Back to Journals » Clinical Interventions in Aging » Volume 18
GNRI, PLR and Stroke-Associated Pneumonia: From Association to Development of a Web-Based Dynamic Nomogram
Authors Wang C, Jiang X, Wu D, Ge M, Deng L
Received 1 August 2023
Accepted for publication 7 November 2023
Published 17 November 2023 Volume 2023:18 Pages 1893—1904
DOI https://doi.org/10.2147/CIA.S433388
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Chunqing Wang, Xiaoyao Jiang, Di Wu, Mengjun Ge, Li Deng
Department of General Practice, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, 221000, People’s Republic of China
Correspondence: Chunqing Wang, Department of General Practice, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, 221000, People’s Republic of China, Email [email protected]
Objective: Discussing the relationship between geriatric nutritional risk index (GNRI) and platelet-to-lymphocyte ratio (PLR) on stroke-associated pneumonia (SAP) in acute ischemic stroke (AIS) patients, developing and validating a web-based dynamic nomogram.
Methods: A total of 996 AIS patients admitted to the Department of General Medicine and Neurology at Xuzhou Medical University Affiliated Hospital were collected. They were divided into Non-SAP group and SAP group based on the occurrence of SAP. The data was randomly divided into training set and validation set in a ratio of 7:3. LASSO regression and multivariable logistic regression analysis were used to screen for independent risk factors and develop a dynamic nomogram. Area under the receiver operating characteristic curve (AUC-ROC), calibration curve, and decision curve analysis (DCA) curve were used to validate the model’s discriminative ability, calibration, and clinical value, respectively.
Results: Among AIS patients, a total of 221 cases (22.19%) developed SAP. Age, NIHSS score, comorbid atrial fibrillation, dysphagia, PLR, and GNRI were identified as independent factors influencing the occurrence of SAP in AIS patients. A web-based dynamic nomogram was developed based on these six variables. The training set showed an AUC-ROC of 0.864 (95% CI: 0.828– 0.892), while the validation set showed an AUC-ROC of 0.825 (95% CI: 0.772– 0.882), indicating good predictive ability and discrimination of the model. The calibration curve demonstrated good calibration of the model, and the DCA curve showed its clinical value. This model can be accessed and utilized by anyone on the website (https://moonlittledoctor.shinyapps.io/ANADPG/).
Conclusion: PLR and GNRI are independent factors influencing the occurrence of SAP in AIS patients, and a dynamic nomogram was constructed to predict the risk of SAP in AIS patients. It can guide clinical decision-making and improve patient prognosis.
Keywords: GNRI, PLR, dynamic nomogram, acute ischemic stroke, stroke-associated pneumonia
Introduction
Acute ischemic stroke (AIS) is an acute brain dysfunction and a leading cause of death and disability worldwide, with an increasing incidence rate of approximately 8.7% per year.1,2 AIS is characterized by high disability rate, high mortality rate, high recurrence rate, and multiple complications. It is often difficult to be promptly detected, and the effective treatment window is short, resulting in poor prognosis for AIS patients.3 Stroke-associated pneumonia (SAP) is a common complication occurring within 7 days after stroke, with reported incidence rates ranging from 7% to 38% among stroke patients.4 Studies have shown that SAP not only severely affects the neurological recovery of AIS patients but also increases mortality rate and prolongs hospital stay, imposing a significant economic burden on the healthcare system.5 Therefore, accurately identifying the risk factors for SAP in AIS patients and timely intervention are crucial for patient prognosis.
Some studies have indicated that the decreased immune function induced by stroke makes patients more susceptible to infections, and immune suppression is also an important mechanism for SAP.6 Therefore, biomarkers that mediate immune changes and systemic inflammatory responses may be related to the occurrence of SAP. The geriatric nutritional risk index (GNRI) is an indicator for assessing the nutritional status of elderly individuals. It evaluates their nutritional risk by considering changes in body weight and the degree of appetite decline.7 Research has shown that GNRI is associated with the occurrence of SAP, and its predictive value for SAP contributes to early intervention and treatment.8 Platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR), as indicators reflecting inflammatory response, have been used for prognostic evaluation in various cancer patients. Previous studies have demonstrated that high PLR and NLR have predictive value for SAP in AIS patients, and can help identify high-risk patients in a timely manner, providing clues for treatment decisions.9–11
The dynamic nomogram is a graphical tool based on websites that allows for the evaluation of the risk of disease diagnosis and prognosis based on relevant risk factors.12 Compared to traditional scoring systems or nomograms, it possesses greater visual appeal and facilitates the rapid and accurate acquisition of predictive results. By analyzing and modeling patient-related data, we create dynamic nomogram, a visualization tool that not only helps us better understand and analyze the patient’s condition but also predicts the risk of SAP occurrence in AIS patients, thereby providing a better basis for medical team decision-making. Previous studies have shown that nomograms can be used to predict stroke-related infections and SAP, aiding in guiding clinical decisions and improving patient prognosis.13,14 Therefore, the purpose of this study is to construct and validate a dynamic nomogram based on GRNI and NLR to predict the risk of SAP occurrence in AIS patients, enhancing the care and management of AIS patients, reducing the incidence of complications, and improving the quality of life for patients.
Materials and Methods
Study Population and Data Source
This study is based on the electronic medical record system of Xuzhou Medical University Affiliated Hospital. The study selected hospitalized patients diagnosed with AIS from January 2018 to December 2022 at Xuzhou Medical University Affiliated Hospital.
The inclusion criteria are as follows: (1). Age ≥ 18; (2). AIS patients who meet the definition of AIS according to the World Health Organization’s standards;15 (3). Complete medical records and admission within 7 days after stroke onset. The exclusion criteria are as follows: (1). Stroke onset time greater than 7 days; (2). Active infection within 2 weeks prior to admission; (3). Use of mechanical ventilation; (4). History of cancer, hematological disorders, or use of immunosuppressive agents; (5). Severe liver or kidney disease; (6). Swallowing difficulties or long-term nasogastric tube placement due to other reasons; (7). Patients with missing or lost follow-up data.
Clinical Endpoints
According to the revised standards of the Centers for Disease Control and Prevention, patients diagnosed with SAP are those who experience lower respiratory tract infections within the first seven days following an acute stroke.4
Data Collection
Through literature review, we have gathered a total of 38 potential risk factors that may be associated with SAP in AIS patients.16–18 These factors include demographic data (age, gender, body mass index (BMI), blood pressure), medical history (smoking history, alcohol consumption, hypertension, diabetes, hyperlipidemia, atrial fibrillation, dysphagia, etc.), hematological indicators (neutrophils, lymphocytes, monocytes, platelets, blood glucose, blood lipids, etc.), as well as relevant scores (national institutes of health stroke (NIHSS), NLR, PLR, lymphocytes-to-monocytes ratio (LMR), GNRI (MLR), GNRI).
The specific calculation methods are as follows: NLR = neutrophils/lymphocytes, PLR = platelets/lymphocytes, LMR = lymphocytes/monocytes, MLR = monocytes/lymphocytes, GNRI = 1.489 × serum albumin (g/L) + 41.7 × admission weight (kg)/ideal weight (kg).
Data Statistics
This study employed R version 3.6.4 and SPSS version 22.0 for subsequent statistical analysis of the collected data from AIS patients. For normally distributed continuous variables, the mean ± standard deviation (x ̅±S) was used to represent the data, while for non-normally distributed patient data, the median (M) and interquartile range (P25, P75) were used. For categorical variables, frequency or percentage (%) was used. Different data indicators between two groups (non-SAP group vs SAP group, training set vs validation set) were compared. For normally distributed continuous variables, independent samples T-test was used for intergroup comparisons; for non-normally distributed continuous variables, non-parametric tests were used. Chi-square test was used for intergroup comparisons of categorical variables. The significance level was set at α=0.05, and P<0.05 was considered statistically significant. The least absolute shrinkage and selection operator (LASSO) regression and multiple logistic regression analysis was used to select independent predictors for SAP. Based on the relative weights of each risk factor, a clinical prediction model was constructed and a dynamic nomogram was drawn. The model’s discriminatory ability was evaluated using receiver operating characteristic (ROC) curve and the area under the curve (AUC) was calculated. In addition, the clinical prediction model was internally validated using the Bootstrap resampling method with B=1000 repetitions, and a calibration curve was drawn. Finally, the clinical validity of the prediction model was assessed using decision clinic curve (DCA) analysis.
Results
Baseline Characteristics
This study included a total of 1086 AIS patients. After exclusion according to the exclusion criteria, the final number of AIS patients was 996, see Figure 1. Based on the occurrence of SAP within 7 days of admission, they were divided into the non-SAP group (n=775) and the SAP group (n=221). Vertebrobasilar cerebral infarction is known for its acute onset, severe symptoms, and poor prognosis. In this study, we identified a total of 164 patients with this type of stroke, accounting for 16.5% of the sample, which is consistent with previous research findings.19 We conducted a 3-month follow-up study to assess the early outcomes of AIS patients in two groups. Among the SAP group patients, 51 (23.1%) cases resulted in death, of which 26 were due to neurological causes. In the non-SAP group, 25 (3.2%) cases resulted in death, with 15 being caused by neurological reasons.
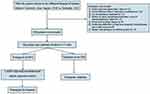 |
Figure 1 Flow chart of patient selection included in this study. Abbreviation: AIS, acute ischemic stroke. |
As indicated in Table 1, we first compared the data of these two groups of patients and found that patients in the SAP group had higher values of age, systolic blood pressure (SBP), NIHSS score, and PLR, while they had lower values of weight, BMI, hemoglobin, albumin, and GNRI. The probability of having hypertension, diabetes, atrial fibrillation (AF), swallowing difficulties, and a history of stroke was higher in the SAP group. The differences in these variables between the two groups were statistically significant (P<0.05).
 |
Table 1 Comparison of Baseline Data Between Non-SAP and SAP Groups |
Clinical Features of the Training Set and Validation Set
In order to prevent overfitting in the analysis of influencing factors, the included 996 AIS patients were randomly divided into a training set (n=697) and a validation set (n=299) in a 7:3 ratio. Comparing the data between the two groups, we found that, except for RBC, there were no statistically significant differences in the remaining variables (P≥0.05), as demonstrated in Table 2. This indicates that our data set partitioning is reasonable and comparable.
 |
Table 2 Comparison of Baseline Data Between Training Set and Validation Set |
LASSO Regression and Multivariate Logistic Analyses
The results of LASSO regression indicate that age, NIHSS score, comorbid AF, dysphagia, albumin, PLR, and GNRI are factors influencing the occurrence of SAP in AIS patients, as shown in Figure 2. We included these seven variables in a multiple logistic regression analysis and found that the model had a sensitivity of 46.7%, specificity of 95.8%, positive predictive value of 75.3%, and negative predictive value of 86.8%. Age (odds ratio (OR): 1.051; 95% confidence interval (CI): 1.024–1.079), NIHSS score (OR:1.397; 95% CI:1.277–1.529), comorbid AF (OR:1.717; 95% CI:1.059–2.784), dysphagia (OR:3.220; 95% CI:1.931–5.367), PLR (OR:1.003; 95% CI:1.000–1.006), and GNRI (OR:0.913; 95% CI:0.878–0.949) are independent factors influencing the occurrence of SAP in AIS patients.
Development and Verification of a Web-Based Dynamic Nomogram
According to the relative weights of the independent risk factors influencing the occurrence of SAP in AIS patients in Table 3, a web-based dynamic nomogram as shown in Figure 3 is plotted. This graphical tool allows for the summation of scores for each risk factor to obtain a total score, which can then be converted into a predictive probability.
 |
Table 3 Multivariate Logistic Analysis for the SAP |
 |
Figure 3 Web-based dynamic nomogram used for predicting SAP in patients with AIS. Input participants age, NIHSS score, comorbid AF, dysphagia, PLR, and GNRI at https://moonlittledoctor.shinyapps.io/ANADPG/, user can get the corresponding probability of SAP. (A) Input page: Enter the patient’s information according to the relevant variables on this page. (B) Graphical summary: This page shows the probability of SAP and its 95% confidence interval in AIS patients. (C) Numerical summary: Display the specific values of the patient’s indicators and predicted outcomes. Abbreviations: SAP, stroke-associated pneumonia; AIS, acute ischemic stroke; NIHSS, national institutes of health stroke; AF, atrial fibrillation; PLR, platelet-to-lymphocyte ratio; GNRI, geriatric nutritional risk index. |
To validate the developed predictive model, ROC curves were first plotted for both the training and validation sets. The AUC-ROC for the training set was 0.864 (95% CI: 0.828–0.892), while for the validation set it was 0.825 (95% CI: 0.772–0.882). This indicates a good discriminatory ability of the model, as shown in Figure 4. Subsequently, using the Bootstrap resampling method with 1000 repetitions, calibration curves were separately plotted for the training and validation sets. The results suggest good consistency between the predicted probabilities outputted by the model and the actual occurrence probabilities, indicating good model calibration, as depicted in Figure 5. Finally, to assess the clinical utility of the model, DCA curves were plotted. The DCA curves demonstrate that the net benefits for both the training and validation sets are significantly higher than the two extremes, indicating good clinical value (see Figure 6).
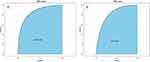 |
Figure 4 ROC curves of dynamic nomogram based on the data of the training set (A) and validation set (B). Abbreviation: AUC, the area under the receiver-operating characteristic. |
 |
Figure 5 Calibration curve of the dynamic nomogram based on the data of training set (A) and validation set (B). |
 |
Figure 6 Decision curve analysis curve of the dynamic nomogram based on the data of training set (A) and validation set (B). |
Discussion
Stroke is the second leading cause of death worldwide, with a high rate of disability. It is the primary cause of permanent disability in adults. Approximately 85% of stroke cases are ischemic strokes, and the incidence is increasing globally.20,21 Pneumonia is a common complication in AIS patients. Studies have shown that 15.6% of stroke patients develop acute lung injury within 36 hours of hospital admission, and between 5% and 22% of patients develop pneumonia or bronchitis during their hospital stay.22 This is consistent with the findings of our study, where 221 cases (22.19%) of SAP were observed. Small artery occlusive stroke, also known as lacunar infarction, has been shown to have a better prognosis compared to other subtypes of stroke, and SAP is less frequent in lacunar infarction, which is consistent with our findings.23 In this retrospective study, we identified age, NIHSS score, comorbid AF, dysphagia, PLR, and GNRI as independent risk factors for SAP in AIS patients. We developed a simple and accurate web-based dynamic nomogram, which was internally validated.
Age is a significant risk factor for SAP in patients, as their immune function and lung defense capabilities decrease with age, making them more susceptible to infections. Studies have shown a significant correlation between higher age and the occurrence of SAP, which is consistent with the findings of this study.24,25 The NIHSS score is an indicator used to assess the severity of stroke, with a higher score indicating more severe brain damage. Research has indicated that AIS patients with higher NIHSS scores are at a greater risk of developing SAP.25,26 We consider the following factors to be related: (i). Patients with higher NIHSS scores exhibit more pronounced immune suppression, leading to increased susceptibility to infections. (ii). Higher NIHSS scores suggest more severe limb paralysis in patients, resulting in prolonged bed rest and an increased chance of developing aspiration pneumonia, leading to a higher incidence of SAP. The analysis of this study’s results indicates that the coexistence of AF and dysphagia is an independent risk factor for SAP in AIS patients. This finding is consistent with previous research and holds significant clinical implications.17,18,25,27–29 Firstly, for stroke patients, especially those with AF or dysphagia, enhanced preventive measures for AIS should be implemented to reduce its incidence. Secondly, AIS patients with AF or dysphagia should be closely monitored for the occurrence of pneumonia and timely interventions should be taken to improve prognosis.
PLR, as an independent prognostic factor for SAP, holds significant clinical significance. It serves as a biomarker reflecting thrombosis formation and inflammatory response, with higher values potentially indicating more severe inflammation.30 Studies have shown that PLR plays a crucial role in the occurrence and prognosis of SAP, with higher PLR values being associated with higher incidence and severity, suggesting compromised immune function and enhanced inflammatory response in patients.10,31,32 Therefore, monitoring PLR allows for early identification of high-risk patients and implementation of corresponding intervention measures. GNRI is used to assess the nutritional status and risk of malnutrition in elderly patients. It takes into account serum albumin levels and body weight, providing a comprehensive indicator of nutritional status. Studies have shown that lower GNRI scores are associated with increased risk and poorer prognosis of SAP.8 This suggests that malnutrition and impaired immune function may contribute to the occurrence and severity of pneumonia in stroke patients.33 Therefore, introducing GNRI assessment in clinical practice can help identify high-risk individuals and guide appropriate nutritional interventions to improve the prognosis of this vulnerable population.
The study has some limitations. Firstly, this study is a retrospective study, which may result in missing values for variables such as IL-6, as some data were missing (>30%). Secondly, we did not collect data on long-term mortality and prognosis outcomes after discharge, therefore we are unable to assess the relationship between GNRI and PLR with long-term outcomes or mortality rates in AIS patients. Thirdly, this study is a single-center study and external validation is needed.
Conclusion
This study found that PLR and GNRI are independent factors influencing the occurrence of SAP in AIS patients, and a dynamic nomogram was constructed to predict the risk of SAP in AIS patients. It can guide clinical decision-making and improve patient prognosis.
We hope to further investigate the impact of novel hematological markers, new drugs, and novel inflammation indices on the occurrence of SAP in AIS patients through multicenter, prospective study in the future. This will help improve prediction models and enhance patient prognosis.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Approval and Informed Consent
The study obtained approval from the Ethics Committee of Xuzhou Medical University Affiliated Hospital (Approval No. XYFY2022-KL241-01). As this study is a single-center retrospective study, the review committee waived the requirement for written informed consent. Patient confidential data was removed from the entire dataset prior to analysis. The study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Applied Basic Research Project of Xuzhou to [grant number: XWKYHT20200058].
Disclosure
The authors declare that they have no competing interests.
References
1. Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6(2):182–187. doi:10.1016/S1474-4422(07)70031-5
2. Zhao D, Liu J, Wang W, et al. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing Project. Stroke. 2008;39(6):1668–1674. doi:10.1161/STROKEAHA.107.502807
3. Feigin VL, Nguyen G, Cercy K, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379(25):2429–2437.
4. Smith CJ, Kishore AK, Vail A, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in Stroke Consensus Group. Stroke. 2015;46(8):2335–2340. doi:10.1161/STROKEAHA.115.009617
5. Tinker RJ, Smith CJ, Heal C, et al. Predictors of mortality and disability in stroke-associated pneumonia. Acta Neurol Belg. 2021;121(2):379–385. doi:10.1007/s13760-019-01148-w
6. Westendorp WF, Dames C, Nederkoorn PJ, Meisel A. Immunodepression, infections, and functional outcome in ischemic stroke. Stroke. 2022;53(5):1438–1448. doi:10.1161/STROKEAHA.122.038867
7. Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783. doi:10.1093/ajcn/82.4.777
8. Dai C, Yan D, Xu M, Huang Q, Ren W. Geriatric nutritional risk index is related to the risk of stroke-associated pneumonia. Brain Behav. 2022;12(8):e2718. doi:10.1002/brb3.2718
9. Nam KW, Kim TJ, Lee JS, et al. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. 2018;49(8):1886–1892. doi:10.1161/STROKEAHA.118.021228
10. Zawiah M, Hayat Khan A, Abu Farha R, Usman A, Bitar AN. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio, and platelet-lymphocyte ratio in stroke-associated pneumonia: a systematic review and meta-analysis. Curr Med Res Opin. 2023;39(3):475–482. doi:10.1080/03007995.2023.2174327
11. Sung KL, Kuo MJ, Hsieh CY, Sung SF. High neutrophil-to-lymphocyte ratio predicts one-year risk of pneumonia post-stroke discharge. Cerebrovasc Dis. 2023;23:1–8.
12. Park SY. Nomogram: an analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg. 2018;155(4):1793. doi:10.1016/j.jtcvs.2017.12.107
13. Lan Y, Sun W, Chen Y, et al. Nomogram including neutrophil-to-lymphocyte ratio for the prediction of stroke-associated infections. Front Neurol. 2020;11:574280. doi:10.3389/fneur.2020.574280
14. Song X, He Y, Bai J, Zhang J. A nomogram based on nutritional status and A2DS2 score for predicting stroke-associated pneumonia in acute ischemic stroke patients with type 2 diabetes mellitus: a retrospective study. Front Nutr. 2022;9:1009041. doi:10.3389/fnut.2022.1009041
15. Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20(10):1407–1431. doi:10.1161/01.STR.20.10.1407
16. Beloborodov VA, Stepanov IA, Scherbatykh AV, et al. Факторы риска неблагоприятных клинических исходов у больных пожилого и старческого возраста с инсульт-ассоциированной пневмонией [Risk factors of adverse clinical outcomes in the elderly and senile patients with stroke-associated pneumonia]. Adv Gerontol. 2021;34(4):586–591. Russian.
17. Eltringham SA, Kilner K, Gee M, et al. Impact of dysphagia assessment and management on risk of stroke-associated pneumonia: a systematic review. Cerebrovasc Dis. 2018;46(3–4):99–107. doi:10.1159/000492730
18. Hoffmann S, Harms H, Ulm L, et al. Stroke-induced immunodepression and dysphagia independently predict stroke-associated pneumonia - The PREDICT study. J Cereb Blood Flow Metab. 2017;37(12):3671–3682. doi:10.1177/0271678X16671964
19. Schulz UG, Fischer U. Posterior circulation cerebrovascular syndromes: diagnosis and management. J Neurol Neurosurg Psychiatry. 2017;88(1):45–53. doi:10.1136/jnnp-2015-311299
20. Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517–584. doi:10.1161/STR.0b013e3181fcb238
21. Haq S, Mathur M, Singh J, Kaur N, Sibia RS, Badhan R. Colour Doppler evaluation of extracranial carotid artery in patients presenting with acute ischemic stroke and correlation with various risk factors. J Clin Diagn Res. 2017;11(3):Tc01–tc5. doi:10.7860/JCDR/2017/25493.9541
22. Yoo AJ, Sheth KN, Kimberly WT, et al. Validating imaging biomarkers of cerebral edema in patients with severe ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22(6):742–749. doi:10.1016/j.jstrokecerebrovasdis.2012.01.002
23. Rudilosso S, Rodríguez-Vázquez A, Urra X, Arboix A. The potential impact of neuroimaging and translational research on the clinical management of lacunar stroke. Int J Mol Sci. 2022;23(3):1497. doi:10.3390/ijms23031497
24. Cieplik F, Wiedenhofer AM, Pietsch V, et al. Oral health, oral microbiota, and incidence of stroke-associated pneumonia-a prospective observational study. Front Neurol. 2020;11:528056. doi:10.3389/fneur.2020.528056
25. Huang GQ, Lin YT, Wu YM, Cheng QQ, Cheng HR, Wang Z. Individualized prediction of stroke-associated pneumonia for patients with acute ischemic stroke. Clin Interv Aging. 2019;14:1951–1962. doi:10.2147/CIA.S225039
26. Zhao D, Zhu J, Cai Q, Zeng F, Fu X, Hu K. The value of diffusion weighted imaging-alberta stroke program early CT score in predicting stroke-associated pneumonia in patients with acute cerebral infarction: a retrospective study. PeerJ. 2022;10:e12789. doi:10.7717/peerj.12789
27. Zhang SY, Huang J, Zhou XL. A meta-analysis of the risk factors for stroke-associated pneumonia. JCPSP. 2023;33(7):799–803.
28. Chang MC, Choo YJ, Seo KC, Yang S. The relationship between dysphagia and pneumonia in acute stroke patients: a systematic review and meta-analysis. Front Neurol. 2022;13:834240. doi:10.3389/fneur.2022.834240
29. Eltringham SA, Kilner K, Gee M, et al. Factors associated with risk of stroke-associated pneumonia in patients with dysphagia: a systematic Review. Dysphagia. 2020;35(5):735–744. doi:10.1007/s00455-019-10061-6
30. Tang X, Cao Y, Liu J, Wang S, Yang Y, Du P. Diagnostic and predictive values of inflammatory factors in pathology and survival of patients undergoing total cystectomy. Mediators Inflamm. 2020;2020:9234067. doi:10.1155/2020/9234067
31. Li W, He C. Association of platelet-to-lymphocyte ratio with stroke-associated pneumonia in acute ischemic stroke. J Healthc Eng. 2022;2022:1033332. doi:10.1155/2022/1033332
32. Yu T, Liu H, Liu Y, Jiang J. Inflammatory response biomarkers nomogram for predicting pneumonia in patients with spontaneous intracerebral hemorrhage. Front Neurol. 2022;13:1084616. doi:10.3389/fneur.2022.1084616
33. Akimoto T, Hara M, Morita A, Uehara S, Nakajima H. Relationship between nutritional scales and prognosis in elderly patients after acute ischemic stroke: comparison of controlling nutritional status score and geriatric nutritional risk index. Ann Nutr Metab. 2021;77(2):116–123. doi:10.1159/000515212
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

