Back to Journals » International Journal of Nanomedicine » Volume 18
Gold Nanoparticles Enhance the Ability of Radiotherapy to Induce Immunogenic Cell Death in Glioblastoma
Authors He C , Ding H, Li L, Chen J, Mo X, Ding Y , Chen W, Tang Q, Wang Y
Received 28 May 2023
Accepted for publication 20 September 2023
Published 9 October 2023 Volume 2023:18 Pages 5701—5712
DOI https://doi.org/10.2147/IJN.S419712
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Chen He,1– 3,* Huiyan Ding,4,* Lubo Li,5 Jing Chen,6 Xiaofei Mo,1– 3 Yinan Ding,4 Wenjing Chen,4 Qiusha Tang,4 Yuetao Wang1– 3
1Department of Nuclear Medicine, the Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu, People’s Republic of China; 2Institute of Clinical Translation of Nuclear Medicine and Molecular Imaging, Soochow University, Changzhou, Jiangsu Province, People’s Republic of China; 3Changzhou Clinical Medical Center, Changzhou, Jiangsu, People’s Republic of China; 4Medical School of Southeast University, Nanjing, People’s Republic of China; 5The Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu, People’s Republic of China; 6Taikang Xianlin Drum Tower Hospital, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiusha Tang; Yuetao Wang, Email [email protected]; [email protected]
Background: Radiation therapy (RT) is commonly used to treat glioblastoma, but its immunomodulatory effect on tumors, through mechanisms such as immunogenic cell death (ICD), is relatively weak. Gold nanoparticles (AuNPs) have been suggested as potential radio-sensitizers, but it is unclear if they can enhance radiation-induced ICD. This study aimed to investigate the potential of AuNPs to improve the effectiveness of radiation-induced ICD.
Methods: G422 cells were treated with a combination of AuNPs and RT to induce cell death. Various assays were conducted to assess cell death, surface expression of CRT, and release of HMGB1 and ATP. In vitro co-culture experiments with bone marrow-derived dendritic cells (BMDCs) were performed to analyze the immunogenicity of dying cancer cells. Flow cytometry was used to measure the maturation rate of BMDCs. An in vivo mouse tumor prophylactic vaccination model was employed to assess immunogenicity.
Results: The study findings presented here confirm that the combination of radiotherapy (RT) with AuNPs can induce a stronger ICD effect on glioblastoma cells compared to using RT alone. Specifically, treatment with AuNPs combined with RT resulted in the emission of crucial damage-associated molecular patterns (DAMPs) such as CRT, HMGB1 (479.41± 165.34pg/mL vs 216.04± 178.16 pg/mL, *P< 0.05) and ATP (The release of ATP in the AuNPs + RT group was 1.2 times higher than in the RT group, *P< 0.05). The proportion of BMDC maturation rate was higher in the group treated with AuNPs and RT compared to the group treated with RT alone. (32.53± 0.52% vs 25.03± 0.28%,***P < 0.001). In the tumor vaccine experiment, dying tumor cells treated with AuNPs and RT effectively inhibited tumor growth in mice when exposed to living tumor cells.
Conclusion: These results indicate that AuNPs have the ability to enhance RT-induced ICD.
Keywords: Immunogenic cell death, Gold nanoparticles, Radiation therapy, Glioblastoma
Introduction
Glioblastoma (GBM) is the most aggressive primary intracranial tumor and one of the most challenging conditions in the field of oncology.1,2 Radiation therapy (RT) is a common treatment method for glioblastoma.3,4 The main determinant of the radiobiological effects in RT is DNA, and the therapeutic rationale is that high-energy ionizing radiations directly cause DNA damage.5,6 Alternatively, they may indirectly induce cell apoptosis by generating ROS.7
Recent studies have demonstrated that radiation therapy (RT) can induce tumor immune regulation through mechanisms such as immunogenic cell death (ICD).8–10 When ICD occurs in tumor cells, calreticulin (CRT) is exposed on the surface of the tumor cell membrane. Furthermore, high mobility group box 1 (HMGB1) and adenosine triphosphate (ATP) are released from the nucleus.8,11–14 CRT serves as an “eat me” signal for phagocytes, leading to dendritic cells (DC) engulfing dying tumor cells. Additionally, HMGB1 binds to toll-like receptor 4 (TLR4), promoting DC maturation and antigen presentation to cytotoxic T lymphocytes (CTLs). ATP stimulates CTL infiltration in tumor tissue.11,15–17 Thus, RT has the potential to convert irradiated dead tumor cells into in situ vaccines, thereby enhancing the immunogenicity of the tumor microenvironment and inducing anti-tumor immunity. However, the ICD effect induced by RT may be weak due to the low sensitivity of glioblastoma cells to radiation therapy.18,19 The weak induction of immunogenic cell death (ICD) by radiotherapy may be attributed to the following reasons: 1) Immune evasion mechanisms of tumor cells: Tumor cells can employ various mechanisms to evade the immune system, such as downregulation of tumor-associated antigens and upregulation of immune inhibitory molecules. These mechanisms can weaken the ICD induction caused by radiotherapy. 2) Immunosuppression: Radiotherapy triggers inflammation and immune responses, but it can also lead to immunosuppression by reducing the number or function of immune cells. These factors can contribute to the weakened ICD induction by radiotherapy. In conclusion, the weak induction of ICD by radiotherapy may be attributed to a combination of factors including immune evasion mechanisms of tumor cells, and immunosuppression caused by radiotherapy. Enhancing the ICD effect induced by RT could greatly enhance the value of RT in tumor therapy. Previous studies have suggested that gold nanoparticles (AuNPs) have radio-sensitization effects.5,20,21 However, it remains unclear whether the increase in radiation dose mediated by AuNPs can also improve the ability of radiation-induced ICD. Therefore, in this study, we aimed to investigate whether AuNPs have the potential to enhance the ability of radiation-induced ICD using G422 cells. We prepared AuNPs and assessed their radiation sensitization ability. Subsequently, we evaluated whether combining AuNPs with radiotherapy would lead to increased release of damage-related molecular patterns (DAMPs), including calreticulin expression, HMGB1 release, and ATP release. In addition, in this study, we evaluated whether nanogold combined with radiotherapy can further induce the maturation of BMDCs (Scheme 1).
 |
Scheme 1 Schematic Diagram of ICD induced by AuNPs combined with radiotherapy. |
Materials and Methods
Preparation and Characterization of AuNPs
AuNPs were prepared by sodium citrate reduction method.22 The details could be find in Supplemental Materials.
Cell Death Assay
We used flow cytometry and CCK8 kit to evaluate the ability of AuNPs combined with radiotherapy to induce cell death. G422 cells used in this study were purchased from National Collection of Authenticated Cell Cultures. The details could be find in Supplemental Materials.
Analysis of CRT Exposure
G422 cells were stimulated with different groups. Laser confocal microscopy and flow cytometry were used to evaluate the expression of CRT on the surface of cell membranes treated with different groups. The details could be find in Supplemental Materials.
Analysis of HMGB1 Release and ATP Release
After treating tumors cells with different treatment groups, the supernatant of each group was collected at a designated time point and dying tumor cells were removed from the supernatant by centrifugation. Subsequently, HMGB1 in the supernatant of different treatment groups was quantified using the ELISA kit. Luminescent ATP Detection kit was used to analyze ATP content. All measurements are carried out according to the instructions of the respective manufacturers.
Generation and Maturity Evaluation of BMDCs
The details could be find in Supplemental Materials.
In vivo Prophylactic Tumor Vaccination
Tumor vaccine experiments are the gold standard for evaluating ICD. To investigate whether tumor cells treated with AuNPs combined with radiotherapy have potential in vivo immunogenicity, we conducted a tumor vaccine vaccination experiment in the Male BALB-c mice. The details could be find in Supplemental Materials.
Evaluation of ROS Production by AuNPs Combined with RT
The sodium terephthalate fluorescent probe was employed to examine the ROS production of the different groups. Fluorescence microscope and flow cytometry were used in this experiment. The details could be find in Supplemental Materials.
Statistical Analysis
The data are presented as the means ± standard deviations (SD) and GraphPad Prism version 7.0 software (GraphPad Software, Inc., San Diego, CA, USA) was used to analyze the data. The data from two groups were compared using Student’s t-test. The differences between the two groups were considered statistically significant at *P < 0.05, and very significant at **P < 0.01, ***P < 0.001 and ****P<0.0001.
Results
Preparation and Characterization of AuNPs
The sodium citrate reduction method was employed to prepare a solution of AuNPs. The solution exhibited a bright wine red color (Figure S1A). TEM images of the AuNPs are presented in Figure S1B, showing uniformly dispersed black spherical particles. The hydrodynamic diameter of the AuNPs was approximately 19.7 nm (Figure S1C). UV-vis absorption spectroscopy was used to characterize the prepared AuNPs, revealing a maximum absorption peak at 520 nm (Figure S1D). The Zeta potential of the AuNPs was determined to be −35.1 ± 0.66 mV (Figure S1E). The solution of AuNPs was stored at 4 °C, and the hydration particle size was measured every 5 days over a period of 4 weeks. As depicted in Figure S1F, no significant changes in particle size were observed. The uptake of AuNPs by G422 cells was examined using TEM. G422 cells were co-incubated with AuNPs for 24 h. As illustrated in Figure S2, the cytoplasm of G422 cells contained a large number of high-density staining particles, with numerous nanoparticles visible. These particles were mostly trapped in the membrane structures or vesicles, particularly near the endoplasmic reticulum (ER) and mitochondria (MT). The endoplasmic reticulum (ER) and mitochondria are important cellular organelles. Through electron microscopy, we investigated the distribution of gold nanoparticles (AuNP) within cells and their relationship with these cellular organelles. This experimental design aimed to provide a deeper understanding of the mechanisms by which AuNP enhance radiotherapy-induced immunogenic cell death (ICD). By utilizing electron microscopy, we could further elucidate the localization of AuNP within cells and determine whether they interact with the endoplasmic reticulum (ER) and mitochondria. This analysis will help to reveal the regulatory mechanisms of AuNP on ICD-related pathways, thereby enhancing our understanding of the role of AuNP. Additionally, the morphology of G422 cells did not significantly change after co-incubation with AuNPs, indicating that the activity of G422 cells remained unaffected. The safety of AuNPs on astrocyte and G422 cells was evaluated using the CCK-8 method. The results presented in Figure S3 demonstrated that more than 80% of cells remained viable after treatment with AuNPs at concentrations up to 8 nM, indicating that AuNPs exhibited low toxicity towards both astrocyte and G422 cells.
AuNPs Combined RT Induce Cell Death of Tumor Cells
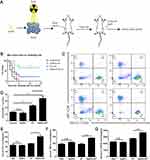
In order to assess the ability of AuNPs in combination with radiotherapy (RT) to induce immunogenic cell death (ICD), the appropriate concentration of AuNPs combined with a 6 Gy dose of RT on G422 cells was determined using CCK-8 kits. The killing efficiency of AuNPs combined with radiotherapy on G422 cells was found to increase as the concentration of AuNPs increased, in comparison to RT alone (Figure 1A). The concentration of IC50 was found to be approximately 8 nM. Based on this result, a concentration of 8 nM was selected for subsequent experiments. Annexin V/PI staining kit was used to detect cell apoptosis (Figure 1B), and the apoptosis rate of G422 cells treated with RT alone and AuNPs combined with RT were 13.43% ± 3.65% and 38.53 ± 2.97% respectively (Figure 1C). These findings indicate that the combination of AuNPs with a 6 Gy dose of RT is capable of enhancing the toxic effect of RT alone.
Evaluation of ICD Induction
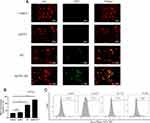
Despite the radio-sensitization effect, we also wondered whether AuNPs could enhance the ability of RT to trigger ICD. The occurrence of ICD in tumor cells was accompanied by the release of DAMPs during the dying process of tumor cells. When ICD occurs in tumor cells, CRT can be exposed on the surface of tumor cell membrane as an “eat me” signal to promote DCs or its precursor somatic cell cells to dying tumor cells, so as to provide rich antigenic substances and promote the maturation of DCs.23 Normally, CRT is expressed in the endoplasmic reticulum. CRT can be translocated and exposed to the surface of dying cell membrane under stress. Releasing “eat me” signals to immune cells and activating the immune response of the body. Furthermore, HMGB1 and ATP were released outside the tumor cells.13,24,25 To investigate the extent of treatment-induced immunogenic cell death (ICD), we employed confocal fluorescence microscopy and immunostaining analysis to detect the expression of cell surface calreticulin (CRT). The cell surface delineation was determined using Dil (red), while the expression of CRT was determined using Alexa Fluor 488-CRT antibody (green). Firstly, we found no green fluorescence signal in the AuNPs group, suggesting that AuNPs did not induce ICD. Secondly, we observed stronger CRT signals in the RT group and the AuNPs+RT group. However, the green fluorescence signal was stronger in the AuNPs+RT group compared to the RT group, indicating that AuNPs could enhance the ability of RT to trigger ICD (Figure 2A). Furthermore, semiquantitative analysis of the fluorescence signals using ImageJ software further supported these conclusions (Figure 2B). In addition, we used flow cytometry to analyze the expression of CRT on the dying tumor cell membrane surface. As shown in Figure 2C, the expression rate of CRT was significantly higher in the AuNPs+RT group than in the RT alone group. These findings indicate that combining AuNPs with RT can induce stronger ICD effects and promote the release of “eat me” signals in G422 cells.
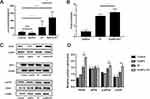
The phagocytosis of bone marrow-derived dendritic cells (BMDCs) on dying tumor cells can be stimulated by HMGB1, which is predominantly located in the nucleus of tumor cells. As illustrated in Figure 3A, the group treated with AuNPs in combination with radiation therapy (RT) exhibited the highest release of HMGB1 from the nucleus in G422 cells. In this group, the average concentration of HMGB1 released from G422 cells was 479.41 pg/mL, which was 2.2 times higher than that of the RT group. Additionally, ATP was released to a greater extent in the AuNPs + RT group, being 1.2 times higher than in the RT group (Figure 3B). Subsequent Western blot analysis (Figure 3C, D) further demonstrated that endoplasmic reticulum stress-related proteins (PERK, p-eIF2α, CHOP) were most abundantly expressed in G422 cells treated with the AuNPs + RT group. Moreover, the combination of RT and AuNPs resulted in markedly higher levels of reactive oxygen species (ROS) compared to radiation alone or AuNPs alone (Figure 4). The production of ROS can also trigger endoplasmic reticulum stress, indicating that the combined treatment of AuNPs and RT induces the strongest endoplasmic reticulum stress. Notably, endoplasmic reticulum stress is commonly considered as a facilitator of immunogenic cell death (ICD). Overall, the aforementioned findings demonstrate that AuNPs can effectively enhance radiation-induced ICD.
Activation and Maturation of BMDCs
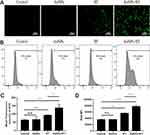
Flow cytometry was used to detect the maturation rate of BMDCs (CD11c+CD86+CD80+)(Figure 5). After incubation with untreated G422 cells for 24 h, the maturation rate of BMDCs was low due to the low immunogenicity of tumor cells. When BMDCs were incubated with RT-pretreated G422 cells, the maturation rate of BMDCs increased about 1.6-fold compared to that of BMDCs incubated with untreated G422 cells. G422 cells pretreated with AuNPs + RT showed the strongest ability to induce maturation rate of BMDCs additionally. The maturation rate of BMDCs further promoted 2.1-fold.
Tumor Cells Treated with AuNPs Combined RT are Effective Vaccines in vivo
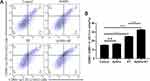
A well-established tumor vaccination experiment was carried out in BALB-c mice to investigate the ability of cancer cells treated with AuNPs combined RT to activate the adaptive immune system (Figure 6A). We immunized BALB-c mice with G422 cells that were dying after four different treatments. Mice immunized with G422 cells treated with AuNPs combined with RT showed strong signs of robust activation from the adaptive immune system and the strongest protection against tumor growth. However, there was tumor growth in most of the mice immunized with PBS or AuNPs treated alone (Figure 6B).Furthermore, some mice immunized with RT treated alone also have tumor growth, which points to the strong immunogenicity of tumor cells treated with AuNPs combined with RT. To investigate whether the anti-tumor immune response of the body could be stimulated after vaccination, the spleens of mice in all groups were harvested and examined for Cytotoxic T lymphocyte (CTL, CD3 + CD8 + CD4 −), which are more beneficial for killing tumor tissue. As shown in Figure 6C and D, after immunized with G422 cells treated by AuNPs combined with RT, the percentage of CTL cells in the spleen was significantly higher than that spleens from mice immunized with only RT (32.53±0.91% vs 25.03±0.49%). Collectively, these results indicated that the combination of AuNPs and RT could promote systemic antitumor immunity for tumor suppression. ELISA kits were also used to detect the levels of IFN-γ, TNF-α, and IL-12/p40 in the serum of mice in each group. The level of IFN-γ in AuNPs+RT group is 206.29±28.45 pg/mL, which is significantly higher than PBS group (117.52±18.82pg/mL), RT group (133.96±4.55pg/mL), and AuNPs group (115.54±7.45pg/mL)(Figure 6E). In addition, we also measured the levels of the IL-12p40 and TNF- α in the serum of each group. As shown in Figure 6F and G, the combination of AuNPs and RT resulted in an enhanced response of immune (compared to the PBS group, both cytokine levels were significantly increased). These results demonstrate the strong immune protective effect induced by the combination of AuNPs and RT.
Discussion
Radiotherapy is a frequently employed anticancer treatment in clinical settings. Its primary therapeutic mechanism involves the irradiation of the body with various rays, which ionize or excite water molecules, resulting in the production of reactive oxygen. This subsequently leads to the disruption of molecular bonds, such as DNA and RNA, in the cell. Ultimately, this process directly or indirectly leads to cell death.26,27 The production of reactive oxygen species can also induce stress on the endoplasmic reticulum, leading to the release of damage-associated molecular patterns (DAMPs) and ultimately causing immunogenic cell death in tumor cells.28–30 Gold nanoparticles have been extensively demonstrated to be effective radiosensitizers. However, the potential of these nanoparticles to enhance the ability of radiotherapy-induced immunogenic cell death (ICD) in tumor cells remains unreported. Therefore, this study aims to investigate whether the combination of AuNPs with radiotherapy exhibits a stronger ICD effect compared to the use of radiotherapy alone.
The findings presented in this study confirm that the combination of radiation therapy (RT) with gold nanoparticles (AuNPs) can induce a stronger effect of immunogenic cell death (ICD) on glioblastoma cells compared to the use of RT alone. The immunogenicity of tumor cells killed by the combination of AuNPs and RT has been demonstrated in vitro. These tumor cells, when treated with AuNPs along with RT, result in the release of crucial damage-associated molecular patterns (DAMPs) such as CRT, HMGB1, and ATP. In this study, we also measured the expression levels of endoplasmic reticulum stress-related proteins after different group treatments. We found that the addition of gold nanoparticles can further enhance the expression levels of endoplasmic reticulum stress-related proteins. Specifically, there was no significant difference in the expression of non-phosphorylated eIF2α between the single radiotherapy group and the combined radiotherapy with gold nanoparticles group, but the combined radiotherapy with gold nanoparticles group significantly increased the expression of phosphorylated eIF2α (p-eIF2α). This result suggests that gold nanoparticles may promote further phosphorylation of the eIF2α protein during radiotherapy. Moreover, when these dying tumor cells are co-cultured with bone marrow mesenchymal stem cells, they are found to induce the maturation of BMDCs. Compared to the group treated with single radiotherapy, the tumor cells treated with the combination of AuNPs and radiotherapy demonstrate a higher proportion of BMDCs maturation rate. It is important to note that mature BMDCs play a significant role in inducing the generation of cytotoxic lymphocytes (CTL).31–33 The results of the tumor vaccine experiment demonstrated that the application of AuNPs in combination with radiotherapy effectively suppressed the growth of tumors in mice when the treated dying tumor cells were subsequently attacked by living tumor cells. Additionally, the immunization of mice with G422 cells treated by AuNPs combined with RT resulted in a higher percentage of CTL cells in the spleen compared to mice immunized with RT alone. This suggests that AuNPs possess the capacity to enhance RT-induced ICD. Recent research has also indicated that the direct injection of dying necroptotic tumor cells into the tumor bed leads to a more efficient control of tumor growth.34 Therefore, it would be an interesting thing to analyze whether tumor cells treated with AuNPs combined with RT can be used as a vaccine in mice with established tumors. However, there are potential limitations in this study: we used subcutaneous vaccination model to evaluate the ability of AuNPs combined with radiotherapy to induce ICD, making it more susceptible to immunotherapy. In fact, due to the existence of the blood-brain barrier, a small number of cytotoxic T lymphocytes can infiltrate into intracranial tumor tissue.35 In the future, we will focus on improving experimental methods and providing more directions for immune-related therapy of glioblastoma.
Conclusion
The combination of AuNPs and RT could induce the eversion of calreticulin to the cell membrane in dying cells. In addition, the combination of AuNPs and RT could promote the release of HMGB1 and ATP from tumor cells and the maturation of BMDCs could also be promoted by AuNPs combined whit RT. Using dying cancer cells induced by AuNPs combined with RT, the efficient vaccination potential of ICD is demonstrated in vivo. These results indicate that AuNPs have the ability to enhance RT-induced ICD.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All animal procedures were performed according to the Institutional Animal Care & Use Committee at the Southeast University. All experiments in this study strictly adhere to the relevant laws, regulations, and standards concerning laboratory animals in China, including the “Regulations on the Management of Laboratory Animals” (revised on March 1, 2017) and the “Guidelines for the Ethical Review of Laboratory Animal Welfare” (GB/T 35892-2018).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Changzhou Sci & Tech Program (Grant No. CJ20235086); Changzhou Applied Basic Research Program (CJ20210080); National Natural Science Foundation of China (Grant No. 82272031); the high level talents (333 Project) of Jiangsu Province (Grant No. BRA2019024).
Disclosure
The authors declare no competing interest in this work.
References
1. Alifieris C, Trafalis DT. Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther. 2015;152:63–82. doi:10.1016/j.pharmthera.2015.05.005
2. Xu HL, Yang JJ, ZhuGe DL, et al. Glioma-Targeted Delivery of a Theranostic Liposome Integrated with Quantum Dots, Superparamagnetic Iron Oxide, and Cilengitide for Dual-Imaging Guiding Cancer Surgery. Adv Healthc Mater. 2018;7(9):e1701130. doi:10.1002/adhm.201701130
3. Janic B, Brown SL, Neff R, et al. Therapeutic enhancement of radiation and immunomodulation by gold nanoparticles in triple negative breast cancer. Cancer Biol Ther. 2021;22(2):124–135. doi:10.1080/15384047.2020.1861923
4. Minniti G, Niyazi M, Alongi F, Navarria P, Belka C. Current status and recent advances in reirradiation of glioblastoma. Radiat Oncol. 2021;16(1):36. doi:10.1186/s13014-021-01767-9
5. Her S, Jaffray DA, Allen C. Gold nanoparticles for applications in cancer radiotherapy: mechanisms and recent advancements. Adv Drug Deliv Rev. 2017;109:84–101. doi:10.1016/j.addr.2015.12.012
6. Elleaume H, Barth RF, Rousseau J, et al. Radiation therapy combined with intracerebral convection-enhanced delivery of cisplatin or carboplatin for treatment of the F98 rat glioma. J Neurooncol. 2020;149(2):193–208. doi:10.1007/s11060-020-03600-x
7. Xie J, Gong L, Zhu S, Yong Y, Gu Z, Zhao Y. Emerging Strategies of Nanomaterial-Mediated Tumor Radiosensitization. Adv Mater. 2019;31(3):e1802244. doi:10.1002/adma.201802244
8. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi:10.1038/nri.2016.107
9. Demaria S, Golden EB, Formenti SC. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1(9):1325–1332. doi:10.1001/jamaoncol.2015.2756
10. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi:10.1084/jem.20052494
11. Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi:10.1038/nm1622
12. Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–1701. doi:10.1084/jem.20050915
13. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875. doi:10.1038/nrc3380
14. Zhou Y, Nan P, Li C, et al. Upregulation of MTA1 in Colon Cancer Drives A CD8(+) T Cell-Rich But Classical Macrophage-Lacking Immunosuppressive Tumor Microenvironment. Front Oncol. 2022;12:825783. doi:10.3389/fonc.2022.825783
15. Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi:10.1038/nm1523
16. Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–1178. doi:10.1038/nm.2028
17. Ren H, Yong J, Yang Q, et al. Self-assembled FeS-based cascade bioreactor with enhanced tumor penetration and synergistic treatments to trigger robust cancer immunotherapy. Acta Pharm Sin B. 2021;11(10):3244–3261. doi:10.1016/j.apsb.2021.05.005
18. Voron T, Marcheteau E, Pernot S, et al. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. doi:10.3389/fonc.2014.00070
19. Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. 2020;21(2):120–134. doi:10.1038/s41590-019-0561-4
20. Chithrani DB, Jelveh S, Jalali F, et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat Res. 2010;173(6):719–728. doi:10.1667/RR1984.1
21. Rippel RA, Seifalian AM. Gold revolution--gold nanoparticles for modern medicine and surgery. J Nanosci Nanotechnol. 2011;11(5):3740–3748. doi:10.1166/jnn.2011.4170
22. Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science. 1997;277(5329):1078–1081. doi:10.1126/science.277.5329.1078
23. Dong X, Cheng R, Zhu S, et al. A Heterojunction Structured WO2.9-WSe2 Nanoradiosensitizer Increases Local Tumor Ablation and Checkpoint Blockade Immunotherapy upon Low Radiation Dose. ACS Nano. 2020;14(5):5400–5416. doi:10.1021/acsnano.9b08962
24. Yamazaki T, Hannani D, Poirier-Colame V, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2014;21(1):69–78. doi:10.1038/cdd.2013.72
25. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31(1):51–72. doi:10.1146/annurev-immunol-032712-100008
26. Farzin L, Sheibani S, Moassesi ME, Shamsipur M. An overview of nanoscale radionuclides and radiolabeled nanomaterials commonly used for nuclear molecular imaging and therapeutic functions. J Biomed Mater Res A. 2019;107(1):251–285. doi:10.1002/jbm.a.36550
27. Salih S, Alkatheeri A, Alomaim W, Elliyanti A. Radiopharmaceutical Treatments for Cancer Therapy, Radionuclides Characteristics, Applications, and Challenges. Molecules. 2022;27(16):5231. doi:10.3390/molecules27165231
28. Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23(4):487–500. doi:10.1038/s41590-022-01132-2
29. Fucikova J, Kepp O, Kasikova L, et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020;11(11):1013. doi:10.1038/s41419-020-03221-2
30. Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14(12):2994–3006. doi:10.1002/1878-0261.12851
31. Knochelmann HM, Dwyer CJ, Bailey SR, et al. When worlds collide: th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. 2018;15(5):458–469. doi:10.1038/s41423-018-0004-4
32. Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234(6):8509–8521. doi:10.1002/jcp.27782
33. Wang B, Hu S, Fu X, Li L. CD4(+) Cytotoxic T Lymphocytes in Cancer Immunity and Immunotherapy. Adv Biol. 2023;7(4):e2200169. doi:10.1002/adbi.202200169
34. Turubanova VD, Balalaeva IV, Mishchenko TA. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J Immunother Cancer. 2019;7(1):350. doi:10.1186/s40425-019-0826-3
35. He C, Ding H, Chen J, et al. Immunogenic Cell Death Induced by Chemoradiotherapy of Novel pH-Sensitive Cargo-Loaded Polymersomes in Glioblastoma. Int J Nanomedicine. 2021;16:7123–7135. doi:10.2147/IJN.S333197
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.