Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 10
Glutathione and its antiaging and antimelanogenic effects
Authors Weschawalit S, Thongthip S, Phutrakool P, Asawanonda P
Received 21 November 2016
Accepted for publication 10 February 2017
Published 27 April 2017 Volume 2017:10 Pages 147—153
DOI https://doi.org/10.2147/CCID.S128339
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Sinee Weschawalit,1 Siriwan Thongthip,2 Phanupong Phutrakool,3 Pravit Asawanonda1
1Department of Medicine, Division of Dermatology, 2Chula Clinical Research Center, 3Chula Data Management Center, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
Background: Previous studies showed that supplementation of reduced form of glutathione (GSH, 500 mg/d) has a skin-lightening efficacy in humans. This study was designed to evaluate the influences of both GSH and oxidized form (GSSG), at doses lower than 500 mg/d, on improving skin properties.
Patients and methods: A randomized, double-blind, placebo-controlled, parallel, three-arm study was conducted. Healthy female subjects were equally randomized into three groups and took GSH (250 mg/d), GSSG (250 mg/d), or placebo orally for 12 weeks. At each visit at baseline and for 12 weeks, skin features including melanin index, wrinkles, and other relevant biophysical properties were measured. Blood samples were collected for safety monitoring.
Results: In generalized estimating equation analyses, melanin index and ultraviolet spots of all sites including face and arm when given GSH and GSSG tended to be lower than placebo. At some sites evaluated, subjects who received GSH showed a significant reduction in wrinkles compared with those taking placebo. A tendency toward increased skin elasticity was observed in GSH and GSSG compared with placebo. There were no serious adverse effects throughout the study.
Conclusion: We showed that oral glutathione, 250 mg/d, in both reduced and oxidized forms effectively influences skin properties. Overall, glutathione in both forms are well tolerated.
Keywords: glutathione, melanin, pigment, aging, wrinkle, whitening
Introduction
The quest for means to alter skin color is endless. Caucasians seek ways to tan their skin, while many darker skin-type individuals are always in search of whitening or lightening agents.
Numerous topical agents available for melasma treatment are also used to lighten the skin color. However, as many people would prefer their skin to be thoroughly fairer, oral or even intravenous agents are administered to obtain these results. One of the widely used, systemic agents is glutathione, a thiol compound and one of the regulators of melanogenic pathway in the human system.
Glutathione is an antioxidant present in almost every cell in the body, playing a role in the detoxification of drugs and xenobiotics.1 Furthermore, reduced glutathione (GSH) acts as a hydrogen donor in the detoxification of hydrogen peroxide.2 As a dietary supplement, GSH possesses various systemic effects such as improvement of liver abnormalities,3,4 improvement of diabetic complication,5 protection from viral infection,6 and antitumor activity.7,8 It is even used to treat autism.9
In vitro experiments have demonstrated that glutathione is related to melanogenesis.10–13 Its antimelanogenic properties result from a variety of mechanisms including stimulation of pheomelanin synthesis rather than darker eumelanin, its antioxidant effects,14 and interference with intracellular trafficking of melanogenic enzymes.15 Glutathione also possesses certain antiaging properties.16
Glutathione is generally a safe ingredient for use as a dietary supplement. An oral acute toxicity study of GSH in mice found that the lethal dose 50 (LD50) was more than 5 g/kg, indicating that glutathione is nontoxic. In many clinical trials, no serious adverse reactions have been observed.9,17–19 On the contrary, it can even reverse the toxic effects following excessive intake of other amino acids.20
In the human body, glutathione exists in two forms, reduced and oxidized (GSSG), which can be readily converted to each other. However, it is not clear whether the two forms are physiologically similar, especially when melanogenesis is concerned. Moreover, efficacy and long-term safety of either form have not been examined systematically.
Glutathione is regarded as food or health supplements in several countries including the Philippines, Malaysia, Taiwan, and Thailand, while it is considered a pharmaceutical agent in Korea, Japan, and People’s Republic of China. Our group previously reported that oral GSH administration (500 mg/d) resulted in lightening of skin color, when given for 4 weeks.21 The main objective of this study was to find out whether glutathione, in the reduced and oxidized forms, maintains its skin-lightening efficacy when given at a dose of 250 mg/d for 12 weeks, a dosage allowed by the Thai and Taiwanese Food and Drug Administrations.
Patients and methods
Study design
The study protocol was conducted in accordance with the Declaration of Helsinki, in compliance with the International Conference on Harmonization - Good Clinical Practice and reviewed and approved by the Institutional Review Board of Faculty of Medicine, Chulalongkorn University. Clinical study was conducted at Chula Clinical Research Center, Faculty of Medicine, Chulalongkorn University. A randomized, double-blind, placebo-controlled, parallel, three-arm study was applied. The subjects were equally block-randomized into three groups: GSSG (250 mg/d), GSH (250 mg/d), or placebo. Each subject received their assigned capsules in identical packages at weeks 0, 4, and 8, with 30 capsules per visit (two extra capsules per package). The subjects were informed to take the study capsules once before bedtime. Subjects returned for evaluations at weeks 4, 8, and 12.
Subjects
Sixty healthy volunteers, aged between 20 and 50 years, were eligible for this study. They were residents of Bangkok and recruited through the dermatology clinic at King Chulalongkorn Memorial Hospital. Only female volunteers were enrolled to reduce gender variability and also because females are by far the majority of individuals who seek skin-whitening agents. All volunteers were found to be healthy based on their medical history and physical and clinical laboratory examination including serology, hematology, and biochemistry tests. All volunteers had to abstain from other medications, supplementary vitamins, and alcohol intake for 2 weeks prior to enrollment and throughout the study. The methods and conditions of the study were clearly explained to all volunteers. Signed written informed consent was obtained from each volunteer before screening processes for this study. However, each subject had the right to withdraw their consent at any time.
Study medications
Daily doses of GSSG (AquaGluta™; 250 mg/d), GSH (Setria®; 250 mg/d), or dibasic calcium phosphate as placebo in identical capsules and packages were provided by Kyowa Hakko Bio Co., Ltd (Tokyo, Japan). The total weight of the three capsules for each group was the same.
Objective evaluation of skin properties
At each visit, subjects rested in a room controlled at a temperature of 21 ± 3°C for 20 minutes before assessments. Prior to the evaluation, they washed their face with soap and water, pat-dried with paper towel, and waited 5–10 minutes for air drying.
Melanin index
For objective evaluation of skin color, melanin index as determined by Mexameter (Courage-Khazaka Electronic, Koln, Germany) was used as the primary outcome. All measurements were done in triplicate at six sites to represent the skin of the sun-exposed and sun-protected areas as follows:
Sun-exposed areas
Face: left and right; 2.5 cm caudally from the lateral canthi.
Extensor surfaces of the forearms, left, and right; 7 cm above the ulnar styloid processes.
Sun-protected areas
Upper, inner arms, left, and right; 10 cm from the axillary vault.
VISIATM CR system
Standardized digital photographs were taken by the VISIATM CR system (Canfield Scientific, Fairfield, NJ, USA), a software which was also used to quantitatively evaluate ultraviolet (UV) spots, pores, and evenness on the left and right sides of the face.
Transepidermal water loss (TEWL)
TEWL was measured using Tewameter® TM300 (Courage+Khazaka Electronic GmbH, Köln, Germany). All measurements were done in triplicate at sites designated for melanin index to represent the skin of the sun-exposed and sun-protected areas.
Water contents (Corneometer)
Water contents were measured by Corneometer® CM825 (Courage+Khazaka) in triplicate at sites as mentioned earlier.
Elasticity
Elasticity was measured by Cutometer MPA580® (Courage+Khazaka) in triplicate at the sites mentioned previously.
Wrinkle
Wrinkle formation was objectively measured by Visioscan® (Courage+Khazaka).
Subjective evaluation of skin properties
For global evaluation, at each visit, subjects were asked to grade the overall response using a 4-point rating scale: 4 = very satisfactory, 3 = moderately satisfactory, 2 = minimally satisfactory, and 1 = not satisfactory. In the questionnaires, the following features/items were addressed: skin lightening, facial skin evenness, pigmented spots lightening, pore size improvement, skin smoothness, wrinkle reduction – crow’s feet, wrinkle reduction – nasolabial folds, wrinkle reduction – forehead, general skin condition, fatigability, sleep (e.g., quality and length)
Safety
To evaluate the safety of subjects, vital signs were determined at each visit. Also, at all visits, a blood aliquot of 7 mL was drawn for complete blood cell counts, chemistry, and lactate dehydrogenase.
Statistical analysis
Paired t-test was used to compare baseline values with those of the final visit. The generalized estimating equation (GEE) was performed to investigate how the efficacies of GSSG, GSH and placebo varied over time before and after treatment. Subjective evaluations of the two glutathione preparations and placebo were compared by analysis of covariance (ANCOVA), with the baseline values as covariates. Statistically significant level was defined as P-value <0.05 (two-tailed). Analyses were performed using STATA software version 11.0 (StataCorp, College Station, TX, USA).
Results
Sixty volunteers were enrolled in the study. Three volunteers had to terminate, two due to elevation in liver function tests and one due to unanticipated start of oral contraceptive pills. Fifty-seven volunteers were included for final analysis. The majority of subjects had skin phototype IV (96.4%). Subjects’ demographic data are summarized in Table 1.
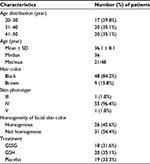  | Table 1 Baseline demographics Abbreviations: SD, standard deviation; GSSG, oxidized glutathione; GSH, reduced glutathione. |
A total of 18 subjects received GSSG, 20 received GSH, and 19 received placebo. Mean baseline measurements (Mexameter, VISIA, Tewameter, Corneometer, Cutometer, Visioscan) in the three groups at six sites were not significantly different (ANCOVA, P > 0.05).
The GEE was performed to investigate how the efficacies of GSSG, GSH, and placebo varied over time during treatment (Table 2). As our subjects were recruited from a broad age range, we also decided to categorize the age groups into those younger and older than 40 years.
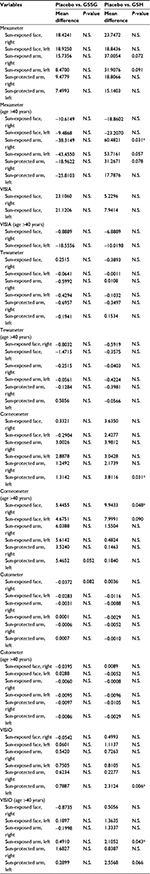  | Table 2 GEE analysis Note: *P<0.05. Abbreviations: GEE, generalized estimating equation; N.S., not significant; GSSG, oxidized glutathione; GSH, reduced glutathione. |
Melanin index
In all subjects with an age range between 20 and 50 years, GEE model showed that melanin index and UV spots of all sites including face and arm from GSSG and GSH groups tended to be lower than placebo group (Table 2) but were not statistically significant (P > 0.05). There were no significant differences between GSSG and GSH groups. The subgroup analysis of middle-aged individuals showed that the melanin index of sun-exposed right forearm of subjects aged >40 years who received GSH (N = 7) was significantly lower than the index of those who received placebo (N = 10, P = 0.031). Melanin index measured at the sun-exposed left forearm from those receiving GSH was also lower than those receiving placebo. However, this did not reach statistically significant level (P = 0.057).
TEWL and water content
TEWL measurement of sun-exposed right forearm of the GSH group was significantly lower than that of GSSG group (P = 0.044). However, the water contents of sun-protected left arm of those who received GSH were lower than that of those who received placebo (P = 0.031). This was also true for subjects aged >40 years, for the sun-exposed right face (P = 0.048).
Wrinkles
Visioscan measurements of sun-protected left arm of subjects in the GSH group were significantly lower than those of the placebo group (P = 0.006). This was also true for those aged >40 years when measurements were taken at the sun-exposed left forearm (P = 0.043). A similar trend was seen for GSH vs. placebo for the sun-protected left arm, in advanced age group (P = 0.066).
Elasticity
Although statistically significant differences could not be demonstrated, GSSG and GSH supplementation tended to increase skin elasticity. Especially, the elasticity of sun-exposed right face of those who received GSSG was notably higher than the elasticity of those who received placebo (P = 0.082).
Subjects were asked to fill the questionnaires to subjectively evaluate skin properties at each visit. Satisfaction was scored as rating scale. There were no statistically significant differences in any of the ratings among the three groups (P > 0.05, ANCOVA).
Compliance
Compliance was not an issue in the present study. All subjects took the capsules as directed and assessed by the protocol throughout the study.
Adverse events
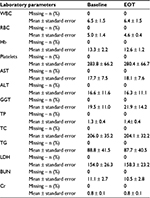
Adverse reactions included pruritus, macular erythema, transient minute red spots on the skin, and tiredness. In the treatment groups combined (GSSG, GSH), these occurred in five patients (13.15%), which included three with pruritus (7.89%), one with erythema (2.63%), three with red spots (7.89%), and one with tiredness (2.63%). In the placebo group, there were two incidents of pruritus (10.52%), one erythema (5.26%), and three red spots (15.79%) (Table 3). No serious adverse events took place. The two incidents of transaminitis were temporary and the liver function tests returned to normal within a few weeks. Detailed blood parameters are described in Table 4.
  | Table 3 Adverse events Abbreviations: AE, adverse event; GSSG, oxidized glutathione; GSH, reduced glutathione. |
Discussion
Our group has previously demonstrated that oral glutathione, 500 mg/d, can reduce skin pigmentation after 4 weeks’ administration in young, otherwise-healthy medical students.21 Watanabe et al also demonstrated that topically applied GSSG can significantly reduce melanin indices.22 Recently, Handog et al investigated the use of intraoral lozenge containing 500 mg of glutathione in an open-label study and demonstrated significant skin lightening after 8 weeks of administration.23
It is well established that glutathione can be transported across the intestinal epithelium after oral supplementation,9,19 yet the fate of orally administered GSH is to be resolved as it is readily oxidized within the human body. On the contrary, its oxidized counterpart is much more stable. Until very recently, most studies were not able to detect blood or plasma glutathione, despite large doses of oral intake.17 However, Park et al demonstrated that although no glutathione could be measured in the whole plasma compartment, GSH could be detected in the protein-bound fraction of the human blood between 60 and 120 minutes after oral intake.24
In this study, we have demonstrated that both GSSG and GSH exerted their effects on melanin indices, which reached statistically significant levels at specific site and higher age group. This is in agreement, yet to some degree dissimilar, to our earlier study, the explanations for which are several-fold. First, the dose of glutathione used in this study is half of that used in the prior study. This is to comply with the daily dosage of L-glutathione allowed in some countries including Thailand. Second, the subjects recruited in this study are all females and of more advanced age. Interestingly, with subgroup analysis, the changes in subjects aged more than 40 were even more pronounced than when the whole group was analyzed. Being affected with more photodamage can definitely affect the final outcomes measured, especially when pigment is concerned.
Our results also showed that GSH was significantly superior to placebo in its ability to improve wrinkles, at least at some anatomic locations. This is an extremely interesting and novel finding as cutaneous aging is a significant problem faced by the majority of people of any age. As the world’s populations are rapidly heading toward an aging society and human life spans are increasing, this problem will certainly be of greater magnitude in the foreseeable future. Having to apply topical antiwrinkle preparation to the entire skin is both costly and in many circumstances impractical for the elderly, especially when compared with popping a pill.
Also of importance are the findings that both forms of glutathione showed trends in increased skin elasticity at various sites, both sun-exposed and sun-protected skin. These findings have never been reported before and deserve further investigations in larger populations.
Because glutathione has regulatory properties on melanogenesis and antioxidants in general are protective against aging process, the “dual” antimelanogenic and antiaging properties demonstrated in Watanabe’s and our studies are not surprising. In fact, the link between melanization and aging has been studied in animal models.25
The strengths of our study are, first, objective and well-standardized measurements. Second, a randomized, double-blind study that analyzes the effect of glutathione in both reduced and oxidized forms in comparison with placebo has never been conducted. Limitations are that our subjects are all female, Asian, and of certain age range.
Overall, glutathione in both forms are well tolerated. No major adverse events took place during the study period. Increases in transaminases occurred in two subjects highlighting the fact that blood chemistry should be performed even when individuals are taking over-the-counter supplements. Nonetheless, these adverse events were transient and the blood parameters promptly returned to their normal values upon cessation of consumption.
Conclusion
In summary, we have shown that oral glutathione, 250 mg/d, in both reduced and oxidized forms have various beneficial effects on skin properties and is possibly an antiaging agent, at least in middle-aged female subjects. Further studies in larger and more diverse populations are warranted.
Acknowledgment
This study was funded by Kyowa Hakko Bio (Tokyo, Japan).
Disclosure
The authors report no conflicts of interest in this work.
References
Chasseaud LF. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. | ||
Burk RF. Glutathione-dependent protection by rat liver microsomal protein against lipid peroxidation. Biochim Biophys Acta. 1983;757(1):21–28. | ||
Gorla N, de Ferreyra EC, Villarruel MC, de Fenos OM, Castro JA. Studies on the mechanism of glutathione prevention of carbon tetrachloride-induced liver injury. Br J Exp Pathol. 1983;64(4):388–395. | ||
Sugimura Y, Yamamoto K. Effect of orally administered reduced- and oxidized-glutathione against acetaminophen-induced liver injury in rats. J Nutr Sci Vitaminol (Tokyo). 1998;44(5):613–624. | ||
Ueno Y, Kizaki M, Nakagiri R, Kamiya T, Sumi H, Osawa T. Dietary glutathione protects rats from diabetic nephropathy and neuropathy. J Nutr. 2002;132(5):897–900. | ||
Magnani M, Fraternale A, Casabianca A, et al. Antiretroviral effect of combined zidovudine and reduced glutathione therapy in murine AIDS. AIDS Res Hum Retroviruses. 1997;13(13):1093–1099. | ||
Schwartz JL, Shklar G. Glutathione inhibits experimental oral carcinogenesis, p53 expression, and angiogenesis. Nutr Cancer. 1996;26(2):229–236. | ||
Shklar G, Schwartz J, Trickler D, Cheverie SR. The effectiveness of a mixture of beta-carotene, alpha-tocopherol, glutathione, and ascorbic acid for cancer prevention. Nutr Cancer. 1993;20(2):145–151. | ||
Kern JK, Geier DA, Adams JB, Garver CR, Audhya T, Geier MR. A clinical trial of glutathione supplementation in autism spectrum disorders. Med Sci Monit. 2011;17(12):CR677–CR682. | ||
Imokawa G. Analysis of initial melanogenesis including tyrosinase transfer and melanosome differentiation through interrupted melanization by glutathione. J Invest Dermatol. 1989;93(1):100–107. | ||
del Marmol V, Solano F, Sels A, et al. Glutathione depletion increases tyrosinase activity in human melanoma cells. J Invest Dermatol. 1993;101(6):871–874. | ||
Benathan M, Virador V, Furumura M, Kobayashi N, Panizzon RG, Hearing VJ. Co-regulation of melanin precursors and tyrosinase in human pigment cells: roles of cysteine and glutathione. Cell Mol Biol (Noisy-le-grand). 1999;45(7):981–990. | ||
Panich U, Onkoksoong T, Limsaengurai S, Akarasereenont P, Wongkajornsilp A. UVA-induced melanogenesis and modulation of glutathione redox system in different melanoma cell lines: the protective effect of gallic acid. J Photochem Photobiol B. 2012;108:16–22. | ||
Villarama CD, Maibach HI. Glutathione as a depigmenting agent: an overview. Int J Cosmet Sci. 2005;27(3):147–153. | ||
Nakajima H, Nagata T, Koga S, Imokawa G. Reduced glutathione disrupts the intracellular trafficking of tyrosinase and tyrosinase-related protein-1 but not dopachrome tautomerase and Pmel17 to melanosomes, which results in the attenuation of melanization. Arch Dermatol Res. 2014;306(1):37–49. | ||
Furukawa T, Meydani SN, Blumberg JB. Reversal of age-associated decline in immune responsiveness by dietary glutathione supplementation in mice. Mech Ageing Dev. 1987;38(2):107–117. | ||
Witschi A, Reddy S, Stofer B, Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43(6):667–669. | ||
Japan Pharmaceutical Information Center. Drugs in Japan: Ethical Drugs. 27th ed. Tokyo: Jiho Inc.; 2004. | ||
Kovacs-Nolan J, Rupa P, Matsui T, et al. In vitro and ex vivo uptake of glutathione (GSH) across the intestinal epithelium and fate of oral GSH after in vivo supplementation. J Agric Food Chem. 2014;62(39):9499–9506. | ||
Tateishi N, Higashi T, Naruse A, Nakashima K, Shiozaki H. Rat liver glutathione: possible role as a reservoir of cysteine. J Nutr. 1977;107(1):51–60. | ||
Arjinpathana N, Asawanonda P. Glutathione as an oral whitening agent: a randomized, double-blind, placebo-controlled study. J Dermatolog Treat. 2012;23(2):97–102. | ||
Watanabe F, Hashizume E, Chan GP, Kamimura A. Skin-whitening and skin-condition-improving effects of topical oxidized glutathione: a double-blind and placebo-controlled clinical trial in healthy women. Clin Cosmet Investig Dermatol. 2014;7:267–274. | ||
Handog EB, Datuin MS, Singzon IA. An open-label, single-arm trial of the safety and efficacy of a novel preparation of glutathione as a skin-lightening agent in Filipino women. Int J Dermatol. 2016;55(2):153–157. | ||
Park EY, Shimura N, Konishi T, et al. Increase in the protein-bound form of glutathione in human blood after the oral administration of glutathione. J Agric Food Chem. 2014;62(26):6183–6189. | ||
Galván I, Alonso-Alvarez C, Negro JJ. Relationships between hair melanization, glutathione levels, and senescence in wild boars. Physiol Biochem Zool. 2012;85(4):332–347. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.