind skills.226,227,236 More and more evidence supports the view that changes in gut microbiota composition can affect cognitive function at multiple levels. The same experiment was done with germ-free mice. The results showed that germ-free mice had an impaired ability to remember familiar objects, and the expression of brain-derived neurotrophic factor (BDNF) in the hippocampus of these animals also changed.237–239 In a study on probiotics in treating cognitive impairment in the elderly, the cognitive test performance of healthy elderly subjects and elderly subjects with cognitive impairment improved after receiving the Lactobacillus strain compared with the placebo group.240 Another study showed that a probiotic mixture containing Bifidobacterium longum and different Lactobacillus strains positively affected the cognitive function and metabolic status in patients with Alzheimer’s disease.241 These results suggest that probiotics can potentially improve the cognitive function in healthy, older people and the clinical population with Alzheimer’s disease.
Obesity
Obesity is a major public health problem that increases the risk of serious health problems and has been causing huge economic losses to society.71 Fundamentally speaking, obesity is an energy imbalance caused by the excess of calories consumed over expenditure.242 According to the Center for Disease Control and Prevention (CDC), the prevalence of obesity among the elderly (in the age range of 60 years and older) was 42.8% in 2017–2018.243 Obesity and obesity-related diseases seem to be related to the acceleration of cellular processes observed during normal aging,244 and inflammation and oxidative stress seem to be important mediators of this connection.242 On the other hand, obesity will also accelerate the onset of age-related diseases such as diabetes, hypertension and dyslipidemia.245,246
As the gut microbiota is highly influenced by diet,247 it plays a vital role in regulating food intake. Therefore, the gut microbiota is also considered a critical factor in obesity. Most previous studies on microbiota and obesity have focused on blood glucose and peripheral regulation of metabolism.80 However, current studies have found that the microbiota-gut-brain axis plays a significant role. The obesity models in both humans and mice showed an increase in the relative abundance of Firmicutes and a decrease in Bacteroidetes compared to the thinner control group. Then, surprisingly, when people followed a low-calorie diet, the observation was the opposite. This result shows that a low-calorie diet that stimulates metabolic potential and improves the utilisation of food energy.248,249 In addition, the retention of food in the gut will trigger a series of signalling events, which is very important in regulating energy balance.80 For example, vagus nerve neurons will resist leptin and reduce the sensitivity to cholecystokinin, which will lead to obesity.250 In the obese rat model, the activation of neuronal activation marker c-fos in the nucleus tractus solitarius decreased after eating, indicating a dropped vagal signal from the gut to the nucleus tractus solitarius.251
Parkinson’s Disease
Aging is the leading risk factor for Parkinson’s disease (PD). PD is the second-largest neurodegenerative disease diagnosed, affecting nearly 1% of people over 60 years.252 Additionally, PD is the fastest-growing disease. As the world population is aging, the number of people affected is expected to increase exponentially, and the number of patients with PD is expected to double from 6.9 million in 2015 to 14.2 million in 2040.253 So far, there is no effective treatment to prevent or reverse the neurodegenerative process of PD. The current treatment depends only on dopaminergic drugs, including levodopa and dopaminergic agonists, which can only temporarily alleviate the symptoms of motor disorders.254–256
The latest evidence from multiple laboratories shows a relationship between the complexity and diversity of gut microbiota and PD.257–260 These studies suggest that changes in the gut microbiota can lead to inflammatory states, which may have harmful effects on the gut and brain.261 One study found that Prevotellaceae decreased significantly and the number of Enterobacteriaceae increased in PD patients when compared with healthy people.262 Another study involving 197 PD patients confirmed that PD drugs had a significant effect on the gut microbiota. The study also confirmed that PD status itself is the cause of significant changes in gut microbiota.259 In the study of PD patients in China, Clostridium IV, Aquabacterium, Holdemania, Sphingomonas, Clostridium XVIII, Butyricicoccus and Anaerotruncus were found to be increased, and Escherichia/Shigella was negatively correlated with the disease duration.263 In addition, probiotic intervention is also meaningful in improving PD symptoms.264 Of course, much research is needed to help understand how changes in the microbiota can alleviate PD symptoms.
Alzheimer’s Disease
Alzheimer’s disease (AD) is another most common neurodegenerative disease and the leading cause of senile dementia. Moreover, until now, there are still unknown or multifactorial causes.265,266 The relationship between gut microbiota and AD has been discussed for many years.267–269 As for the microbiota composition of AD patients, studies have shown that the richness and diversity of gut microbiota in AD patients have decreased, showing a decrease in Firmicutes, an increase in Bacteroidetes and a reduction in Bifidobacteria.270 In the study using the AD mouse model, the researchers found that treating a probiotic cocktail with a mixture of lactic acid bacteria and Bifidobacteria can reduce the oxidative stress response of AD mice.271 Amyloid protein may play the role of an antimicrobial peptide in the brain. Like PD, the relationship between gut protein and cognitive ability has attracted much attention, indicating that amyloid protein can be produced by bacteria and increase α-synuclein pathology in vasectomised older rats.272,273 It is undeniable that there are still many questions about the treatment of AD patients with microbiota-gut-brain axis therapy. Moreover, there is still much work to determine whether targeting the axis can lead to significant clinical improvement to slow down or prevent AD’s progress.
Conclusions and Prospects
With the continuous progress of the international aging process, the problem of aging has been widely discussed. How to age healthily has become the most noticeable question now. Gut microbiota mainly affects the physiological and pathological functions of the human body by regulating immune function and inflammatory state. At the same time, it also has positive or negative effects on aging. For example, as we mentioned earlier, the gut microbiota can change with the aging of the host. We believe that the impact of age-related changes on these microorganisms is of great significance, which is also illustrated by many model animal experiments.
The progress in Modern Medicine allows for a clearer and more accurate exploration on the relationship between gut microbiota and aging. However, the role of ancient and profound TCM cannot be ignored. Compared with Modern Medicine, the diagnosis and treatment concepts and methods of TCM and the tested traditional herbal medicine have shown unique advantages. In particular, a series of studies on the molecules of traditional herbal medicine seems to have become the hub of the connection between TCM and Modern Medical research. Many studies have confirmed that TCM indirectly affects the production and metabolism of SCFA, LPS, BA and other substances by regulating gut microbiota. (Table 1) Because of this, the characteristics of multi-component and multi-target effects, low drug toxicity and few side effects constitute a major advantage of TCM. The regulation of these microscopic substances is the correct way to explain the macro theory of TCM to prolong life. With the progress of China’s national rejuvenation plan, the research on TCM also shows a trend of becoming more popular and international. Therefore, the infiltration of TCM into scientific research is inevitable.
However, despite many new insights gained from the exploration in recent years, the research on gut microbiota in human aging is still full of challenges. Changes in lifestyle, such as diet, exercise, medication and environment, considerably impact the progress of gut microbiota and aging status. In the research without hierarchical discussion, these unstable factors determine the trend of the outcome to a certain extent. For example, on one hand, the use of various drugs (such as antibiotics) may significantly affect the population abundance of gut microbiota,274 while on the other hand, the microbiota will affect the bioavailability of drugs in the host.275 Therefore, properly controlling the deviation caused by hybrid factors is essential. In addition, the causal relationship between gut microbiota and aging status needs to be further explained through more powerful longitudinal and intervention studies. As far as the research on gut microbiota is concerned, most of the current research focuses on bacterial species; however, the research on viruses, fungi and eukaryotes, as well as their mutual functions, need to be further clarified.15
Similarly, in the field of TCM, it is precisely because of the complexity of TCM decoction that its mechanism of action against gut microbial and aging has become more challenging to explore. Relevant research reports only talk about the intervention results in general. Although exploring and researching a single medicine/monomer can remedy this defect, it seems that the study of TCM without prescription lacks the very soul of TCM. In the future, research involving TCM may seek more extraordinary breakthroughs in the aspect of decoction, despite this being a challenging and arduous subject.
In summary, through this review, we introduced the known relationship between gut microbiota and aging under the research of TCM and Modern Medicine, attempting to explore a new way to combine TCM with Modern Medicine to adjust gut microbiota and delay aging and providing some references for similar research and clinical application.
Acknowledgments
This paper was supported by the project Special Disease Prevention Project of Integrated Traditional Chinese, Western Medicine of Shandong Provincial Health Commission (Diabetes Prevention Project, No. 37000021P28000210022R) and National Key R&D Program of China (2021YFA 0805100).
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO. Maximizing potential of traditional medicines through modern science and technology; 2022. Available from: https://www.who.int/zh/news/item/25-03-2022-who-establishes-The-global-centre-for-traditional-medicine-in-india.
2. DeJong EN, Surette MG, Bowdish DME. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe. 2020;28(2):180–189. doi:10.1016/j.chom.2020.07.013
3. Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561(7721):45–56. doi:10.1038/s41586-018-0457-8
4. Jagger C, Gillies C, Moscone F, et al. Inequalities in healthy life years in the 25 countries of the European Union in 2005: a cross-national meta-regression analysis. Lancet. 2008;372(9656):2124–2131. doi:10.1016/S0140-6736(08)61594-9
5. Conway JA, Duggal N. Ageing of the gut microbiome: potential influences on immune senescence and inflammageing. Ageing Res Rev. 2021;68:101323. doi:10.1016/j.arr.2021.101323
6. Pepper GV, Nettle D. Perceived extrinsic mortality risk and reported effort in looking after health: testing a behavioral ecological prediction. Hum Nat. 2014;25(3):378–392. doi:10.1007/s12110-014-9204-5
7. Pei H, Ma L, Cao Y, et al. Traditional Chinese medicine for Alzheimer’s disease and other cognitive impairment: a review. Am J Chin Med. 2020;48(3):487–511. doi:10.1142/S0192415X20500251
8. Zhang HY, Tian JX, Lian FM, et al. Therapeutic mechanisms of traditional Chinese medicine to improve metabolic diseases via the gut microbiota. Biomed Pharmacother. 2021;133:110857. doi:10.1016/j.biopha.2020.110857
9. Liu Y, Yang S, Wang K, et al. Cellular senescence and cancer: focusing on traditional Chinese medicine and natural products. Cell Prolif. 2020;53(10):e12894. doi:10.1111/cpr.12894
10. Shenghua P, Ziqin Z, Shuyu T, et al. An integrated fecal microbiome and metabolome in the aged mice reveal anti-aging effects from the intestines and biochemical mechanism of FuFang zhenshu TiaoZhi(FTZ). Biomed Pharmacother. 2020;121:109421. doi:10.1016/j.biopha.2019.109421
11. Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14(1):20–32. doi:10.1038/nrmicro3552
12. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. doi:10.1038/s41579-020-0433-9
13. Evenepoel P, Poesen R, Meijers B. The gut-kidney axis. Pediatr Nephrol. 2017;32(11):2005–2014. doi:10.1007/s00467-016-3527-x
14. Mosterd CM, Kanbay M, van den Born BJH, et al. Intestinal microbiota and diabetic kidney diseases: the Role of microbiota and derived metabolites inmodulation of renal inflammation and disease progression. Best Pract Res Clin Endocrinol Metab. 2021;35(3):101484. doi:10.1016/j.beem.2021.101484
15. Lynch SV, Pedersen O, Phimister EG. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–2379. doi:10.1056/NEJMra1600266
16. Ivey KL, Hodgson JM, Kerr DA, et al. The effects of probiotic bacteria on glycaemic control in overweight men and women: a randomised controlled trial. Eur J Clin Nutr. 2014;68(4):447–452. doi:10.1038/ejcn.2013.294
17. Liu X, Chen Y, Zhang S, Dong L. Gut microbiota-mediated immunomodulation in tumor. J Exp Clin Cancer Res. 2021;40(1):221. doi:10.1186/s13046-021-01983-x
18. Zhang T, Cheng JK, Hu YM. Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res Rev. 2022;81:101739. doi:10.1016/j.arr.2022.101739
19. Białecka-Dębek A, Granda D, Szmidt MK, et al. Gut microbiota, probiotic interventions, and cognitive function in the elderly: a review of current knowledge. Nutrients. 2021;13(8):2514. doi:10.3390/nu13082514
20. Walrath T, Dyamenahalli KU, Hulsebus HJ, et al. Age-related changes in intestinal immunity and the microbiome. J Leukoc Biol. 2021;109(6):1045–1061. doi:10.1002/JLB.3RI0620-405RR
21. Shi N, Li N, Duan X, et al. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 2017;4:14. doi:10.1186/s40779-017-0122-9
22. Wang J, Wong YK, Liao F. What has traditional Chinese medicine delivered for modern medicine? Expert Rev Mol Med. 2018;20:e4. doi:10.1017/erm.2018.3
23. Wang W-Y, Zhou H, Wang Y-F, et al. Current policies and measures on the development of traditional Chinese medicine in China. Pharmacol Res. 2021;163:105187. doi:10.1016/j.phrs.2020.105187
24. Hu K, Guan WJ, Bi Y, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85:153242. doi:10.1016/j.phymed.2020.153242
25. Jiang WY. Therapeutic wisdom in traditional Chinese medicine: a perspective from modern science. Trends Pharmacol Sci. 2005;26(11):558–563. doi:10.1016/j.tips.2005.09.006
26. Dong J. The relationship between traditional Chinese medicine and modern medicine. Evid Based Complement Alternat Med. 2013;2013:153148. doi:10.1155/2013/153148
27. Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130(5):769–774. doi:10.1016/j.cell.2007.08.021
28. Li JW-H, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325(5937):161–165. doi:10.1126/science.1168243
29. Tu Y. Tu Youyou biographical; 2015. https://www.nobelprize.org/prizes/medicine/2015/tu/biographical/.
30. Lin TL, Lu CC, Lai WF, et al. Role of gut microbiota in identification of novel TCM-derived active metabolites. Protein Cell. 2021;12(5):394–410. doi:10.1007/s13238-020-00784-w
31. Li LC, Kan LD. Traditional Chinese medicine for pulmonary fibrosis therapy: progress and future prospects. J Ethnopharmacol. 2017;198:45–63. doi:10.1016/j.jep.2016.12.042
32. Liu P, Zhao H, Luo Y. Anti-aging implications of Astragalus Membranaceus (Huangqi): a well-known Chinese tonic. Aging Dis. 2017;8(6):868–886. doi:10.14336/AD.2017.0816
33. Zhao X, Zhou S, Yan R, et al. Parishin from gastrodia elata ameliorates aging phenotype in mice in a gut microbiota-related manner. Front Microbiol. 2022;13:877099. doi:10.3389/fmicb.2022.877099
34. Hu HC, Zheng LT, Yin HY, et al. A significant association between rhein and diabetic nephropathy in animals: a systematic review and meta-analysis. Front Pharmacol. 2019;10:1473. doi:10.3389/fphar.2019.01473
35. Liu M, Zhao Q, Liu J, Huang A, Xia X. Buyang Huanwu decoction affects gut microbiota and lipid metabolism in a ZDF rat model of co-morbid type 2 diabetes mellitus and obesity: an integrated metabolomics analysis. Front Chem. 2022;10:1036380. doi:10.3389/fchem.2022.1036380
36. Chen J, Hu Y, Chen L, Liu W, Mu Y, Liu P. The effect and mechanisms of Fuzheng Huayu formula against chronic liver diseases. Biomed Pharmacother. 2019;114:108846. doi:10.1016/j.biopha.2019.108846
37. Zhang R, Gao X, Bai H, et al. Traditional Chinese medicine and gut microbiome: their respective and concert effects on healthcare. Front Pharmacol. 2020;11:538. doi:10.3389/fphar.2020.00538
38. Fulde M, Hornef MW. Maturation of the enteric mucosal innate immune system during the postnatal period. Immunol Rev. 2014;260(1):21–34. doi:10.1111/imr.12190
39. Neuman H, Debelius JW, Knight R, Koren O, Banin E. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev. 2015;39(4):509–521. doi:10.1093/femsre/fuu010
40. Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–591. doi:10.1038/nrendo.2015.128
41. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
42. Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Leeuwenhoek. 2020;113(12):2019–2040. doi:10.1007/s10482-020-01474-7
43. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–1836. doi:10.1042/BCJ20160510
44. Perry RJ, Peng L, Barry NA, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–217. doi:10.1038/nature18309
45. Martinez-Guryn K, Hubert N, Frazier K, et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe. 2018;23(4):458–469.e5. doi:10.1016/j.chom.2018.03.011
46. Kübeck R, Bonet-Ripoll C, Hoffmann C, et al. Dietary fat and gut microbiota interactions determine diet-induced obesity in mice. Mol Metab. 2016;5(12):1162–1174. doi:10.1016/j.molmet.2016.10.001
47. Mardinoglu A, Shoaie S, Bergentall M, et al. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol. 2015;11(10):834. doi:10.15252/msb.20156487
48. Wesemann DR, Portuguese AJ, Meyers RM, et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013;501(7465):112–115. doi:10.1038/nature12496
49. Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi:10.1038/nature11319
50. Huang WC, Chen YH, Chuang HL, Chiu CC, Huang CC. Investigation of the effects of microbiota on exercise physiological adaption, performance, and energy utilization using a gnotobiotic animal model. Front Microbiol. 2019;10:1906. doi:10.3389/fmicb.2019.01906
51. Nikolova VL, Smith MRB, Hall LJ, et al. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry. 2021;78(12):1343–1354. doi:10.1001/jamapsychiatry.2021.2573
52. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi:10.1038/nature08821
53. von Martels JZH, Sadaghian Sadabad M, Bourgonje AR, et al. The role of gut microbiota in health and disease: in vitro modeling of host-microbe interactions at the aerobe-anaerobe interphase of the human gut. Anaerobe. 2017;44:3–12. doi:10.1016/j.anaerobe.2017.01.001
54. Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20(4):214–223. doi:10.1016/j.molmed.2013.08.004
55. Biagi E, Franceschi C, Rampelli S, et al. Gut microbiota and extreme longevity. Curr Biol. 2016;26(11):1480–1485. doi:10.1016/j.cub.2016.04.016
56. Bischoff SC. Microbiota and aging. Curr Opin Clin Nutr Metab Care. 2016;19(1):26–30. doi:10.1097/MCO.0000000000000242
57. Salazar N, Arboleya S, Fernández-Navarro T, et al. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: a cross-sectional study. Nutrients. 2019;11(8):1765. doi:10.3390/nu11081765
58. Vaiserman AM, Koliada AK, Marotta F. Gut microbiota: a player in aging and a target for anti-aging intervention. Ageing Res Rev. 2017;35:36–45. doi:10.1016/j.arr.2017.01.001
59. O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350(6265):1214–1215. doi:10.1126/science.aac8469
60. Ghosh TS, Das M, Jeffery IB, et al. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. Elife. 2020;9:e50240. doi:10.7554/eLife.50240
61. Jeffery IB, Lynch DB, O’Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10(1):170–182. doi:10.1038/ismej.2015.88
62. Amato KR, Sanders J, Song SJ, et al. Evolutionary trends in host physiology outweigh dietary niche in structuring primate gut microbiomes. ISME J. 2019;13(3):576–587. doi:10.1038/s41396-018-0175-0
63. Elderman M, Sovran B, Hugenholtz F, et al. The effect of age on the intestinal mucus thickness, microbiota composition and immunity in relation to sex in mice. PLoS One. 2017;12(9):e0184274. doi:10.1371/journal.pone.0184274
64. Rampelli S, Candela M, Turroni S, et al. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging . 2013;5(12):902–912. doi:10.18632/aging.100623
65. Blacher E, Levy M, Tatirovsky E, Elinav E. Microbiome-modulated metabolites at the interface of host immunity. J Immunol. 2017;198(2):572–580. doi:10.4049/jimmunol.1601247
66. Smith P, Willemsen D, Popkes M, et al. Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. Elife. 2017;6:e27014. doi:10.7554/eLife.27014
67. Mossad O, Nent E, Woltemate S, et al.. Microbiota-dependent increase in δ-valerobetaine alters neuronal function and is responsible for age-related cognitive decline. Nat Aging 1. 2021;1127–1136. doi:10.1038/s43587-021-00141-4
68. Boehme M, Guzzetta KE, Bastiaanssen TFS, et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat Aging 1. 2021;1(8):666–676. doi:10.1038/s43587-021-00093-9
69. Collado MC, Rautava S, Aakko J, et al. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6(1):23129. doi:10.1038/srep23129
70. Jiménez E, Marín ML, Martín R, et al. Is meconium from healthy newborns actually sterile? Res Microbiol. 2008;159(3):187–193. doi:10.1016/j.resmic.2007.12.007
71. Aagaard K, Ma J, Antony KM, et al. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. doi:10.1126/scitranslmed.3008599
72. Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. doi:10.1126/scitranslmed.3009759
73. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
74. Chu DM, Ma J, Prince AL, et al. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017;23(3):314–326. doi:10.1038/nm.4272
75. Yassour M, Vatanen T, Siljander H, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8(343):343ra81. doi:10.1126/scitranslmed.aad0917
76. Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–4585. doi:10.1073/pnas.1000081107
77. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi:10.1038/nature11053
78. Hollister EB, Riehle K, Luna RA, et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 2015;3:36. doi:10.1186/s40168-015-0101-x
79. Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut. 2001;48(2):198–205. doi:10.1136/gut.48.2.198
80. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi:10.1152/physrev.00018.2018
81. Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–4591. doi:10.1073/pnas.1000097107
82. Zapata HJ, Quagliarello VJ. The microbiota and microbiome in aging: potential implications in health and age-related diseases. J Am Geriatr Soc. 2015;63(4):776–781. doi:10.1111/jgs.13310
83. Wilmanski T, Diener C, Rappaport N, et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans [published correction appears in Nat Metab. 2021 Apr;3(4):586]. Nat Metab. 2021;3(2):274–286. doi:10.1038/s42255-021-00348-0
84. McMurdie PJ, Holmes S, Watson M. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217. doi:10.1371/journal.pone.0061217
85. Lewis KN, Rubinstein ND, Buffenstein R. A window into extreme longevity; the circulating metabolomic signature of the naked mole-rat, a mammal that shows negligible senescence [published correction appears in Geroscience. 2018 May 31]. Geroscience. 2018;40(2):105–121. doi:10.1007/s11357-018-0014-2
86. Biagi E, Nylund L, Candela M, et al.. Correction: through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(6). doi:10.1371/annotation/df45912f-d15c-44ab-8312-e7ec0607604d
87. Giron M, Thomas M, Dardevet D, Chassard C, Savary-Auzeloux I. Gut microbes and muscle function: can probiotics make our muscles stronger? J Cachexia Sarcopenia Muscle. 2022;13(3):1460–1476. doi:10.1002/jcsm.12964
88. Lahiri S, Kim H, Garcia-Perez I, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. 2019;11(502):eaan5662. doi:10.1126/scitranslmed.aan5662
89. Feng W, Ao H, Peng C, et al. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. 2019;142:176–191. doi:10.1016/j.phrs.2019.02.024
90. Peng Y, Zhang S, Liu Z, et al. Gut microbiota and Chinese medicine syndrome: altered fecal microbiotas in spleen (Pi)-deficient patients. J Tradit Chin Med. 2020;40(1):137–143.
91. Yang J, Dong H, Wang Y, et al. Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. Int J Biol Macromol. 2020;163:442–456. doi:10.1016/j.ijbiomac.2020.06.153
92. Han W, Ma Q, Liu Y, et al. Huangkui capsule alleviates renal tubular epithelial-mesenchymal transition in diabetic nephropathy via inhibiting NLRP3 inflammasome activation and TLR4/NF-κB signaling. Phytomedicine. 2019;57:203–214. doi:10.1016/j.phymed.2018.12.021
93. Zhu L, Han J, Yuan R, Xue L, Pang W. Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway. Biol Res. 2018;51(1):9. doi:10.1186/s40659-018-0157-8
94. Wang JH, Kim BS, Han K, Kim H. Ephedra-treated donor-derived gut microbiota transplantation ameliorates high fat diet-induced obesity in rats. Int J Environ Res Public Health. 2017;14(6):555. doi:10.3390/ijerph14060555
95. Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. 2020;156:104761. doi:10.1016/j.phrs.2020.104761
96. Zhang X, Zhao Y, Xu J, et al. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. doi:10.1038/srep14405
97. Shao J, Liu Y, Wang H, et al. An integrated fecal microbiome and metabolomics in T2DM rats reveal antidiabetes effects from host-microbial metabolic axis of EtOAc extract from Sophora flavescens. Oxid Med Cell Longev. 2020;2020:1805418. doi:10.1155/2020/1805418
98. Sun H, Wang N, Cang Z, et al. Modulation of microbiota-gut-brain axis by berberine resulting in improved metabolic status in high-fat diet-fed rats. Obes Facts. 2016;9(6):365–378. doi:10.1159/000449507
99. Liu Y, Li J, Yu J, et al. Disorder of gut amino acids metabolism during CKD progression is related with gut microbiota dysbiosis and metagenome change. J Pharm Biomed Anal. 2018;149:425–435. doi:10.1016/j.jpba.2017.11.040
100. Xiao S, Zhang Z, Chen M, et al. Xiexin Tang ameliorates dyslipidemia in high-fat diet-induced obese rats via elevating gut microbiota-derived short chain fatty acids production and adjusting energy metabolism. J Ethnopharmacol. 2019;241:112032. doi:10.1016/j.jep.2019.112032
101. Wei X, Tao J, Xiao S, et al. Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Sci Rep. 2018;8(1):3685. doi:10.1038/s41598-018-22094-2
102. Chen M, Liao Z, Lu B, et al. Huang-Lian-Jie-Du-decoction ameliorates hyperglycemia and insulin resistant in association with gut microbiota modulation. Front Microbiol. 2018;9:2380. doi:10.3389/fmicb.2018.02380
103. Hussain A, Yadav MK, Bose S, et al. Daesiho-Tang is an effective herbal formulation in attenuation of obesity in mice through alteration of gene expression and modulation of intestinal microbiota. PLoS One. 2016;11(11):e0165483. doi:10.1371/journal.pone.0165483
104. Xu J, Lian F, Zhao L, et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015;9(3):552–562. doi:10.1038/ismej.2014.177
105. Leng J, Huang F, Hai Y, et al. Amelioration of non-alcoholic steatohepatitis by Qushi Huayu decoction is associated with inhibition of the intestinal mitogen-activated protein kinase pathway. Phytomedicine. 2020;66:153135. doi:10.1016/j.phymed.2019.153135
106. Cui HX, Zhang LS, Luo Y, Yuan K, Huang ZY, Guo Y. A purified anthraquinone-glycoside preparation from rhubarb ameliorates type 2 diabetes mellitus by modulating the gut microbiota and reducing inflammation. FrontMicrobiol. 2019;10:1423. doi:10.3389/fmicb.2019.01423
107. Wang R, Zang P, Chen J, et al. Gut microbiota play an essential role in the antidiabetic effects of rhein. Evid Based Complement Alternat Med. 2018;2018:6093282. doi:10.1155/2018/6093282
108. Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
109. Wang K, Bao L, Zhou N, et al. Structural modification of natural product ganomycin I leading to discovery of a α-glucosidase and HMG-CoA reductase dual inhibitor improving obesity and metabolic dysfunction in vivo. J Med Chem. 2018;61(8):3609–3625. doi:10.1021/acs.jmedchem.8b00107
110. Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. 2011;34(2):392–397. doi:10.2337/dc10-1676
111. Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi:10.1016/j.cell.2016.05.041
112. Tan J, McKenzie C, Potamitis M, et al. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119.
113. Yoshida H, Ishii M, Akagawa M. Propionate suppresses hepatic gluconeogenesis via GPR43/AMPK signaling pathway. Arch Biochem Biophys. 2019;672:108057. doi:10.1016/j.abb.2019.07.022
114. Hernández MAG, Canfora EE, Jocken JWE, et al. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients. 2019;11(8):1943. doi:10.3390/nu11081943
115. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. doi:10.1038/nri2925
116. He K, Hu Y, Ma H, et al. Rhizoma Coptidis alkaloids alleviate hyperlipidemia in B6 mice by modulating gut microbiota and bile acid pathways. Biochim Biophys Acta. 2016;1862(9):1696–1709. doi:10.1016/j.bbadis.2016.06.006
117. Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28(4):573–583. doi:10.1016/j.bpg.2014.07.004
118. Li T, Chiang JYL, Ma Q. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66(4):948–983. doi:10.1124/pr.113.008201
119. Fiorucci S, Distrutti E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol Med. 2015;21(11):702–714. doi:10.1016/j.molmed.2015.09.001
120. Wang YS, Shen CY, Jiang JG. Antidepressant active ingredients from herbs and nutraceuticals used in TCM: pharmacological mechanisms and prospects for drug discovery. Pharmacol Res. 2019;150:104520. doi:10.1016/j.phrs.2019.104520
121. Yin D, Chen K. The essential mechanisms of aging: irreparable damage accumulation of biochemical side-reactions. Exp Gerontol. 2005;40(6):455–465. doi:10.1016/j.exger.2005.03.012
122. Panza F, D’introno A, Capurso C, et al. Lipoproteins, vascular-related genetic factors, and human longevity. Rejuvenation Res. 2007;10(4):441–458. doi:10.1089/rej.2007.0537
123. Cao H, Zhang A, Zhang H, et al. The application of metabolomics in traditional Chinese medicine opens up a dialogue between Chinese and Western medicine. Phytother Res. 2015;29(2):159–166. doi:10.1002/ptr.5240
124. Wiegant FA, Surinova S, Ytsma E, et al. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology. 2009;10(1):27–42. doi:10.1007/s10522-008-9151-9
125. Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106(6):661–673. doi:10.1016/S0092-8674(01)00492-5
126. Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448(7155):767–774. doi:10.1038/nature05985
127. Oberdoerffer P, Sinclair DA. The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol. 2007;8(9):692–702. doi:10.1038/nrm2238
128. Westphal CH, Dipp MA, Guarente L. A therapeutic role for sirtuins in diseases of aging? Trends Biochem Sci. 2007;32(12):555–560. doi:10.1016/j.tibs.2007.09.008
129. Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi:10.1038/nature01960
130. Li X, Li J, Wang L, et al. The role of metformin and resveratrol in the prevention of hypoxia-inducible factor 1α accumulation and fibrosis in hypoxic adipose tissue. Br J Pharmacol. 2016;173(12):2001–2015. doi:10.1111/bph.13493
131. Steelman LS, Chappell WH, Abrams SL, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging. 2011;3(3):192–222. doi:10.18632/aging.100296
132. Lin K, Hsin H, Libina N, et al. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28(2):139–145. doi:10.1038/88850
133. Hansen M, Taubert S, Crawford D, et al. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6(1):95–110. doi:10.1111/j.1474-9726.2006.00267.x
134. Kaeberlein M, Powers RW, Steffen KK, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310(5751):1193–1196. doi:10.1126/science.1115535
135. Kapahi P, Zid BM, Harper T, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14(10):885–890. doi:10.1016/j.cub.2004.03.059
136. Sasazawa Y, Sato N, Umezawa K, et al. Conophylline protects cells in cellular models of neurodegenerative diseases by inducing mammalian target of rapamycin (mTOR)-independent autophagy. J Biol Chem. 2015;290(10):6168–6178. doi:10.1074/jbc.M114.606293
137. Helliwell RM, ShioukHuey CO, Dhuna K, et al. Selected ginsenosides of the protopanaxdiol series are novel positive allosteric modulators of P2X7 receptors. Br J Pharmacol. 2015;172(13):3326–3340. doi:10.1111/bph.13123
138. Liu J, Peng L, Huang W, et al. Balancing between aging and cancer: molecular genetics meets traditional Chinese medicine. J Cell Biochem. 2017;118(9):2581–2586. doi:10.1002/jcb.25898
139. Kapoor V, Aggarwal S, Das SN. 6-gingerol mediates its anti tumor activities in human oral and cervical cancer cell lines through apoptosis and cell cycle arrest. Phytother Res. 2016;30(4):588–595. doi:10.1002/ptr.5561
140. Apfeld J, O’Connor G, McDonagh T, et al. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18(24):3004–3009. doi:10.1101/gad.1255404
141. Greer EL, Dowlatshahi D, Banko MR, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17(19):1646–1656. doi:10.1016/j.cub.2007.08.047
142. Mahran RI, Hagras MM, Sun D, et al. Bringing curcumin to the clinic in cancer prevention: a review of strategies to enhance bioavailability and efficacy. AAPS J. 2017;19(1):54–81. doi:10.1208/s12248-016-0003-2
143. Salminen A, Kauppinen A, Kaarniranta K. Phytochemicals suppress nuclear factor-κB signaling: impact on health span and the aging process. Curr Opin Clin Nutr Metab Care. 2012;15(1):23–28. doi:10.1097/MCO.0b013e32834d3ae7
144. Wang T, Guo R, Zhou G, et al. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: a review. J Ethnopharmacol. 2016;188:234–258. doi:10.1016/j.jep.2016.05.005
145. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. doi:10.1371/journal.pbio.1002533
146. Sinha T, Vich Vila A, Garmaeva S, et al. Analysis of 1135 gut metagenomes identifies sex-specific resistome profiles. Gut Microbes. 2019;10(3):358–366. doi:10.1080/19490976.2018.1528822
147. Chen KL, Madak-Erdogan Z. Estrogen and microbiota crosstalk: should we pay attention? Trends Endocrinol Metab. 2016;27(11):752–755. doi:10.1016/j.tem.2016.08.001
148. Gomez A, Luckey D, Taneja V. The gut microbiome in autoimmunity: sex matters. Clin Immunol. 2015;159(2):154–162. doi:10.1016/j.clim.2015.04.016
149. Paterni I, Bertini S, Granchi C, Macchia M, Minutolo F. Estrogen receptor ligands: a patent review update. Expert Opin Ther Pat. 2013;23(10):1247–1271. doi:10.1517/13543776.2013.805206
150. Shin JH, Park YH, Sim M, et al. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol. 2019;170(4–5):192–201. doi:10.1016/j.resmic.2019.03.003
151. Liu R, Zhang C, Shi Y, et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol. 2017;8:324. doi:10.3389/fmicb.2017.00324
152. Barford A, Dorling D, Davey Smith G, et al. Life expectancy: women now on top everywhere. BMJ. 2006;332(7545):808. doi:10.1136/bmj.332.7545.808
153. Gordon EH, Peel NM, Samanta M, et al. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40. doi:10.1016/j.exger.2016.12.021
154. Hägg S, Jylhävä J. Sex differences in biological aging with a focus on human studies. Elife. 2021;10:e63425. doi:10.7554/eLife.63425
155. Turnbaugh PJ, Bäckhed F, Fulton L, et al. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–223. doi:10.1016/j.chom.2008.02.015
156. Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res. 2017;179:223–244. doi:10.1016/j.trsl.2016.10.002
157. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi:10.1038/nature12820
158. Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137(5):1716–24.e242. doi:10.1053/j.gastro.2009.08.042
159. Tsivgoulis G, Psaltopoulou T, Wadley VG, et al. Adherence to a Mediterranean diet and prediction of incident stroke. Stroke. 2015;46(3):780–785. doi:10.1161/STROKEAHA.114.007894
160. Wu L, Sun D. Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci Rep. 2017;7:41317. doi:10.1038/srep41317
161. Marlow G, Ellett S, Ferguson IR, et al. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum Genomics. 2013;7(1):24. doi:10.1186/1479-7364-7-24
162. Greco T, Glenn TC, Hovda DA, et al. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J Cereb Blood Flow Metab. 2016;36(9):1603–1613. doi:10.1177/0271678X15610584
163. Olson CA, Vuong HE, Yano JM, et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728–1741.e13. doi:10.1016/j.cell.2018.04.027
164. Aon MA, Bernier M, Mitchell SJ, et al. Untangling determinants of enhanced health and lifespan through a multi-omics approach in mice. Cell Metab. 2020;32(1):100–116.e4. doi:10.1016/j.cmet.2020.04.018
165. Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2(11):659–666. doi:10.1016/S1473-3099(02)00437-1
166. Ong SM, Hadadi E, Dang TM, et al. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis. 2018;9(3):266. doi:10.1038/s41419-018-0327-1
167. Di Mitri D, Azevedo RI, Henson SM, et al. Reversible senescence in human CD4+CD45RA+CD27- memory T cells. J Immunol. 2011;187(5):2093–2100. doi:10.4049/jimmunol.1100978
168. Arsenović-Ranin N, Petrović R, Živković I, et al. Influence of aging on germinal centre reaction and antibody response to inactivated influenza virus antigens in mice: sex-based differences. Biogerontology. 2019;20(4):475–496. doi:10.1007/s10522-019-09811-8
169. Eberl G, Lochner M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2009;2(6):478–485. doi:10.1038/mi.2009.114
170. Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–241. doi:10.1038/nature11551
171. Elson CO, Cong Y, McCracken VJ, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206(1):260–276. doi:10.1111/j.0105-2896.2005.00291.x
172. Kim CH. Immune regulation by microbiome metabolites. Immunology. 2018;154(2):220–229. doi:10.1111/imm.12930
173. Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52(1):1–8. doi:10.1007/s00535-016-1242-9
174. Zhang M, Zhou Q, Dorfman RG, et al. Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 2016;16(1):84. doi:10.1186/s12876-016-0500-x
175. Rosser EC, Piper CJM, Matei DE, et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. 2020;31(4):837–851.e10. doi:10.1016/j.cmet.2020.03.003
176. Campbell C, McKenney PT, Konstantinovsky D, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature. 2020;581(7809):475–479. doi:10.1038/s41586-020-2193-0
177. Haselow K, Bode JG, Wammers M, et al. Bile acids PKA-dependently induce a switch of the IL-10/IL-12 ratio and reduce proinflammatory capability of human macrophages. J Leukoc Biol. 2013;94(6):1253–1264. doi:10.1189/jlb.0812396
178. Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–1283. doi:10.1136/gutjnl-2013-304833
179. Tamanai-Shacoori Z, Smida I, Bousarghin L, et al. Roseburia spp.: a marker of health? Future Microbiol. 2017;12:157–170. doi:10.2217/fmb-2016-0130
180. Kim S, Jazwinski SM. The gut microbiota and healthy aging: a mini-review. Gerontology. 2018;64(6):513–520. doi:10.1159/000490615
181. Ha EM, Lee KA, Park SH, et al. Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev Cell. 2009;16(3):386–397. doi:10.1016/j.devcel.2008.12.015
182. Leulier F, Royet J. Maintaining immune homeostasis in fly gut. Nat Immunol. 2009;10(9):936–938. doi:10.1038/ni0909-936
183. Fulop T, Larbi A, Dupuis G, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2018;8:1960. doi:10.3389/fimmu.2017.01960
184. Gomez CR. Role of heat shock proteins in aging and chronic inflammatory diseases. Geroscience. 2021;43(5):2515–2532. doi:10.1007/s11357-021-00394-2
185. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi:10.1111/j.1749-6632.2000.tb06651.x
186. Piber D, Olmstead R, Cho JH, et al. Inflammaging: age and systemic, cellular, and nuclear inflammatory biology in older adults. J Gerontol a Biol Sci Med Sci. 2019;74(11):1716–1724. doi:10.1093/gerona/glz130
187. Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3):319–329. doi:10.1016/j.arr.2010.11.002
188. Pansarasa O, Pistono C, Davin A, et al. Altered immune system in frailty: genetics and diet may influence inflammation. Ageing Res Rev. 2019;54:100935. doi:10.1016/j.arr.2019.100935
189. Zhu X, Chen Z, Shen W, et al. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduct Target Ther. 2021;6(1):245. doi:10.1038/s41392-021-00646-9
190. Straub RH, Schradin C. Chronic inflammatory systemic diseases: an evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol Med Public Health. 2016;2016(1):37–51. doi:10.1093/emph/eow001
191. Neves J, Sousa-Victor P. Regulation of inflammation as an anti-aging intervention. FEBS J. 2020;287(1):43–52. doi:10.1111/febs.15061
192. Gorgoulis V, Adams PD, Alimonti A, et al. Cellular senescence: defining a path forward. Cell. 2019;179(4):813–827. doi:10.1016/j.cell.2019.10.005
193. de Keizer PL. The fountain of youth by targeting senescent cells? Trends Mol Med. 2017;23(1):6–17. doi:10.1016/j.molmed.2016.11.006
194. Meyer P, Maity P, Burkovski A, et al. A model of the onset of the senescence associated secretory phenotype after DNA damage induced senescence. PLoS Comput Biol. 2017;13(12):e1005741. doi:10.1371/journal.pcbi.1005741
195. Latz E, Duewell P. NLRP3 inflammasome activation in inflammaging. Semin Immunol. 2018;40:61–73. doi:10.1016/j.smim.2018.09.001
196. Waters DW, Schuliga M, Pathinayake PS, et al. A senescence bystander effect in human lung fibroblasts. Biomedicines. 2021;9(9):1162. doi:10.3390/biomedicines9091162
197. Jeon OH, David N, Campisi J, et al. Senescent cells and osteoarthritis: a painful connection. J Clin Invest. 2018;128(4):1229–1237. doi:10.1172/JCI95147
198. Xu M, Bradley EW, Weivoda MM, et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J Gerontol a Biol Sci Med Sci. 2017;72(6):780–785. doi:10.1093/gerona/glw154
199. Jeon OH, Kim C, Laberge RM, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775–781. doi:10.1038/nm.4324
200. Weaver LK, Minichino D, Biswas C, et al. Microbiota-dependent signals are required to sustain TLR-mediated immune responses. JCI Insight. 2019;4(1):e124370. doi:10.1172/jci.insight.124370
201. Thevaranjan N, Puchta A, Schulz C, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455–466.e4. doi:10.1016/j.chom.2017.03.002
202. Fransen F, van Beek AA, Borghuis T, et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol. 2017;8:1385. doi:10.3389/fimmu.2017.01385
203. Abdelhamid L, Luo XM. Retinoic acid, leaky gut, and autoimmune diseases. Nutrients. 2018;10(8):1016. doi:10.3390/nu10081016
204. Volynets V, Rings A, Bárdos G, et al. Intestinal barrier analysis by assessment of mucins, tight junctions, and α-defensins in healthy C57BL/6J and BALB/cJ mice. Tissue Barriers. 2016;4(3):e1208468. doi:10.1080/21688370.2016.1208468
205. Lyu Y, Wu L, Wang F, et al. Carotenoid supplementation and retinoic acid in immunoglobulin A regulation of the gut microbiota dysbiosis. Exp Biol Med. 2018;243(7):613–620.
206. Robinson K, Deng Z, Hou Y, et al. Regulation of the intestinal barrier function by host defense peptides. Front Vet Sci. 2015;2:57. doi:10.3389/fvets.2015.00057
207. Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020;16(4):206–222. doi:10.1038/s41581-019-0234-4
208. Cheng W, Lu J, Lin W, et al. Effects of a galacto-oligosaccharide-rich diet on fecal microbiota and metabolite profiles in mice. Food Funct. 2018;9(3):1612–1620. doi:10.1039/C7FO01720K
209. Wilms E, Jonkers DM, Savelkoul HFJ, et al. The impact of pectin supplementation on intestinal barrier function in healthy young adults and healthy elderly. Nutrients. 2019;11(7):1554. doi:10.3390/nu11071554
210. Bernard C. Lectures on Phenomena of Life Common to Animals and Plants. Paris: JB Balliere and Son; 1878.
211. Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–466. doi:10.1038/nrn3071
212. Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160(5):1486–1501. doi:10.1053/j.gastro.2020.10.066
213. Farmer AD, Aziz Q. Mechanisms and management of functional abdominal pain. J R Soc Med. 2014;107(9):347–354. doi:10.1177/0141076814540880
214. Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20(1):64–72. doi:10.1111/j.1365-2982.2008.01104.x
215. Konturek SJ, Konturek JW, Pawlik T, et al. Brain-gut axis and its role in the control of food intake. J Physiol Pharmacol. 2004;55(1 Pt 2):137–154.
216. Bernstein CN. The brain-gut axis and stress in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46(4):839–846. doi:10.1016/j.gtc.2017.08.006
217. Breit S, Kupferberg A, Rogler G, et al. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44. doi:10.3389/fpsyt.2018.00044
218. Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125(3):926–938. doi:10.1172/JCI76304
219. Dinan TG, Cryan JF. Gut-brain axis in 2016: brain-gut-microbiota axis - mood, metabolism and behaviour. Nat Rev Gastroenterol Hepatol. 2017;14(2):69–70. doi:10.1038/nrgastro.2016.200
220. Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi:10.1016/j.ynstr.2017.03.001
221. Gao X, Cao Q, Cheng Y, et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci U S A. 2018;115(13):E2960–E2969. doi:10.1073/pnas.1720696115
222. Maniscalco JW, Rinaman L. Vagal interoceptive modulation of motivated behavior. Physiology. 2018;33(2):151–167. doi:10.1152/physiol.00036.2017
223. Marin IA, Goertz JE, Ren T, et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep. 2017;7:43859.
224. Arentsen T, Khalid R, Qian Y, et al. Sex-dependent alterations in motor and anxiety-like behavior of aged bacterial peptidoglycan sensing molecule 2 knockout mice. Brain Behav Immun. 2018;67:345–354. doi:10.1016/j.bbi.2017.09.014
225. Antonucci TC, Ajrouch KJ, Manalel JA. Social relations and technology: continuity, context, and change. Innov Aging. 2017;1(3):igx029. doi:10.1093/geroni/igx029
226. Charles ST, Carstensen LL. Social and emotional aging. Annu Rev Psychol. 2010;61(1):383–409. doi:10.1146/annurev.psych.093008.100448
227. Carstensen LL, DeLiema M. The positivity effect: a negativity bias in youth fades with age. Curr Opin Behav Sci. 2018;19:7–12. doi:10.1016/j.cobeha.2017.07.009
228. Buffington SA, Di Prisco GV, Auchtung TA, et al. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762–1775.
229. Desbonnet L, Clarke G, Shanahan F, et al. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19(2):146–148. doi:10.1038/mp.2013.65
230. Sgritta M, Dooling SW, Buffington SA, et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101(2):246–259.e6. doi:10.1016/j.neuron.2018.11.018
231. Stilling RM, Moloney GM, Ryan FJ, et al. Social interaction-induced activation of RNA splicing in the amygdala of microbiome-deficient mice. Elife. 2018;7:e33070. doi:10.7554/eLife.33070
232. Degroote S, Hunting DJ, Baccarelli AA, et al. Maternal gut and fetal brain connection: increased anxiety and reduced social interactions in Wistar rat offspring following peri-conceptional antibiotic exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:76–82. doi:10.1016/j.pnpbp.2016.06.010
233. Desbonnet L, Clarke G, Traplin A, et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. doi:10.1016/j.bbi.2015.04.004
234. Arentsen T, Qian Y, Gkotzis S, et al. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol Psychiatry. 2017;22(2):257–266. doi:10.1038/mp.2016.182
235. Poutahidis T, Kearney SM, Levkovich T, et al. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS One. 2013;8(10):e78898. doi:10.1371/journal.pone.0078898
236. Machanda ZP, Rosati AG. Shifting sociality during primate ageing. Philos Trans R Soc Lond B Biol Sci. 2020;375(1811):20190620. doi:10.1098/rstb.2019.0620
237. Luk B, Veeraragavan S, Engevik M, et al. Postnatal colonization with human “infant-type” Bifidobacterium species alters behavior of adult gnotobiotic mice. PLoS One. 2018;13(5):e0196510. doi:10.1371/journal.pone.0196510
238. Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609.e6093. doi:10.1053/j.gastro.2011.04.052
239. Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. doi:10.1073/pnas.1010529108
240. Chung YC, Jin HM, Cui Y, et al. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J Funct Foods. 2014;10:465–474. doi:10.1016/j.jff.2014.07.007
241. Akbari E, Asemi Z, Daneshvar Kakhaki R, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. doi:10.3389/fnagi.2016.00256
242. Santos AL, Sinha S. Obesity and aging: molecular mechanisms and therapeutic approaches. Ageing Res Rev. 2021;67:101268. doi:10.1016/j.arr.2021.101268
243. CDC. Obesity is a common, serious, and costly disease; 2020. Available from: https://www.cdc.gov/obesity/data/adult.html.
244. Ahima RS. Connecting obesity, aging and diabetes. Nat Med. 2009;15(9):996–997. doi:10.1038/nm0909-996
245. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9(5):667–684. doi:10.1111/j.1474-9726
246. Salmon AB. Beyond diabetes: does obesity-induced oxidative stress drive the aging process? Antioxidants. 2016;5(3):24. doi:10.3390/antiox5030024
247. Marungruang N, Kovalenko T, Osadchenko I, et al. Lingonberries and their two separated fractions differently alter the gut microbiota, improve metabolic functions, reduce gut inflammatory properties, and improve brain function in ApoE-/- mice fed high-fat diet. Nutr Neurosci. 2020;23(8):600–612. doi:10.1080/1028415X.2018.1536423
248. Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi:10.1038/nature05414
249. Turnbaugh PJ, Ridaura VK, Faith JJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. doi:10.1126/scitranslmed.3000322
250. de Lartigue G, Barbier de la Serre C, Espero E, et al. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS One. 2012;7(3):e32967. doi:10.1371/journal.pone.0032967
251. Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept. 2000;86(1–3):83–88. doi:10.1016/S0167-0115(99)00084-1
252. Blauwendraat C, Heilbron K, Vallerga CL, et al. Parkinson’s disease age at onset genome-wide association study: defining heritability, genetic loci, and α-synuclein mechanisms. Mov Disord. 2019;34(6):866–875. doi:10.1002/mds.27659
253. Dorsey ER, Bloem BR. The Parkinson pandemic-a call to action. JAMA Neurol. 2018;75(1):9–10. doi:10.1001/jamaneurol.2017.3299
254. Obeso JA, Stamelou M, Goetz CG, et al. Past, present, and future of Parkinson’s disease: a special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord. 2017;32(9):1264–1310. doi:10.1002/mds.27115
255. Olanow CW. Levodopa is the best symptomatic therapy for PD: nothing more, nothing less. Mov Disord. 2019;34(6):812–815. doi:10.1002/mds.27690
256. Olanow CW, Schapira AH. Therapeutic prospects for Parkinson disease. Ann Neurol. 2013;74(3):337–347. doi:10.1002/ana.24011
257. Bedarf JR, Hildebrand F, Coelho LP, et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017;9(1):39. doi:10.1186/s13073-017-0428-y
258. Hasegawa S, Goto S, Tsuji H, et al. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS One. 2015;10(11):e0142164. doi:10.1371/journal.pone.0142164
259. Hill-Burns EM, Debelius JW, Morton JT, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32(5):739–749. doi:10.1002/mds.26942
260. Unger MM, Spiegel J, Dillmann KU, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi:10.1016/j.parkreldis.2016.08.019
261. Perez-Pardo P, Dodiya HB, Engen PA, et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut. 2019;68(5):829–843. doi:10.1136/gutjnl-2018-316844
262. Scheperjans F, Aho V, Pereira PA, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–358. doi:10.1002/mds.26069
263. Qian Y, Yang X, Xu S, et al. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun. 2018;70:194–202. doi:10.1016/j.bbi.2018.02.016
264. Barichella M, Pacchetti C, Bolliri C, et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology. 2016;87(12):1274–1280. doi:10.1212/WNL.0000000000003127
265. Xu Z, Xiao N, Chen Y, et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol Neurodegener. 2015;10:58. doi:10.1186/s13024-015-0056-1
266. O’Bryant SE, Mielke MM, Rissman RA, et al. Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement. 2017;13(1):45–58. doi:10.1016/j.jalz.2016.09.014
267. Balin BJ, Gérard HC, Arking EJ, et al. Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med Microbiol Immunol. 1998;187(1):23–42. doi:10.1007/s004300050071
268. Balin BJ, Little CS, Hammond CJ, et al. Chlamydophila pneumoniae and the etiology of late-onset Alzheimer’s disease. J Alzheimers Dis. 2008;13(4):371–380. doi:10.3233/JAD-2008-13403
269. Itzhaki RF, Lathe R, Balin BJ, et al. Microbes and Alzheimer’s disease. J Alzheimers Dis. 2016;51(4):979–984. doi:10.3233/JAD-160152
270. Vogt NM, Kerby RL, Dill-McFarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537. doi:10.1038/s41598-017-13601-y
271. Bonfili L, Cecarini V, Cuccioloni M, et al. SLAB51 probiotic formulation activates SIRT1 pathway promoting antioxidant and neuroprotective effects in an AD mouse model. Mol Neurobiol. 2018;55(10):7987–8000. doi:10.1007/s12035-018-0973-4
272. Van Gerven N, Van der Verren SE, Reiter DM, et al. The role of functional amyloids in bacterial virulence. J Mol Biol. 2018;430(20):3657–3684. doi:10.1016/j.jmb.2018.07.010
273. Friedland RP. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J Alzheimers Dis. 2015;45(2):349–362. doi:10.3233/JAD-142841
274. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152(1–2):39–50. doi:10.1016/j.cell.2012.10.052
275. Haiser HJ, Gootenberg DB, Chatman K, et al. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science. 2013;341(6143):295–298. doi:10.1126/science.1235872
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
