Back to Journals » International Journal of Nanomedicine » Volume 12
Gold-quercetin nanoparticles prevent metabolic endotoxemia-induced kidney injury by regulating TLR4/NF-kB signaling and Nrf2 pathway in high fat diet fed mice
Authors Xu MX, Wang M, Yang WW
Received 29 June 2016
Accepted for publication 20 September 2016
Published 5 January 2017 Volume 2017:12 Pages 327—345
DOI https://doi.org/10.2147/IJN.S116010
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
This paper has been retracted
Min-Xuan Xu,1,2,* Ming Wang,3,* Wei-Wei Yang4
*These authors contributed equally to this work
Abstract: High-fat diet-induced metabolic syndrome followed by chronic kidney disease caused by intestinal endotoxemia have received extensive attention. Toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB) and oxidative stress-related Nrf2/Keap1 were regarded as the key target points involved in metabolic inflammation and kidney injury. However, the molecular mechanism of interaction between TLR4/NF-κB and Nrf2 activation in high-fat diet-induced renal injury is not absolutely understood. Quercetin, a natural product, has been reported to possess antitumor and anti-inflammatory effects. In this regard, this study attempted to prepare poly(d,l-lactide-co-glycolide)-loaded gold nanoparticles precipitated with quercetin (GQ) to investigate the anti-inflammatory and anti-oxidative stress effects in high-fat diet-induced kidney failure. For this study, C57BL/6 mice fed fat-rich fodder were used as the metabolic syndrome model to evaluate the protective effects of GQ on kidney injury and to determine whether TLR4/NF-κB and Nrf2 pathways were associated with the process. Moreover, histological examinations, enzyme-linked immunosorbent assay, Western blot, and basic blood tests and systemic inflammation-related indicators were used to investigate the inhibitory effects of GQ and underlying molecular mechanism by which it may reduce renal injury. Of note, podocyte injury was found to participate in endotoxin-stimulated inflammatory response. TLR4/NF-κB and Nrf2 pathways were upregulated with high-fat diet intake in mice, resulting in reduction of superoxide dismutase activity and increase in superoxide radical, H2O2, malondialdehyde, XO, XDH, and XO/XDH ratio. In addition, upregulation of TLR4/NF-κB and oxidative stress by endotoxin were observed in vitro, which were suppressed by GQ administration, ultimately alleviating podocyte injury. These findings indicated that GQ could restore the metabolic disorders caused by high-fat diet, which suppresses insulin resistance, lipid metabolic imbalance, and proinflammatory cytokine production. Also, it may prevent kidney injury by inhibition of TLR4/NF-κB and oxidative stress, further increasing superoxide dismutase activity.
Keywords: gold-quercetin nanoparticles, kidney injury, podocytes, TLR4/NF-κB, Nrf2
Introduction
Accumulating evidence has demonstrated that excess intake of fat may result in metabolic symptoms, including hyperleptinemia, insulin resistance, and neuroinflammation, thus becoming a high risk factor of developing chronic kidney disease (CKD) in humans and animals.1–3 Lately, high-fat diet (HFD)-induced inflammatory responses in the peripheral tissues, especially in liver, cardiac muscle, and kidney, have been well characterized.4–6 In a previous research, HFD was found to be capable of causing metabolic disorders, changing the structure of intestinal flora, promoting the formation of inflammation, resulting in endotoxemia, and so on.3
CKD, a progressive loss in renal function over a period of months or years, has been regarded as a threat to people all over the world.7,8 As the final manifestation of CKD, renal fibrosis is a common pathway toward kidney failure.9,10 It is characterized by excessive accumulation and deposition of extracellular matrix components. However, the molecular mechanism of high fat intake-induced CKD is not explained clearly. Recent studies have further demonstrated that HFD could affect the peripheral tissues, as well as cause systematic inflammation by directly or indirectly activating Toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB) pathway.11,12 Of note, on one hand, biomechanical experiments and clinical observations have illustrated that long-term intake of HFD results in lipid accumulation and endotoxemia, which can cause increase in inflammatory cytokines in the serum and organs and inflammation-related signaling activation, promoting the development of nonalcoholic fatty liver disease (NAFLD) and systemic disorder.11,13–15 On the other hand, HFD significantly increases the production of reactive oxygen species (ROS), which further release superoxide anion and cause oxidative stress in the kidney by stimulating podocyte injury.15–17 Hence, antioxidant therapy may be involved in the pathological process of kidney injury. Previous reports show that TLR4/NF-κB and mitogen-activated protein kinase (MAPK) pathways are involved in cellular inflammation in response to oxidative stress.18–20 Nrf2/Keap1 reactions could also be increased when oxidative stress occurs in the kidney.21,22 To our knowledge, HFD increases renal inflammation by stimulating podocyte injury, which may be the key target for the treatment of CKD.23,24
Recently, biotechnological application of nanomaterials conjugated with plant molecules has been continuously gaining importance in the therapeutic and medical field.25,26 Nanomaterials used as carriers of plant secondary metabolites are characterized by chemical stability, octahedral symmetry, rigid structure, large surface area, and low cost of production.26 Studies suggest that quercetin, an important flavonoid antioxidant thought to promote health, partly due to its ability to act as an antioxidant against ROS, helps in maintaining the blood pressure, fighting asthma and allergies, preventing angiocardiopathy and tumor progression, and so on.27–33 Indeed, quercetin as a well-known flavonoid with various biological effects has been widely used in many disease models. However, the molecular mechanisms underlying the protective actions of quercetin against kidney injury in mice fed fat-rich diet are not yet understood. Therefore, this study attempted to prepare poly(D,L-lactide-co-glycolide) (PLGA)-loaded gold nanoparticles precipitated with quercetin (GQ) to investigate the anti-inflammatory and antioxidant effects in mice fed HFD.
Materials and methods
Gold-quercetin (GQ) nanoparticle preparation
Quercetin (C15H10O7; relative molecular mass: 302.23; CAS: 117-39-5; HPLC ≥98%) was purchased from HiMedia Laboratories (Mumbai, India) in an anhydrous powdered form and characterized as described. The protocol of PLGA-capsulated GQ preparation was in accordance with a previous report,33 but with certain modifications. Briefly, GQ was prepared by mixing 1 mM gold chloride with the quercetin solution prepared in absolute ethyl alcohol, until the light yellow solution changed to deep red finally. Subsequently, 50 mg PLGA (Sigma-Aldrich, Shanghai, People’s Republic of China) was added to the final solution. Then, the mixture was added slowly to 20 mL of 1% polyoxyethylene-polyoxypropylene (F68) stabilizer and stirred continuously at 500 rpm and 4°C for at least 4 h, until the organic solvent evaporated completely. The remaining material was harvested by re-washing and centrifugation (15,000 rpm and 4°C for 40 min), and the beads were resuspended in double-distilled water and then stored at 4°C until use.
Animals and drug administration
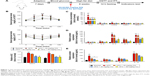
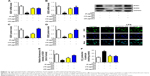
Male C57BL/6 mice aged 6–8 weeks, weighing 20–25 g, were purchased from the Experimental Animal Center of Nanjing Medical University (Nanjing, People’s Republic of China). All animal experiments were performed taking proper care of the animals in accordance with the Guide for the Care and Use of Laboratory Animals, which was issued by the National Institutes of Health in 1996. Before the experiments, all mice were housed in a specific pathogen-free, temperature- and humidity-controlled environment (25°C±2°C, 50%±5% humidity) with a standard 12 h light/12 h dark cycle with food and water in their cages. The Institutional Animal Care and Use Committee at Huai’an First People’s Hospital, Nanjing Medical University approved the animal study protocols. Mice were administered a standard diet containing the most essential nutrients such as vitamins A (≥14,000 IU), D (≥1,500 IU), E (≥120 IU), K (≥5 mg), B1 (≥13 mg), B2 (≥12 mg), B6 (≥12 mg), B12 (≥0.022 mg), biotin (≥0.2 mg), and niacin (≥60 mg) per kg. The mice were divided into five groups: normal (without any treatment), HFD group, HFD + 20 mg/kg GQ, HFD + 40 mg/kg GQ, and HFD + 80 mg/kg GQ. During the period of study, the fodder was changed with HFD (60 kcal% fat, typical analysis of cholesterol [CHOL] in lard =0.95 mg/g, cholesterol (mg)/kg =300.8, D12492; Research Diets, New Brunswick, NJ, USA) until the mice were sacrificed for further study. The GQ solution as drug was given by gavage once a day for 8 weeks with HFD simultaneously. Body weight, blood pressure, and serum endotoxin were measured during the treatment. At the end of the experiments, eyeball blood was harvested for measurement of the levels of insulin, leptin (Mouse enzyme-linked immunosorbent assay [ELISA]-kits; Alpco Inc., Salem, NH, USA; Crystal Chem, Downers Grove, IL, USA), interleukin (IL)-1β, IL-6, and tumor necrosis factor alpha (TNF-α). Subsequently, the liver and kidney tissues were rapidly removed and/or in part dissected for mRNA and protein analyses. The detailed process is shown in Figure 1A.
Biochemical analysis
At the end of the experiments, all mice were fasted for 12 h and blood samples were collected for biochemical analysis including fasting blood glucose (GLU), plasma insulin, and lipid assay. Oral glucose tolerance testing and insulin tolerance testing (ITT) were performed to evaluate the insulin resistance. Mice were given an intraperitoneal injection of glucose (2 g/kg body weight). Blood samples were collected from the tail vein immediately before and 0, 30, 60, 90, and 120 min after glucose administration, and blood GLU levels were measured with o-toluidine reagent (Sigma). For ITT, the animals were fasted for 4 h before administering an intraperitoneal injection of insulin (1 U/kg body weight). Blood samples were withdrawn from the tail vein at 0, 30, 60, 90, and 120 min postinjection. Finally, all animals were sacrificed with a high dose of sodium thiopentone (100 mg/kg body weight). In addition, the blood samples were investigated for routine blood analysis including whole blood viscosity, plasma viscosity, packed cell volume (PCV), erythrocyte aggregation index, platelet counts, and platelet aggregation rate. Shanghai Biohelper, Co., Ltd analyzed the collected serum or kidney tissue for GLU, triglyceride (TG), CHOL, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, superoxide dismutase (SOD), glutathione S-transferase (GST), glutathione peroxidase (GPx), and lipid peroxidation (LPO). Moreover, another serum sample was analyzed for the expression of IL-2, IL-4, IL-6, IL-1, IL-1β, interferon-γ, TNF-α, IL-10, and IL-17 using ELISA kits purchased from R&D Systems (Shanghai, People’s Republic of China). Blood pressure was measured by a noninvasive blood pressure meter (Smiths medical, Dublin, OH, Wisconsin, USA). Malondialdehyde (MDA) level and O2− and H2O2 production in tissues were determined by commercially available kits (Beyotime Institute of Biotechnology, Nantong, People’s Republic of China) according to the manufacturer’s instructions. In all other cellular assays, the cells were treated with a concentration of 0–70 μg/mL GQ for 24 h or were cultured with 70 μg/mL GQ for 0–96 h. Using Cell Counting Kit 8 (Zoman Biotechnology Co., Ltd, Beijing, People’s Republic of China), the WST-8 assay was carried out to examine the cell viability according to the manufacturer’s instructions.
Histological analysis
The liver and kidney samples were subjected to oil red staining and were examined for injury by light microscopy. In brief, tissues were fixed with 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4). After dehydration, thin sections were strictly evaluated under light microscopy. The oil red staining tests were performed by Jiangsu Biotechnology, Co., Ltd (Nanjing, People’s Republic of China). Furthermore, the kidney tissues were subjected to immunohistochemical (IHC) staining for the measurement of Nrf2 and p-IκBα expression. The sections were stained with anti-rabbit Nrf2 or anti-rabbit p-IκBα. All the histological protocols were in accordance with the standard procedures demonstrated previously.34,35
Cell culture
The mouse immortalized podocyte line was obtained from Shanghai Sxbid Biotechnology Co., Ltd (Shanghai, People’s Republic of China) and maintained in uncoated culture flasks containing medium (Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum, 1×105 U/L streptomycin sulfate, pH 7.2 [GIBCO Corporation, Gaithersburg, MD, USA] and recombinant interferon-γ [R&D, Minneapolis, MN, USA]) at a concentration of 2.5×105/mL at 33°C in the presence of 5% CO2. Then, confluent cultures were passaged by trypsinization and cultured in 12-well plates at a density of 1×105/mL without interferon, and maintained in a constant environment of 37°C, 5% CO2 for 14 days. Differentiated podocytes were incubated with 5 μg/mL lipopolysaccharide (LPS) with or without 15 or 30 μg/mL GQ for 24 h. Then, the cells were harvested and detected by Western blot and reverse transcription polymerase chain reaction, p-IkBα and SOD1 were tested using immunofluorescent assay.
Quantitative real-time PCR (qPCR)
Total RNA was extracted from the tissues and cells by using Trizol reagent (Sigma-Aldrich, St Louis, MO, USA) following the manufacturer’s instructions and treated with deoxyribonuclease I. Then, the mRNA was converted into complementary DNA for real-time PCR. Real-time PCR was carried out for 35 cycles at 95°C for 20 s, 54°C for 30 s, and 72°C for 30 s. Fold changes in mRNA levels of the target gene relative to endogenous cyclophilin control were calculated. Briefly, the cycle threshold (Ct) values of each target gene were subtracted from the Ct values of the housekeeping gene cyclophilin (ΔCt). Target gene ΔΔCt was calculated as ΔCt of the target gene minus ΔCt of control. The fold change in mRNA expression was calculated as 2−ΔΔCt. The sequences used in this study are shown in Table 1.
  | Table 1 The sequences of RT-PCR used in this study |
Western blot analysis
Tissues and cells were homogenized in 10% (wt/vol) hypotonic buffer (25 mM Tris-HCl, pH 8.0, 1 mM ethylenediaminetetraacetic acid, 5 μg/mL leupeptin, 1 mM Pefabloc SC, 50 μg/mL aprotinin, 5 μg/mL soybean trypsin inhibitor, 4 mM benzamidine) to yield a homogenate. Then, the final supernatants were obtained by centrifugation at 12,000 rpm for 20 min. Protein concentration was determined using BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) with bovine serum albumin as a standard. Then, the same amount of total protein was subjected to 10% or 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by immunoblotting using the following antibodies (1:1,000): rabbit anti-TLR4, p-IκBα, p-IKKα, p-NF-κB, NF-κB, SOD1/2, XO, Nrf2, Keap1, HO-1, GAPDH (Cell Signaling Technology, Inc., Massachusetts, MA, USA), and NQO1 (Abcam). Western blot bands were observed using GE Healthcare ECL Western Blotting Analysis System and exposed to X-ray film of Kodak. Every protein expression level would be defined as the gray value (Version 1.4.2b, Mac OS X, ImageJ; National Institutes of Health, Bethesda, MD, USA) and standardized to housekeeping genes (GAPDH) and expressed as a fold of control.
Statistical analysis
Data were expressed as mean ± standard error of the mean. Statistical analyses were performed using GraphPad PRISM (version 6.0; GraphPad Software) by analysis of variance with Dunnet’s least significant difference post hoc tests. A P-value <0.05 was considered significant.
Results
GQ nanoparticles inhibited metabolic disorder in HFD-induced mice
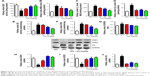
HFD-induced mice were treated with different concentrations of GQ in order to evaluate the possible protective effects on systemic metabolism disorder. As found in Figure 1B, the oral glucose tolerance testing and ITT data showed that insulin resistance could be significantly observed in the experimental mice. However, insulin resistance was inhibited in a dose-dependent manner in the GQ-treated groups, compared with the model group. Moreover, GQ has the ability to decrease the body weight and fat percent in the long-term HFD administration. Also, controls were more resistant than the treated mice, compared to all groups, with area under curve suggesting that GQ administration makes the mice more sensitive to insulin and restrains the insulin resistance caused by fat-rich diet. Meanwhile, we also investigated the lipid metabolism and inflammatory cytokine levels using ELISA and biochemical analysis. It is observed in Figure 1C that HFD-induced mice had a typical metabolism disorder in the blood lipid levels. TG, CHOL, and GLU were found to be in high concentrations in them. In the treated group, these symptoms we analyzed have been significantly down regulated. Meanwhile, regarding the inflammatory cytokine analysis, the data presented in Figure 1D and E show that fat diet promotes the production and release of proinflammatory cytokines. Thus, GQ has the ability to suppress inflammatory cytokine production, decrease blood lipid abnormality and insulin resistance by regulating the metabolic system. The effects of GQ on the general parameters in HFD-fed mice were further studied. Table 2 shows that HFD-induced mice have a higher body weight, fat tissue, serum insulin, aspartate transaminase, alanine transaminase, alkaline phosphatase (AKP), uric acid, and leptin than the normal animals, which were significantly suppressed in a dose-dependent manner by GQ treatment. These findings indicated that treatment of GQ displayed potential effects to inhibit fat-rich diet-induced metabolic disorder. Furthermore, as found in previous reports, obese patients always have unbalanced and abnormal homeostasis in blood.36,37 Hence, it is necessary to conduct routine blood tests. As shown in Tables 3 and 4, the analysis of indicators including whole blood viscosity, whole blood reduction viscosity, plasma viscosity, erythrocyte aggregation index, and PCV indicated that, due to elevated levels of blood sugar than normal, HFD could lead to increase in blood viscosity in mice, slow blood flow, increase in platelet aggregation factor, and thus promotes the development of metabolic syndrome. Meanwhile, the SOD activity and serum LPO were further investigated. Apparently, Table 5 shows that HFD could suppress the activation of SOD and promote the formation and development of LPO. Compared with the control group, GQ helps to improve the SOD activity and significantly downregulates LPO levels in serum. Importantly, as shown in Figure 2A and B, consistent with the previous reports,38,39 increases in serum endotoxin and mean blood pressure were significantly observed in HFD-induced mice, which could be inhibited dose-dependently by GQ. HFD may upregulate serum endotoxin and further promote inflammation-related signaling activation and inflammatory cytokine production and increase the blood pressure. In contrast, GQ treatment limited the upregulation of endotoxin and increase of blood pressure, suggesting it has the potential to be a key therapeutic drug for the treatment of fat-rich diet–stimulated metabolic disorder.
GQ nanoparticles suppressed HFD-induced lipid accumulation, kidney injury, and inflammation-related signaling activation
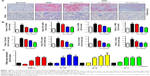
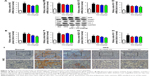
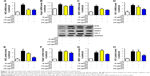
Previous researches have reported that HFD can promote lipid accumulation in the liver tissue. Hereby, next histological changes in the liver tissue were examined. As shown in Figure 3A, lipid accumulation was observed in HFD-induced liver and kidney tissue, which was alleviated by GQ administration in a dose-dependent manner, suggesting that moderate GQ restrained HFD-induced lipid accumulation. Hepatic pathology evaluation further holds that inflammation, ballooning score, non-alcoholic steatohepatitis score, and NAFLD score in HFD mice were higher than normal, but this was inhibited by GQ administration dose-dependently (Table 6). Also, the detached kidney tissues were analyzed using qPCR and ELISA to evaluate proinflammatory cytokine levels. As shown in Figure 3B, significant inhibitory effects of GQ on mRNA and proteins levels of IL-1β, TNF-α, IL-1, and IL-6 were observed. GQ displayed anti-inflammatory role in HFD-induced kidney injury, which was in a dose-dependent manner in the tissues. Meanwhile, the dynamic changes in proinflammatory cytokine levels between 0 and 8 weeks were also investigated. Figure 3C further shows that the mRNA levels of IL-1β, TNF-α, IL-1, and IL-6 were upregulated with HFD, showing increase with time, compared with 0 week.
Besides, HFD is reported to upregulate kidney TLR4/NF-κB signaling pathway and inflammation-related chemokines.40,41 In this regard, Emr-1, MCP-1, MIP-1α, Cxcr4, and TLR4/NF-κB signaling-related indicators were tested. As shown in Figure 4A–D, significant increases in mRNA of inflammation-related factors, including Emr-1, MCP-1, MIP-1α, and Cxcr4, were observed in the HFD group, suggesting that fat-rich diet activates the inflammatory responses and cellular infiltration. In contrast, GQ administration can restrain inflammatory chemokine expression in a dose-dependent manner, and is able to inhibit inflammatory indicators expression in mRNA levels. Also, TLR4/NF-κB as an important inflammatory pathway was investigated using qPCR and Western blot. The results showed that TLR4/NF-κB signaling mediators, including TLR4, MyD88, IKKα, and IKKβ mRNA levels, were increased in HFD mice, but downregulated by GQ treatment in typical dose-dependent manner (Figure 4E–H). Meanwhile, it was further found that p-IKKα, p-IκBα, and p-NF-κB were upregulated in the HFD group, resulting in the activation of TLR4/NF-κB inflammatory pathway. Importantly, IHC analysis of p-IκBα staining in the kidney tissue also proved that TLR4/NF-κB was involved in HFD-induced kidney inflammatory responses (Figure 5A–E). The above findings indicated that the inhibition of TLR4/NF-κB signaling by GQ administration may be the key point in suppressing HFD-induced kidney injury.
GQ nanoparticles restrained HFD-induced oxidative stress and Nrf2 activation in the kidney
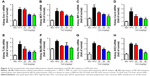
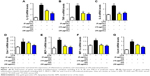
Oxidative stress may be involved in the pathological process of kidney injury. Accordingly, in this regard, a significant decrease in SOD, GST, and GPx activation and upregulation of superoxide radical, MDA, H2O2, xanthine oxidase (XO), xanthine dehydrogenase (XDH), and XO/XDH ratio in the kidney tissues caused by HFD were observed. In contrast, oxidative stress-related indicators were significantly suppressed by an increase in dose of GQ (Figure 6A–G). Meanwhile, downregulation of SOD1, SOD2 and increase in XO protein expression were also evidenced by Western blot analysis, which were inhibited by GQ administration dose-dependently (Figure 6H–J). XO activity as an important source of ROS production has obtained wide attention. Excess ROS formation in the tissues can upregulate Nrf2 pathway activation. Accordingly, the Nrf2-related protein and mRNA expression in HFD-induced kidney tissues were investigated. As shown in Figure 7A–H, indeed, the expression levels of Nrf2, Keap1, NQO1, and HO-1 mRNA and protein were increased in HFD-induced kidney, suggesting that Nrf2 pathway was involved in HFD-induced kidney injury. Significant inhibitory effects of GQ on limitation of Nrf2 activation were observed, suggesting that oxidative stress suppressed by GQ treatment can help to downregulate Nrf2 activation. Also, IHC test presented in Figure 7I further proves that Nrf2 activation was enhanced in HFD-induced kidney tissue, but significantly limited by GQ administration in a dose-dependent manner.
GQ nanoparticles suppressed LPS-induced podocyte inflammation and TLR4/NF-κB pathway activation
As mentioned above, excess intake of fat-rich diet may increase the LPS levels in serum, resulting in occurrence of metabolic endotoxemia. HFD increases renal inflammation by stimulation of podocytes, which may be the key target for the treatment of CKD. Accordingly, the effects of dose-dependent and time-dependent GQ administration on podocytes’ viability were first tested (Figure 8A and B). Cell viability showed no significant toxicity in the cells, which further suggests that GQ nanoparticles do not affect the activity of cells in a dose-dependent and time-dependent manner. Next, the mRNA expression levels of inflammatory cytokines and chemokines, including Emr-1, MCP-1, MIP-1α, Cxcr4, TNF-α, IL-1, and IL-6 in LPS-challenged podocytes were investigated. As shown in Figure 9A–G, a significant activation of Emr-1, MCP-1, MIP-1α, Cxcr4, TNF-α, IL-1, and IL-6 gene expression was observed in the podocytes. GQ (15 and 30 μg/mL) significantly suppressed 5 μg/mL LPS-induced podocyte injury dose-dependently, suggesting that inflammatory responses were involved in LPS-stimulated podocyte activation, but were limited by GQ treatment. Besides, whether TLR4/NF-κB signaling participated in LPS-induced podocyte injury need to be known. As shown in Figure 10A–D, qPCR analysis indicated that, on one hand, TLR4/NF-κB signaling contributed to inflammation and podocyte injury by upregulation of proinflammatory cytokine expression; on the other hand, GQ administration can suppress TLR4/NF-κB related indicators expression in mRNA levels. Indeed, Western blot and immunofluorescence staining of p-IκBα also showed that TLR4/NF-κB was associated with LPS-induced podocytes, but was inhibited dose-dependently by GQ nanoparticles (Figure 10E–G).
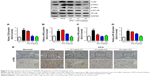
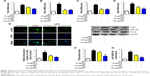
GQ nanoparticles reduced oxidative stress and Nrf2 activation in LPS-exposed podocytes
Previous reports demonstrated that oxidative stress production was suppressed by SOD activation and upregulated by Nrf2 pathway-related gene expression.42 A significant reduction in the mRNA and protein expression levels of SOD1 and SOD2 and an increase in hydrogen peroxide were observed in LPS-exposed podocytes (Figure 11A–F), suggesting that oxidative stress contributed to LPS-induced podocyte injury. Moreover, the immunofluorescence staining of SOD1 further showed that upregulation of oxidative stress levels by LPS stimulation in podocytes is suppressed by GQ administration in a dose-dependent manner. As mentioned above, it was believed that Nrf2 pathway participated in LPS-induced podocyte inflammation. Accordingly, the mRNA and protein expression levels of Nrf2-related factors, including Nrf2, Keap1, NQO1, and HO-1, were examined using qPCR and Western blot. As shown in Figure 12A–H, LPS challenge contributed to increase in Nrf2 activation and downstream genes’ expression, suggesting that Nrf2 pathway was involved in LPS-induced podocyte injury. For another, GQ nanoparticles are able to restrain LPS induced cells injury via regulation of Nrf2 activation. These results suggest that GQ nanoparticles protect the podocytes against LPS stimulation, in part, by regulating Nrf2-related pathway activation.
Discussion
CKDs have become one of the important diseases jeopardizing public health.43 CKD as well as heart attacks, strokes, and uremia cause 80% deaths in Asia, and they are projected to rise considerably over the coming decade.43–45 Particularly, pervious researches have suggested that excess intake of fat-rich diet may cause hyperlipemia and intestinal endotoxemia, resulting in blood lipid metabolism disorder and systemic inflammation, ultimately contributing to the development of CKD and NAFLD.46–48 Thus, kidney disease caused by eating habits has widely received enough attention by the researchers. HFD-induced metabolic abnormalities would change kidney-related gene expression and increase metabolic inflammation and oxidative stress.48 Therefore, inhibition of metabolic disorder at an early stage is considered a feasible strategy for curing chronic renal disease. Recently, quercetin, a beneficial bioflavonoid thought to promote health, has been shown to aid in the prevention of cancer, hepatitis, atherosclerosis, organic lesions, and other diseases.27–33
In this study, HFD-stimulated metabolic endotoxemia followed by kidney injury was used to study the protective effects of GQ nanoparticles on lipid metabolism dysfunction and systemic inflammatory response. Indeed, a long-term intake of fat-rich diet results in hepatic dysfunction and lipid accumulation, accompanied with intestinal endotoxemia. LPS directly triggers kidney injury by pathways that involve inflammatory cells including podocytes, as well as chemical mediators, such as superoxide and nitric oxide. In this study, mice fed fat-rich diet were found to have increased levels of serum ALT, AST, AKP, uric acid, leptin, TG, TC, CHOL, low-density lipoprotein cholesterol, GLU, and inflammation-related cytokines. Moreover, excess fat ingestion may increase the blood pressure, accompanied with increase in serum endotoxin levels. In addition, routine blood test conducted in mice of all groups further showed that a significant increase in whole blood viscosity, whole blood reduction viscosity, plasma viscosity, PCV, erythrocyte aggregation index, and platelet aggregation rate was observed in model mice. These findings suggest that HFD has the ability to change body metabolic balance, glycometabolism, and lipid metabolism, ultimately leading to endotoxemia, lipid accumulation in the liver tissue, increase in systemic inflammatory cytokines, and kidney injury. NF-κB, a transcription factor that displays vital roles in inflammation, immunity, cell proliferation, differentiation, and survival, has been proved to be a major signaling pathway in the development of various inflammation-related diseases, which is mediated by TLR4 to perform regulation of inflammation.49–52 NF-κB has been treated as a central link in the pathogenic processes of systemic inflammatory response and kidney injury to high fat exposure.53,54 As expected, in the present study, on one hand, HFD-fed mice were found to have a significant upregulation in TLR4/NF-κB activation, resulting in the expression of proinflammatory cytokines and related chemokines, including IL-β, IL-6, TNF-α, MCP-1, Cxcr4, Emr-1, and MIP-1α. On the other hand, endotoxin increase in serum can be upregulated by HFD; here podocytes were used as the model to investigate the effect of NF-κB activation in LPS-exposed podocytes. Occurrence of significant podocyte injury in LPS-induced group was found. TLR4/NF-κB activation was upregulated during podocyte injury, suggesting that podocyte injury may contribute to fat-diet-induced kidney inflammation. In addition, oxidative stress is a major contributing factor to the onset of kidney injury and it is typically associated with a decrease in the antioxidant defense. Endotoxin increase may upregulate oxidative stress levels in tissues and cells, which further increase superoxide radical, H2O2, and MDA levels and further reduce SOD activation, GST, and GPx in the kidney tissue or serum. Of note, a previous study demonstrated that Nrf2 pathway was activated by oxidative stress, suggesting that Nrf2 and its downstream genes expression are essential and helpful to the anti-oxidative stress process.55 In the present study, a significant increase in Nrf2 activation, NQO1, and HO-1 and reduction in SOD1 and SOD2 levels were observed in HFD-induced kidney tissue, demonstrating that Nrf2-related genes were activated and associated with kidney injury. Moreover, Nrf2 pathway was also enhanced in LPS-exposed podocytes in vitro, resulting in the upregulation of HO-1 and NQO1 expression. These results further indicate that TLR4/NF-κB activation and Nrf2 signaling were involved in HFD-induced kidney injury.
Quercetin, an important antioxidant with various bioactivities, has been widely studied in a number of disease models, such as arteriosclerosis, diabetes, and cancer.27–33 In this regard, it was found that GQ administration can downregulate HFD-induced kidney injury by suppression of proinflammatory cytokines and chemokines. Of note, insulin resistance, lipid metabolism dysfunction, and serum inflammatory cytokine increase were further inhibited by GQ treatment, suggesting that GQ restrained HFD-stimulated metabolic syndrome in a dose-dependent manner. Also, in vitro, GQ dose-dependently suppressed LPS-exposed podocytes expression of inflammatory cytokines, including IL-β, IL-6, and TNF-α. All the data further indicate that quercetin nanoparticles are capable of ameliorating HFD-induced metabolic disorder and systemic inflammation. Importantly, as mentioned above, HFD-caused endotoxemia may lead to upregulation of TLR4/NF-κB signaling and Nrf2 activation, which often aggravate the inflammatory response and oxidative stress. Therefore, the protective effects of GQ on inhibition of NF-κB activation and Nrf2 pathway were determined. On one hand, qPCR and Western blot analysis showed that significant reduction in superoxide radical, H2O2, and MDA levels and increase in SOD activity were observed in GQ-treated kidney tissues and podocytes, suggesting that GQ displayed anti-oxidative stress effect in HFD-induced kidney injury or LPS-treated podocytes. On the other hand, oxidative stress upregulated Nrf2 pathway activation and downstream antioxidant-related genes’ expression. Indeed, Nrf2 expression was increased with upregulation of oxidative stress and inflammatory cytokines. But GQ administration can reduce Nrf2 and downstream antioxidants, including HO-1 and NQO1, suggesting that suppression of oxidative stress and inflammatory cytokines by GQ administration may lead to downregulation of Nrf2 pathway.
To summarize, this present study showed that HFD-induced endotoxemia contributed to kidney inflammation and podocyte injury by upregulation of oxidative stress and systemic inflammation. Of note, TLR4/NF-κB and Nrf2 activation were the key targets involved in kidney injury. GQ nanoparticles protect mice against podocyte injury and restore the lipid metabolism by inhibiting TLR4/NF-κB-stimulated proinflammatory cytokine production and oxidative stress, which were associated with the HFD-induced kidney failure in mice. These findings bring new insight into our knowledge of the molecular mechanisms that link metabolic disorder and endotoxemia to kidney injury caused by intake of various fat-rich food items. Also, quercetin, a bioactive product generated from plants with high dependability, reliability, and potential anti-inflammatory effects, was found to inhibit the inflammatory pathways and oxidative stress induced by excess fat intake. Hence, inhibition of inflammation and oxidative stress by GQ nanoparticles may provide a potential therapeutic strategy to prevent kidney injury.
Disclosure
The authors report no conflicts of interest in this work.
References
Li L, Zhao Z, Xia J, et al. A long-term high-fat/high-sucrose diet promotes kidney lipid deposition and causes apoptosis and glomerular hypertrophy in bama minipigs. PLoS One. 2015;10(11):e0142884. | ||
Yang G, Lee HE, Lee JY. A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet. Sci Rep. 2016;6:24399. | ||
Coomans CP, van den Berg SA, Houben T, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27(4):1721–1732. | ||
Roychowdhury S, McCullough RL, Sanz-Garcia C, et al. Receptor interacting protein 3 protects mice from high fat diet-induced liver injury. Hepatology. Epub 2016 Jun 15. | ||
Howarth FC, Qureshi MA, Sobhy ZH, et al. Structural lesions and changing pattern of expression of genes encoding cardiac muscle proteins are associated with ventricular myocyte dysfunction in type 2 diabetic Goto-Kakizaki rats fed a high-fat diet. Exp Physiol. 2011;96(8):765–777. | ||
Aliou Y, Liao MC, Zhao XP, et al. Post-weaning high-fat diet accelerates kidney injury, but not hypertension programmed by maternal diabetes. Pediatr Res. 2016;79(3):416–424. | ||
Jiang Y, Zhang J, Yuan Y, et al. Association of increased serum leptin with ameliorated anemia and malnutrition in stage 5 chronic kidney disease patients after parathyroidectomy. Sci Rep. 2016;6:27918. | ||
Busch M, Nadal J, Schmid M, et al. Glycaemic control and antidiabetic therapy in patients with diabetes mellitus and chronic kidney disease – cross-sectional data from the German Chronic Kidney Disease (GCKD) cohort. BMC Nephrol. 2016;17(1):59. | ||
Boor P, Floege J. Chronic kidney disease growth factors in renal fibrosis. Clin Exp Pharmacol Physiol. 2011;38(7):441–450. | ||
Sharma K. Obesity, oxidative stress, and fibrosis in chronic kidney disease. Kidney Int Suppl (2011). 2014;4(1):113–117. | ||
Ferreira PS, Spolidorio LC, Manthey JA, Cesar TB. Citrus flavanones prevent systemic inflammation and ameliorate oxidative stress in C57BL/6J mice fed high-fat diet. Food Funct. 2016;7(6):2675–2681. | ||
Santos SH, Andrade JM, Fernandes LR, et al. Oral Angiotensin-(1–7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-kappaB in rats fed with high-fat diet. Peptides. 2013;46:47–52. | ||
Peng X, Nie Y, Wu J, Huang Q, Cheng Y. Juglone prevents metabolic endotoxemia-induced hepatitis and neuroinflammation via suppressing TLR4/NF-kappaB signaling pathway in high-fat diet rats. Biochem Biophys Res Commun. 2015;462(3):245–250. | ||
Wang C, Tao Q, Wang X, Wang X, Zhang X. Impact of high-fat diet on liver genes expression profiles in mice model of nonalcoholic fatty liver disease. Environ Toxicol Pharmacol. 2016;45:52–62. | ||
Jarukamjorn K, Jearapong N, Pimson C, Chatuphonprasert W. A high-fat, high-fructose diet induces antioxidant imbalance and increases the risk and progression of nonalcoholic fatty liver disease in mice. Scientifica (Cairo). 2016;2016:5029414. | ||
Arany I, Hall S, Reed DK, Reed CT, Dixit M. Nicotine enhances high-fat diet-induced oxidative stress in the kidney. Nicotine Tob Res. 2016;18(7):1628–1634. | ||
Maithili Karpaga Selvi N, Sridhar MG, Swaminathan RP, Sripradha R. Curcumin attenuates oxidative stress and activation of redox-sensitive kinases in high fructose- and high-fat-fed male wistar rats. Sci Pharm. 2015;83(1):159–175. | ||
Zhang R, Yu Y, Deng J, et al. Sesamin ameliorates high-fat diet-induced dyslipidemia and kidney injury by reducing oxidative stress. Nutrients. 2016;8(5):E276. | ||
Wang L, Liu XH, Chen H, et al. Picroside II protects rat kidney against ischemia/reperfusion-induced oxidative stress and inflammation by the TLR4/NF-kappaB pathway. Exp Ther Med. 2015;9(4):1253–1258. | ||
Hou X, Shen YH, Li C, et al. PPARalpha agonist fenofibrate protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress and MAPK activity. Biochem Biophys Res Commun. 2010;394(3):653–659. | ||
Soetikno V, Sari FR, Lakshmanan AP, et al. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol Nutr Food Res. 2013;57(9):1649–1659. | ||
Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83(6):1029–1041. | ||
Bao L, Cai X, Dai X, et al. Grape seed proanthocyanidin extracts ameliorate podocyte injury by activating peroxisome proliferator-activated receptor-gamma coactivator 1alpha in low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Food Funct. 2014;5(8):1872–1880. | ||
Boini KM, Xia M, Abais JM, et al. Activation of inflammasomes in podocyte injury of mice on the high fat diet: effects of ASC gene deletion and silencing. Biochim Biophys Acta. 2014;1843(5):836–845. | ||
Gismondi A, Reina G, Orlanducci S, et al. Nanodiamonds coupled with plant bioactive metabolites: a nanotech approach for cancer therapy. Biomaterials. 2015;38:22–35. | ||
Gismondi A, Nanni V, Reina G, Orlanducci S, Terranova ML, Canini A. Nanodiamonds coupled with 5,7-dimethoxycoumarin, a plant bioactive metabolite, interfere with the mitoticprocess in B16F10 cells altering the actin organization. Int J Nanomedicine. 2016;11:557–574. | ||
Larson A, Witman MA, Guo Y, et al. Acute, quercetin-induced reductions in blood pressure in hypertensive individuals are not secondary to lower plasma angiotensin-converting enzyme activity or endothelin-1: nitric oxide. Nutr Res. 2012;32(8):557–564. | ||
Oliveira TT, Campos KM, Cerqueira-Lima AT, et al. Potential therapeutic effect of Allium cepa L. and quercetin in a murine model of Blomia tropicalis induced asthma. Daru. 2015;23:18. | ||
Shaik YB, Castellani ML, Perrella A, et al. Role of quercetin (a natural herbal compound) in allergy and inflammation. J Biol Regul Homeost Agents. 2006;20(3–4):47–52. | ||
Abarikwu SO. Protective effect of quercetin on atrazine-induced oxidative stress in the liver, kidney, brain, and heart of adult wistar rats. Toxicol Int. 2014;21(2):148–155. | ||
Zhao J, Liu J, Wei T, et al. Quercetin-loaded nanomicelles to circumvent human castration-resistant prostate cancer in vitro and in vivo. Nanoscale. 2016;8(9):5126–5138. | ||
Chen LC, Chen YC, Su CY, Hong CS, Ho HO, Sheu MT. Development and characterization of self-assembling lecithin-based mixed polymeric micelles containing quercetin in cancer treatment and an in vivo pharmacokinetic study. Int J Nanomedicine. 2016;11:1557–1566. | ||
Bishayee K, Khuda-Bukhsh AR, Huh SO. PLGA-loaded gold-nanoparticles precipitated with quercetin downregulate HDAC-Akt activities controlling proliferation and activate p53-ROS crosstalk to induce apoptosis in hepatocarcinoma cells. Mol Cells. 2015;38(6):518–527. | ||
Li JM, Ge CX, Xu MX, et al. Betaine recovers hypothalamic neural injury by inhibiting astrogliosis and inflammation in fructose-fed rats. Mol Nutr Food Res. 2015;59(2):189–202. | ||
Wu SJ, Tam KW, Tsai YH, Chang CC, Chao JC. Curcumin and saikosaponin a inhibit chemical-induced liver inflammation and fibrosis in rats. Am J Chin Med. 2010;38(1):99–111. | ||
Silverberg JI, Becker L, Kwasny M, Menter A, Cordoro KM, Paller AS. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151(2):144–152. | ||
Dudchenko IA, Pristupa LN, Ataman AV, Garbuzova V. [Genetic dependency of blood pressure and heart rate in patients with arterial hypertension and obesity]. Vestn Ross Akad Med Nauk. 2014;(5–6):40–46. Russian. | ||
Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142(5):1100–1101.e2. | ||
Leite RD, Durigan Rde C, de Souza Lino AD, et al. Resistance training may concomitantly benefit body composition, blood pressure and muscle MMP-2 activity on the left ventricle of high-fat fed diet rats. Metabolism. 2013;62(10):1477–1484. | ||
Benzler J, Ganjam GK, Pretz D, et al. Central inhibition of IKKbeta/NF-kappaB signaling attenuates high-fat diet-induced obesity and glucose intolerance. Diabetes. 2015;64(6):2015–2027. | ||
Wu Q, Li S, Li X, et al. Inhibition of advanced glycation endproduct formation by lotus seedpod oligomeric procyanidins through RAGE-MAPK signaling and NF-kappaB activation in high-fat-diet rats. J Agric Food Chem. 2015;63(31):6989–6998. | ||
Kamiya T, Hara H, Yamada H, Imai H, Inagaki N, Adachi T. Cobalt chloride decreases EC-SOD expression through intracellular ROS generation and p38-MAPK pathways in COS7 cells. Free Radic Res. 2008;42(11–12):949–956. | ||
Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014;13(8):823–833. | ||
Nitta K. Chronic kidney diseases – recent advances in clinical and basic research. Preface. Contrib Nephrol. 2015;185:VII–XI. | ||
Jha V. Current status of chronic kidney disease care in southeast Asia. Semin Nephrol. 2009;29(5):487–496. | ||
Hao L, Lu X, Sun M, Li K, Shen L, Wu T. Protective effects of L-arabinose in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Food Nutr Res. 2015;59:28886. | ||
Luo Y, Burrington CM, Graff EC, et al. Metabolic phenotype and adipose and liver features in a high-fat Western diet-induced mouse model of obesity-linked NAFLD. Am J Physiol Endocrinol Metab. 2016;310(6):E418–E439. | ||
Stemmer K, Perez-Tilve D, Ananthakrishnan G, et al. High-fat-diet-induced obesity causes an inflammatory and tumor-promoting microenvironment in the rat kidney. Dis Model Mech. 2012;5(5):627–635. | ||
Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. | ||
Lai S. Chronic kidney disease and diabetes – a potential causal link. EBioMedicine. 2016;6:10–11. | ||
Yang Y, Wang JK. The functional analysis of MicroRNAs involved in NF-kappaB signaling. Eur Rev Med Pharmacol Sci. 2016;20(9):1764–1774. | ||
Rinkenbaugh AL, Baldwin AS. The NF-kappaB pathway and cancer stem cells. Cells. 2016;5(2):E16. | ||
Zhao H, Zheng Q, Hu X, Shen H, Li F. Betulin attenuates kidney injury in septic rats through inhibiting TLR4/NF-kappaB signaling pathway. Life Sci. 2016;144:185–193. | ||
Vykhovanets EV, Shankar E, Vykhovanets OV, Shukla S, Gupta S. High-fat diet increases NF-kappaB signaling in the prostate of reporter mice. Prostate. 2011;71(2):147–156. | ||
Kerns ML, Hakim JM, Lu RG, et al. Oxidative stress and dysfunctional NRF2 underlie pachyonychia congenita phenotypes. J Clin Invest. 2016;126(6):2356–2366. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.