Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 15
Glomerulus-on-a-Chip: Current Insights and Future Potential Towards Recapitulating Selectively Permeable Filtration Systems
Authors Doi K, Kimura H , Matsunaga YT, Fujii T, Nangaku M
Received 16 October 2021
Accepted for publication 14 February 2022
Published 10 March 2022 Volume 2022:15 Pages 85—101
DOI https://doi.org/10.2147/IJNRD.S344725
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pravin Singhal
Kotaro Doi,1 Hiroshi Kimura,2 Yukiko T Matsunaga,1 Teruo Fujii,3 Masaomi Nangaku4
1Institute of Industrial Science, The University of Tokyo, Tokyo, Japan; 2Department of Mechanical Engineering, School of Engineering, Tokai University, Kanagawa, Japan; 3The University of Tokyo, Tokyo, Japan; 4Division of Nephrology and Endocrinology, The University of Tokyo Graduate School of Medicine, Tokyo, Japan
Correspondence: Masaomi Nangaku, Division of Nephrology and Endocrinology, The University of Tokyo Graduate School of Medicine, 7-3-1, Hongo, Bunkyo-ku, Tokyo, 113-8655, Japan, Tel/Fax +81358009736, Email [email protected]
Abstract: Glomerulopathy, characterized by a dysfunctional glomerular capillary wall, results in proteinuria, leading to end-stage renal failure and poor clinical outcomes, including renal death and increased overall mortality. Conventional glomerulopathy research, including drug discovery, has mostly relied on animal experiments because in-vitro glomerulus models, capable of evaluating functional selective permeability, was unavailable in conventional in-vitro cell culture systems. However, animal experiments have limitations, including time- and cost-consuming, multi-organ effects, unstable reproducibility, inter-species reliability, and the social situation in the EU and US, where animal experiments have been discouraged. Glomerulus-on-a-chip, a new in-vitro organ model, has recently been developed in the field of organ-on-a-chip research based on microfluidic device technology. In the glomerulus-on-a-chip, the podocytes and endothelial cells are co-cultured in a microfluidic device with physical stimuli that mimic the physiological environment to enhance cell function to construct a functional filtration barrier, which can be assessed by permeability assays using fluorescently labeled molecules including inulin and albumin. A combination of this glomerulus-on-a chip technology with the culture technology to induce podocytes and endothelial cells from the human pluripotent stem cells could provide an alternative organ model and solve the issue of animal experiments. Additionally, previous experiments have verified the difference in the leakage of albumin using differentiated podocytes derived from patients with Alport syndrome, such that it could be applied to intractable hereditary glomerulopathy models. In this review, we provide an overview of the features of the existing glomerulus-on-a-chip systems, focusing on how they can address selective permeability verification tests, and the challenges they involved. We finally discuss the future approaches that should be developed for solving those challenges and allow further improvement of glomerulus-on-a-chip technologies.
Keywords: glomerulus, podocyte, selective permeability, microfluidic device, organ-on-a-chip
Plain Language Summary
This review article discusses an overview of the features of the existing glomerulus-on-a-chip techniques used for studying glomerulopathy, focusing on their selective permeability verification tests. Moreover, it discusses the challenges involved in the implementation of this technology as well as the approaches for solving those challenges. Glomerulopathy with high proteinuria is associated with end-stage renal failure requiring renal replacement therapy. It can also lead to an increased overall mortality and has implications for the global health economy. The glomerulus-on-a-chip method develops a new in-vitro organ model through which a functional filtration barrier is constructed by co-culturing podocytes and endothelial cells in a unique micro 3D space with physical stimuli that mimic the physiological environment. In addition, leakage assays can evaluate the selective permeability of filtration barrier with fluorescently labeled molecules including inulin and albumin. The integration of podocytes and endothelial cells derived from human pluripotent stem cells, including those from patients with intractable genetic diseases, into glomerulus-on-a-chip is expected to solve the problems of animal experiments and enable the development of efficient treatments for intractable genetic glomerular disorders.
Introduction
In recent years, kidney diseases have become a worldwide social problem. Kidney diseases reportedly affect approximately 10% of the world population, incurring high expenditure between $35,000 to $100,000 annually for dialysis treatment and kidney transplantation for patients with end-stage kidney disease.1 Over the past two decades, guidelines, including diet (eg, low salt intake) to organ protection strategies (eg, renin-angiotensin-aldosterone system inhibitors), have significantly improved kidney disease management.2,3 However, little progress has been made in the management and treatment of glomerular diseases, especially for those with a poor prognosis, such as idiopathic focal segmental sclerosis and Alport syndrome.4,5 Although there have been advances, such as the indication of rituximab for treating nephrotic syndrome,6 the options for the treatment of glomerulopathy with massive proteinuria remain limited to immunosuppressive therapy and renin-angiotensin-aldosterone system inhibitors.7 Thus, there is a serious demand for the development of effective and preventative therapies to improve the prognosis of patients with glomerulopathy.
The health of the filtration barrier in the glomerular capillary wall is directly linked to proteinuria, which is a clinically important surrogate endpoint. Therefore, it is necessary to evaluate the selective permeability function of the filtration barrier with high performance for studying the glomerular disease.8–10 Traditionally, approaches are limited to 2D cell culture system11–14 and experiments with animals15–18 in research fields of nephrology and non-clinical drug discovery. The conventional 2D cell culture system has been used to study gene and protein expression changes in response to chemical and culture condition stimuli.11–14 Although there have been reports of the construction of glomerular filtration barriers by co-culturing podocytes and endothelial cells in transwellTM culture inserts and their use in leakage assays,19,20 it has not been possible to reproduce the physiological and physical environment that is considered beneficial to the cells.21 In contrast, animal experiments are used to study the roles of specific genes, chemical efficacy in diseased conditions, and drug testing in non-clinical tests to monitor the degree of proteinuria.15–18 Nevertheless, animal experiments are not only time- and cost-consuming but can also lead to uncontrolled multi-organ effects, individual variation, unstable reproducibility, inter-species poor reliability, and low throughput.22,23 Moreover, considering environmental pollution, adverse impacts on biodiversity, and public health associated with animal experiments, the US Environmental Protection Agency has proposed to eliminate all the animal experiments by 2035.24 Subsequently, the past decade has witnessed the emergence and development of culture methods of lineage-specific differentiation with pluripotent stem cells like kidney organoid25–30 and organ-on-a-chip (OoC) technology.31–38 The kidney organoid culture approaches are advantageous over the 2D cell culture system as they highly express tissue-specific phenotypes in general. However, it has not attained an adequate level for evaluating the selectively permeable function of the filtration barrier owing to the lack of glomerular endothelial cell (GEnC) infiltration beneath the podocytes and capillary loop formation in the glomerulus.39
The OoC based on microfluidic device technology has been recently developed in micro-total analysis systems (µTAS) as an application of in-vitro analysis to provide novel organ models.31–38 Among the many types of OoC, such as lung-on-a-chip,31 liver-on-a-chip,34 brain-on-a-chip,35 glomerulus-on-a-chip (GoC) have also been under development in the last decade. Researchers have been focusing on the development of GoCs, which are capable of recapitulating the filtration barrier in the glomerular capillary wall by co-culturing podocytes and endothelial cells in various forms, either on both sides of a porous membrane or on a gel wall containing an extracellular matrix (ECM) while allowing the perfusion of medium in a three-dimensional micro-space in order to mimic the body fluids flow, but also the actuation of physical stimulations, such as stretching of the cell base. Selective permeability can be measured by perfusing the device with a medium containing fluorescently labeled molecules with various molecular sizes from the vascular to urinary compartments through the filtration barrier.40–46 Furthermore, reports of the integration of podocytes and endothelial cells derived from human stem cells, including those from patients with hereditary diseases, into a GoC are remarkable in that they suggest the feasibility of human GoC applications, including models of refractory inherited rare diseases.42,43,46 This review aims to enhance the understanding of the current level of technology and the challenges raised for assessing the permeability using GoCs and finally discusses the approaches for further improvement.
The Glomerular Selective Permeability
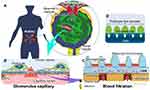
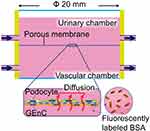
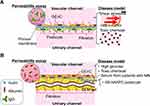
Glomerular tissue is a ball-like mass of capillaries (Figure 1A). The glomerular capillary wall is three-layered inside out, comprising GEnC, glomerular basement membrane (GBM), and the podocyte layer (Figure 1B).47,48 The histological and structural characteristics of each of the three layers are as follows: the GEnCs, lining the vessel walls with a thin cytoplasm, bear numerous septum-free fenestrations of about 100 nm in diameter.49,50 The GBM is a rigid gel plate bearing several extracellular matrices with a thickness of approximately 300 nm in humans (Figure 1C).51,52 Podocytes have several primary processes extending from the cell body floating in the primary urinary space (Figure 1B), and numerous foot processes branching from the primary processes and form a scrum structure with their neighbors (Figure 1A).53,54 The slit diaphragms are located between the foot processes, comprising several membrane proteins, as shown by electron microscopy and immunofluorescence staining (Figure 1C).55–57 Each of these three layers acts as a barrier for the selective filtration of blood.58–61 The effective glomerular filtration pressure acting through the filtration barrier has been reported to be around 20 mmHg.62,63 This filtration pressure affects the sieving coefficient of molecules passing through the filtration barrier (Figure 1).64
There are several theories as to how these three layers contribute to the selective permeability of the filtration barrier. Selective permeability refers to that smaller molecules, such as water and electrolytes can cross the barrier, whereas larger molecules such as albumin are prevented.58,63,65–69 One of the theories has suggested that selective permeability consists of a negatively charged properties of the GEnC and GBM and a size-selective properties of the basement membrane and podocyte slit diaphragm (Figure 1C).58,67,68 In contrast, one of the other theories has suggested that the size-selective properties are mostly due to the basement membrane, with no role of the podocyte slit diaphragm. In this view, podocytes assist in maintaining the sieving coefficient of the basement membrane by pressing down on it with their foot processes (Figure 1D).63,69
Previous in-vivo studies have described the histopathological changes associated with impaired glomerular capillary walls to have abnormalities in proteinuria such as fusion of numerous branched podocyte foot processes, decreased expression and altered localization of slit diaphragm proteins, irregularities in the glomerular basement membranes with large and small thickening, loss of endothelial cell fenestrations, and hypertrophy of the endothelial cells.49,70
Various diseases and drugs damage the glomerular filtration barrier. Although glomerular diseases have been classified histologically and clinically into various types, all of them can develop the symptom of proteinuria. Regardless of the causes and pathological changes in glomerulopathy, the symptom of proteinuria has been clinically considered as one of the few early detectable markers for recognizing the damaged glomeruli filtration barrier in asymptomatic kidney diseases,70–72 and its severity has been widely recognized as a predictor of future decline of the glomerular filtration rate. Moreover, a decreased glomerular filtration rate eventually leads to end-stage renal failure, which incurs high total mortality and necessary renal replacement therapy.8,10
Organ-on-a-Chip; Glomerulus-on-a-Chip
The microfluidic device technology used in OoCs has been developed over the past two decades in the µTAS field. The technology is based on micro-electromechanical systems (MEMS), employing photolithography and soft lithography to mold microscale channels, pillar structures, and micropores. Polydimethylsiloxane (PDMS), a type of silicone rubber, is mainly used for ease of handling, transparency and biocompatibility.73,74 Recently, other materials have been developed to build the devices, such as the use of resins to avoid drug sorption in PDMS,43,44,75 and the use of alginate hollow fibers fabricated by sheathed microfluidic devices as topographically accurate culture platforms.45 In the OoC research field, it has been shown that the physical stimuli and physiological environment reproduced by unique specifications of the device can accelerate cell functionalities in the device.33,37,76,77 Therefore, it is also expected to construct more matured filtration barriers in GoC by promoting cell functionalities and applying them to selective permeability assays.
The difficulty in GoC research lies in culturing the glomerular cells, especially podocyte cell sources in relevant culture conditions. As a result, progress in this research field has been relatively behind other organs. Although the devices used in the GoC discussed in this review are similar to those used in other organs, the development of the GoC in the last decade involves strong expertise in culturing human pluripotent stem cell-derived podocytes and kidney organoids.42,43,46 Moreover, the study of applying the podocyte derived from Alport syndrome patients into the device, reproducing a pathological filtration barrier, and testing its selective permeability, suggests the feasibility of establishing a model for refractory inherited rare diseases.43
Microfluidic Devices of Glomerulus-on-a-Chip
Since most of the devices employed in GoC have been widely used for OoC in other organ models, it is important to understand not only the structural features of each device but also how it has been used (ie, how the filtration barrier has been reproduced and how the selective permeability has been tested in the device) in order to understand the current GoC technology, its remaining challenges and further developments. Hereafter, the previously reported microfluidic devices in GoC study were classified into four types of simplifications (Table 1, Figures 2–5).
 |
Table 1 Summary of Glomerulus-on-A-Chips Reported in Literature |
Two-Layered with Porous Membrane Device
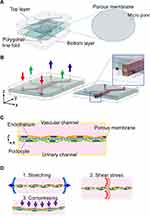
The device is characterized by a porous membrane sandwiched between two layers of vertically aligned microfluidic channels (Figure 2A). This porous membrane forms the basis for the cultured cells, coated on both sides into an ECM (Figure 2B and C). The podocytes and endothelial cells are cultured on both sides of the porous membrane to mimic the glomerular filtration barrier, with the podocyte-side channels assigned to the urinary channel and the endothelial cell-side to the vascular channel.40,42,46 The porous membranes used here are generally made of PDMS,78 polycarbonate (PC),79 or polyethylene terephthalate.80 The device employed by Musah et al and Roye et al has microchannels on either side of the central channel that can be deformed by intermittent negative pressure, allowing the porous membrane to stretch to mimic vascular pulsation (Figure 2D1).42,46
Bilateral Flow with Central Gel Channels Device
This device features a central ECM gel with a thickness of several hundred micrometers, which serves as a scaffold for cultured cells. Two perfusion channels are arranged on both sides of the central gel channel in the XY direction (Figure 3A). Pillar-like guides are placed to sandwich the gel, whereby the gel is retained only in the central channel and interfaces between the medium perfusion channel and the gel are formed along the guides (Figure 3A1 and A2). This structure allows the cultured cells seeded through the perfusion channel to adhere to the gel scaffold.41,43 The isolated glomeruli can be placed between the pillars (Figure 3B1),41 or cells can be seeded into one perfusion channel in the order of the first podocytes, then endothelial cells to mimic the glomerular filtration barrier in the device (Figure 3B2).43 Accordingly, the medium perfusion channel in which glomeruli or cultured cells are embedded is assigned to the vascular channel and the cell-free one to the urinary channel (Figure 3A).41,43
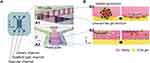
Hollow Alginate Fiber Devices
Hollow alginate fibers, also widely used in cell fiber applications,81 can be produced by a microfluidic device with a sheath structure and a calcium ion solution (Figure 4). To mimic a glomerular topography, Xie et al have modified a hollow alginate fiber fragment with a knot in the center made of multiple micro-convex surfaces. The hollow alginate fiber is placed in the chamber such that its ends are connected to the inlet and outlet chamber (Figure 4B1). The endothelial cells are seeded from the inlet into the hollow alginate fiber and podocytes onto the surface of the fiber using a hanging drop technique to recapitulate the glomerular filtration barrier. This device mimics the glomerular topography more than the other GoC devices (Figure 4B2). Meanwhile, this device does not allow for the exogenous coating of the ECM and requires the continuous addition of 5 mM CaCl2 to the cell culture medium to maintain the alginate cross-linking.45
Larger Scale Fluidic Devices
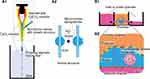
This device is characterized by a scale larger than the other devices wherein the cylindrical chamber is separated from the top and bottom by a porous membrane of around 20 mm in diameter. The porous membrane is tightly sandwiched between the elastic sealing elements and removable. The upper and lower cylinders, mostly made of resin, are connected to inlet and outlet medium perfusing tubes, driven by peristaltic pumps (Figure 5). The large scale of this device facilitates the operation compared to the other microfluidic devices.44
Cell Culture in Glomerulus-on-a-Chip
The use of various types of podocytes has been attempted in GoC development. Additionally, the choice of ECM is important because the ultrastructural changes in the GBM in Alport’s syndrome lead to a deterioration of the glomerular filtration barrier.82,83 We present an overview of cell culture in GoC, focusing on the podocytes.
Temperature-sensitive immortalized podocytes transfected with the SV40 virus-derived T antigen are widely used due to their potential to proliferate. However, the immortalized podocytes are generally not recognized as a valid cell source owing to the loss of maturation markers such as the slit diaphragm proteins, even at culture conditions around 37°C where the proliferation is expected to be inhibited, and the differentiation to be induced.84,85
The primary cultured podocytes obtained from the isolated glomeruli might be more beneficial than the immortalized podocytes due to retention of the maturation markers. However, not only are the sources of primary podocytes limited ethically but also their mitotic potential is negligible. In addition, there is an inevitable loss of the maturation markers during the cryopreservation and passaging;85 hence, they are unlikely to be a useful source. Here, the development of a culture technique induces lineage-specific cells, including glomeruli in kidney orgaonid25–30 or podocytes42,43,86–89 from human induced pluripotent stem (hiPS) cells, which is a breakthrough in the last decade concerning the problem of cell sources. Previous reports on GoC by Musah et al42 and Petrosyan et al43 have exemplified the use of stem cell-derived podocytes.
Musah et al reported the induction of podocytes from hiPS cells via nephron progenitor cells using chemical compounds such as Activin A, CHIR99021, BMP7, retinoic acid, and VEGFA.42 In contrast, Petrosyan et al showed the podocytes induced from the human stem-like cells in the amniotic fluid using calcitriol, retinoic acid, and dexamethasone.43,89 Compared to the human immortalized podocytes using flow cytometry, both the podocytes were found to express the mature podocyte markers significantly.42,89 Additionally, various methods induce podocytes from hiPS cells,86–88 although none have been used for GoC, as we will discuss later in the section of the future issue and perspectives.
The GEnCs cooperate with podocytes forming the glomerular filtration barrier. The fenestrated structure of GEnCs contributes to the sieving coefficient of the glomerular filtration barrier49 and requires the cultured GEnCs. The primary GEnCs employed by Petrosyan et al demonstrate fenestration structures by scanning electron microscopy.43
Although difficult in humans, isolated glomeruli from rats can be directly seeded into devices.41 This method is reasonable considering podocytes generally lose their maturation markers during outgrowth from isolated glomeruli.85
ECM used in GoC is chosen considering both the mature GBM components and the geometry of GoC. Devices, where the cell adhesion base is a porous membrane, are compatible with both liquid and gel types ECM. The bilateral flow with central gel channels device is only of gel type. Nowadays, ECM gels are available as basement membrane gel, Matrigel (Corning), or CultrexTM Basement Membrane Extract (BME) (R&D systems) derived from mouse Engelbreth-Holm-Swarm (EHS) sarcoma,90 fibrin gel,91 and type I collagen gel.92 Some heterogeneities, however, exist between components of mature GBM and basement membrane gel derived from mouse EHS sarcoma concerning the laminin and type IV collagen: laminin α5β2γ1, type IV collagen α3α4α5 in mature GBM,51,52 and the laminin α1β1γ1, type IV collagen α1α2α3 in gel derived from mouse EHS sarcoma,90 while heparan sulfate and nidogen are almost common between them.51,52,90 In a previous practical example, CultrexTM BME and Matrigel, human laminin α5β1γ1 (BioLamina), and rat type I collagen gel (R&D systems) have been used. Although the type I collagen gels in the bilateral flow with the central gel channels device seem unreasonable as they are not part of the normal GBM,93 they can be argued to be merely a foothold for podocytes and endothelial cells as the ECM of the GBM components accumulates between them in a direct contact co-culture environment.43
Selective Permeability Assays in Glomerulus-on-a-Chip
In previous GoC studies, permeability assays as well as molecular biological assessments have been performed to evaluate the selective permeability function and maturity of the filtration barrier reproduced by physiological and physical stimuli in the device. Here, we first outline the methodology of permeability assay in GoC devices and then discuss the current technology level of GoC, highlighting individual reports.
In the permeability assays, fluorescently labeled molecules, such as inulin (5 kDa), albumin (70 kDa), and IgG (150 kDa), were frequently used for analyzing the selective permeability concerning molecular weight (Figure 6).40–44,46 Likewise, the fluorescently-labeled FicollTM and dextran of various molecular sizes are also used in some cases.45 The procedure involved infusing the media mixed with the fluorescently labeled molecules from only vascular channels40–44,46 and subsequently sample media in both channels, which can be used for measuring the fluorescence intensity by means of a microplate reader. The clearance of each fluorescently labeled molecule is calculated from its concentration in the vascular and urinary channels to assess the function of the filtration barrier.42,46 Various diseased models of pathological filtration barriers, such as transplanting the podocytes derived from patients with the hereditary disease of Alport syndrome,43,44 perfusing media containing cytotoxic drugs of puromycin aminonucleoside or doxorubicin,42,43,46 or hyperglycemia41 have also been studied.
The analyses of the filtration barrier function have been reported in detail in studies by Petrosyan et al and Musah et al using podocytes derived from human stem cells and focused on these for providing an understanding of the current technology level.
Musah et al employed a GoC where the podocytes derived from human pluripotent stem cells and primary human GEnCs were transplanted into all PDMS two-layers with porous membrane device, together with an extracellular matrix of laminin 511 (Table 1). Interestingly, the podocytes were transplanted into the device at the intermediate mesoderm stage and were induced to differentiate into podocytes in the device. The cells were cultured under conditions of medium perfusion corresponding to a shear stress of 0.07 mPa for podocytes and 1.7 mPa for GEnCs, respectively, and 10%, 1 Hz cyclic straining of cell base for a minimum of 8 days (Figure 2D1 and D2), including the first 2 days of static culture to promote maturation of the filtration barrier. Fluorescence immunostaining of podocytes demonstrated the basal stretching stimulation above to promote the expression of nephrin and type IV collagen by less than two-fold compared to the unstimulated control. In addition, the ELISA revealed that the combined stimulation of shear stress and stretching above could accelerate the secretion of VEGFA in the podocytes by two-fold compared to no stimulation. Previously, shear stress of 50 mPa has been shown to promote the maturation of podocytes regarding their height in the cytoskeleton and the upregulation of various differentiation markers in monoculture fluidic device by Yang et al.21 In contrast, shear stress alone was not shown to be superior to no shear stress in terms of the maturity of the filtration barrier by Musah et al, but only one-seven hundredth of the shear stress by Yang et al was found to be applicable here. In the permeability assay, the urinary clearances of inulin and albumin (Figure 6A) were comparable to those of in-vivo, and the same clearances could not be reproduced under experimental conditions of the endothelial cells only fibroblasts and endothelial cells, or the co-cultured proximal tubular and endothelial cells. This result suggests that clearances in GoC are comparable to those in-vivo, taking into account the effective filtration area of GoC and that the filtration barrier constructed by podocytes and endothelial cells is distinctive.42 Using immortalized mice cells, Zhou et al studied the permeability of inulin and albumin using a two-layered PC porous membrane device without strain, comparing no cells, podocytes alone, endothelial cells alone, and co-cultures of podocytes and endothelial cells. The results demonstrated that the co-culture had the lowest permeability for both molecules and significant differences in permeability among all the groups compared.40 Thus, the filtration barrier constructed from podocytes and endothelial cells has a unique permeability. Furthermore, doxorubicin, used as a model for drug-induced nephrotic syndrome, increased the albumin clearance in a concentration-dependent manner. However, the clearances were measured only 6 h after the initiation of the fluorescently labeled molecular perfusion, and the daily course remains unknown. Nevertheless, the study by Musah et al demonstrating the activation of cell function by mechanical stimulation using a GoC incorporating podocytes derived from hiPS cells is remarkable.42
Using the same microfluidic device, a study by Roye et al reported that both podocytes and endothelial cells were derived from a single patient-derived pluripotent stem cell and were transplanted into a GoC, in an experiment similar to that by Musah et al (Table 1).46 This work provides a blueprint for creating personalized organ models considering genetic polymorphisms.
Petrosyan et al employed the microfluidic device with a bilateral flow with central gel channels (Figure 3A2) and a rat type I collagen gel scaffold. The device was allowed to culture the primary cultured GEnCs with fenestration and podocytes derived from the human stem-like cells in the amniotic fluid (Table 1). The medium for the endothelial cells was filled in the vascular channel and for the podocytes in the urinary channel, as the podocytes faced the urinary channel through the gel. The medium was perfused using a see-saw principle, where the tilt of the device would switch every 10 min, thereby eliminating the need for a perfusion pump. In this experimental system, the cellular organism of the glomerular filtration barrier was cultured for up to 28 days, and the western blotting and immunostaining results demonstrated that the type IV collagen protein would accumulate in the filtration barrier of the glomeruli-on-a-chip. This finding indicates the possibility of the cells to form and maintain their GBMs in GoC. In addition, as mentioned by the authors, the crosstalk between the podocytes and endothelial cells was important for forming the basement membranes, and the microfluidic devices in which the porous membranes separate the podocytes and endothelial cells might be inferior in this respect. The albumin leakage study first compared the three combinations of podocytes derived from the amniotic fluid stem-like cells, primary cultured podocytes, and immortalized podocytes against the GEnCs, suggesting that the primary cultured podocytes had the lowest albumin leakage and retained stability for 28 days. In contrast, the immortalized podocytes showed the highest albumin leakage. This albumin leakage suggested that the immortalized podocytes are unsuitable for assessing the albumin leakage and that the technique for deriving podocytes from the stem cells could be improved. The studies of albumin leakage under pathological conditions included those under exposure to puromycin aminonucleoside and serum from the patients with membranous nephropathy and using podocytes from the patients with Alport’s syndrome (Figure 6B). All of them showed higher levels of albumin leakage than those in the normals. It is a breakthrough that the GoC model of nephrotic syndrome can be achieved by exposure to toxic test substances and the immunological mechanisms and genetic abnormalities.
Other interesting studies included a work in which glomeruli isolated from rats were cultured in a bilateral flow with central matrigel channels device for filtration barrier construction and demonstrated increased permeability of their filtration barriers with exposure to hyperglycemia to build a model of diabetic nephropathy reported by Wang et al (Figure 3A1 and B1),41 a filtration barrier leakage assay with hyperperfusion of media to mimic hypertensive glomerular injury in a two-layered with porous membrane device reported by Zhou et al (Figure 2D2 and D3),40 and encouragement effect of topographical alginate hollow fiber on the interdigitation and elongation of podocyte foot processes (Figure 4B2).45 These experimental systems using animal-derived glomerular cells will also be replicated in the future using human cells.
Future Issues and Perspectives
From a biological point of view, the main challenge of the current GoC involves the maturity of the filtration barrier, particularly concentrated in the cell source of the podocytes and their culture conditions.
The human podocytes derived from the stem cells discussed in this review have shown some advantages over the immortalized podocytes, but their maturation remains a question that has not been compared with that of the human adults in-vivo.42,43,89 Among the various reports on the induction of podocytes and kidney organoids from the stem cells, the culture technique reported by Yoshimura et al for inducing the podocyte aggregates from the nephron progenitor cells is noteworthy. Their report demonstrated the high efficiency of podocyte induction and expression of maturation markers at a protein level comparable to that of the adult human glomeruli in-vivo.87 Although most of the problems with the podocyte cell sources are due to incompatibility between the podocyte maturation and cell number, this induction technique can solve this problem.85
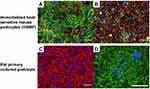
Additionally, there is room for improving the culture conditions for the podocytes in the GoCs, as the interdigitated foot processes and the slit diaphragm formed between the foot processes have not been found to be reproduced in the GoCs, as the cell cycle arrest94 and migration quiescence.95 In cellular biology, the foot processes of the cultured podocytes have been reported to be reproduced under static planar culture conditions, together with the cytoskeletal reorganization of the intermediate diameter filaments structured into the dendritic processes (Figure 7A–C).96–98 The nephrin and podocin linear staining between the foot processes has been confirmed in the primary rat podocytes (Figure 7D), suggesting that the serum-free and laminin 521 scaffolds are essential for the structure of the podocyte foot processes.96,98 The application of these approaches for culturing the podocytes in GoCs and demonstrating the localization of the dendritic intermediate diameter filaments and slit diaphragm proteins between the foot processes and cell cycle migration quiescence would be useful for improving the GoCs.
Based on the cell types constituting the glomerulus, the mesangial cells are eliminated from the current GoCs. The mesangial cells as pericytes mainly provide structural support to the glomerular capillary tuft and do not directly impart a filtration barrier.99,100 Moreover, their importance in reconstituting the highly organized filtration barrier has not been evident. However, the participation of the mesangial cells in the GoC requires the establishment of a model of mesangial proliferative nephritis such as IgA nephropathy.101
The structural specification of the microfluidic device needs to clarify whether the podocytes and endothelial cells should be separated by a porous membrane or cultured in direct contact with each other and whether the pressure is differential across the filtration barrier (approximately 20 mmHg in vivo)62 should be reproduced. These issues are related to the physical stimuli that can be reproduced in the device, the cell–cell interactions that drive the maturation of the filtration barrier, and the differences in the leakage test results between the different molecular sizes. The performance of the filtration barriers and the leakage test results of each device should be compared with established evaluation criteria, which are currently confined to the criteria of individual research groups varying from each other. Interdisciplinary collaborations should be established with mathematicians for developing new mathematical models, thereby complementing and interpreting the differences between the in-vivo and in-vitro data to achieve this. This collaboration would ensure the definition of the confidence intervals for the data of GoC, the establishment of the predictive models for drug discovery research, and thus, the reduction of the development costs in drug discovery research.102,103
We believe that the current intensive collaboration between several research disciplines—engineer, cell biologists, clinicians, pharmacologists, and mathematicians—might evolve practical GoC applications, reconciling the problem of reducing animal testing and drug development costs, to help patients with rare and incurable diseases by providing preventive and therapeutic applications.
Acknowledgments
We thank Dr. Eishin Yaoita for providing images of rat primary podocytes with induced interdigitating foot processes, Dr. Takehiko Wada, and Dr. Taiji Matsuzaka for supporting our arranging of the latest scientific findings of glomerular capillary’s selective permeability, Dr. Jean Cacheux for English proofreading. This review was partially supported by the Japanese Agency for Medical Research and Development (Grant No. 20be0304204h9904).
Disclosure
Kotaro Doi, Hiroshi Kimura, Masaomi Nangaku, and Teruo Fujii report a patent PCT/JP2020/029676 pending. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kotaro Doi, Masaomi Nangaku, and Teruo Fujii report financial support was provided from MEXT/JSPS KAKENHI Grant Number JP 16K15464. Kotaro Doi, Hiroshi Kimura, Masaomi Nangaku, and Teruo Fujii report financial support is provided from AMED under Grant Number JP 20be0304204h9904, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Levin A, Tonelli M, Bonventre J, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet Lond Engl. 2017;390:1888–1917. doi:10.1016/S0140-6736(17)30788-2
2. Levin A, Hemmelgarn B, Culleton B, et al. Guidelines for the management of chronic kidney disease. Can Med Assoc J. 2008;179:1154–1162. doi:10.1503/cmaj.080351
3. Cheung AK, Chang TI, Cushman WC, et al. Executive summary of the KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99:559–569. doi:10.1016/j.kint.2020.10.026
4. Jacobs–Cachá C, Vergara A, García–Carro C, et al. Challenges in primary focal segmental glomerulosclerosis diagnosis: from the diagnostic algorithm to novel biomarkers. Clin Kidney J. 2021;14:482–491. doi:10.1093/ckj/sfaa110
5. Jang HM, Baek HS, Park SH, et al. Clinical characteristics and long-term prognosis of Alport syndrome: a retrospective single-center study. Child Kidney Dis. 2020;24:91–97. doi:10.3339/jkspn.2020.24.2.91
6. Ruggenenti P, Ruggiero B, Cravedi P, et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol. 2014;25:850–863. doi:10.1681/ASN.2013030251
7. Bagga A, Sinha A. Individualizing treatment of steroid-resistant nephrotic syndrome: registries to the fore. Clin J Am Soc Nephrol. 2020;15:920–922. doi:10.2215/CJN.08080520
8. de Jong PE, Curhan GC. Screening, monitoring, and treatment of albuminuria: public health perspectives. J Am Soc Nephrol. 2006;17:2120–2126. doi:10.1681/ASN.2006010097
9. Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–2984. doi:10.1681/ASN.2006040377
10. Gaitonde DY, Cook DL, Rivera IM. Chronic kidney disease: detection and evaluation. Am Fam Physician. 2017;96:776–783.
11. Shankland SJ, Pippin JW, Reiser J, Mundel P. Podocytes in culture: past, present, and future. Kidney Int. 2007;72:26–36. doi:10.1038/sj.ki.5002291
12. Krtil J, Pláteník J, Kazderová M, Tesař V, Zima T. Culture methods of glomerular podocytes. Kidney Blood Press Res. 2007;30:162–174. doi:10.1159/000102520
13. Lal MA, Young KW, Andag U. Targeting the podocyte to treat glomerular kidney disease. Drug Discov Today. 2015;20:1228–1234. doi:10.1016/j.drudis.2015.06.003
14. Lee HW, Khan SQ, Faridi MH, et al. A podocyte-based automated screening assay identifies protective small molecules. J Am Soc Nephrol. 2015;26:2741–2752. doi:10.1681/ASN.2014090859
15. Jefferson JA, Pippin JW, Shankland SJ. Experimental models of membranous nephropathy. Drug Discov Today Dis Models. 2010;7:27–33. doi:10.1016/j.ddmod.2010.11.001
16. Foster MH. Optimizing the translational value of animal models of glomerulonephritis: insights from recent murine prototypes. Am J Physiol-Ren Physiol. 2016;311:F487–F495. doi:10.1152/ajprenal.00275.2016
17. Cianciolo RE, Jennette JC. Glomerulonephritis in animal models and human medicine: discovery, pathogenesis, and diagnostics. Toxicol Pathol. 2018;46:898–903. doi:10.1177/0192623318800714
18. Bomback AS, Appel GB, Gipson DS, et al. Improving clinical trials for anticomplement therapies in complement-mediated glomerulopathies: report of a scientific workshop sponsored by the National Kidney Foundation. Am J Kidney Dis. 2021. doi:10.1053/j.ajkd.2021.07.025
19. Li M, Corbelli A, Watanabe S, et al. Three-dimensional podocyte–endothelial cell co-cultures: assembly, validation, and application to drug testing and intercellular signaling studies. Eur J Pharm Sci. 2016;86:1–12. doi:10.1016/j.ejps.2016.02.013
20. Li M, Alfieri CM, Morello W, et al. Assessment of increased glomerular permeability associated with recurrent focal segmental glomerulosclerosis using an in vitro model of the glomerular filtration barrier. J Nephrol. 2020;33:747–755. doi:10.1007/s40620-019-00683-2
21. Yang SH, Choi JW, Huh D, et al. Roles of fluid shear stress and retinoic acid in the differentiation of primary cultured human podocytes. Exp Cell Res. 2017;354:48–56. doi:10.1016/j.yexcr.2017.03.026
22. Van Norman GA. Limitations of animal studies for predicting toxicity in clinical trials. JACC Basic Transl Sci. 2019;4:845–854. doi:10.1016/j.jacbts.2019.10.008
23. Ebefors K, Lassén E, Anandakrishnan N, Azeloglu EU, Daehn IS. Modeling the glomerular filtration barrier and intercellular crosstalk. Front Physiol. 2021;12. doi:10.3389/fphys.2021.689083
24. Groff K, Bachli E, Lansdowne M, Capaldo T. Review of evidence of environmental impacts of animal research and testing. Environments. 2014;1:14–30. doi:10.3390/environments1010014
25. Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 2015;33:1193–1200. doi:10.1038/nbt.3392
26. Takasato M, Er PX, Chiu HS, Little MH. Generation of kidney organoids from human pluripotent stem cells. Nat Protoc. 2016;11:1681–1692. doi:10.1038/nprot.2016.098
27. Taguchi A, Nishinakamura R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell. 2017;21:730–746.e6. doi:10.1016/j.stem.2017.10.011
28. Low JH, Li P, Chew EGY, et al. Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell. 2019;25:373–387.e9. doi:10.1016/j.stem.2019.06.009
29. Uchimura K, Wu H, Yoshimura Y, Humphreys BD. Human pluripotent stem cell-derived kidney organoids with improved collecting duct maturation and injury modeling. Cell Rep. 2020;33:108514. doi:10.1016/j.celrep.2020.108514
30. Tsujimoto H, Kasahara T, Sueta S, et al. A modular differentiation system maps multiple human kidney lineages from pluripotent stem cells. Cell Rep. 2020;31:107476. doi:10.1016/j.celrep.2020.03.040
31. Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi:10.1126/science.1188302
32. Pauty J, Usuba R, Cheng IG, et al. A vascular endothelial growth factor-dependent sprouting angiogenesis assay based on an in vitro human blood vessel model for the study of anti-angiogenic drugs. EBioMedicine. 2018;27:225–236. doi:10.1016/j.ebiom.2017.12.014
33. Kimura H, Sakai Y, Fujii T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab Pharmacokinet. 2018;33:43–48. doi:10.1016/j.dmpk.2017.11.003
34. Deng J, Wei W, Chen Z, et al. Engineered liver-on-a-chip platform to mimic liver functions and its biomedical applications: a review. Micromachines. 2019;10:676. doi:10.3390/mi10100676
35. Bang S, Jeong S, Choi N, Kim HN. Brain-on-a-chip: a history of development and future perspective. Biomicrofluidics. 2019;13:051301. doi:10.1063/1.5120555
36. Cho KW, Lee WH, Kim BS, Kim DH. Sensors in heart-on-a-chip: a review on recent progress. Talanta. 2020;219:121269. doi:10.1016/j.talanta.2020.121269
37. Donkers JM, Eslami Amirabadi H, van de Steeg E. Intestine-on-a-chip: next level in vitro research model of the human intestine. Curr Opin Toxicol. 2021;25:6–14. doi:10.1016/j.cotox.2020.11.002
38. Shinohara M, Arakawa H, Oda Y, et al. Co-culture with hiPS-derived intestinal cells enhanced human hepatocyte functions in a pneumatic-pressure-driven two-organ microphysiological system. Sci Rep. 2021;11:5437. doi:10.1038/s41598-021-84861-y
39. Sharmin S, Taguchi A, Kaku Y, et al. Human induced pluripotent stem cell–derived podocytes mature into vascularized glomeruli upon experimental transplantation. J Am Soc Nephrol. 2016;27:1778–1791. doi:10.1681/ASN.2015010096
40. Zhou M, Zhang X, Wen X, et al. Development of a functional glomerulus at the organ level on a chip to mimic hypertensive nephropathy. Sci Rep. 2016;6:31771. doi:10.1038/srep31771
41. Wang L, Tao T, Su W, Yu H, Yu Y, Qin J. A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab Chip. 2017;17:1749–1760. doi:10.1039/C7LC00134G
42. Musah S, Mammoto A, Ferrante TC, et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng. 2017;1:0069. doi:10.1038/s41551-017-0069
43. Petrosyan A, Cravedi P, Villani V, et al. A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat Commun. 2019;10:3656. doi:10.1038/s41467-019-11577-z
44. Iampietro C, Bellucci L, Arcolino FO, et al. Molecular and functional characterization of urine‐derived podocytes from patients with Alport syndrome. J Pathol. 2020;252:88–100. doi:10.1002/path.5496
45. Xie R, Korolj A, Liu C, et al. h-FIBER: microfluidic topographical hollow fiber for studies of glomerular filtration barrier. ACS Cent Sci. 2020;6:903–912. doi:10.1021/acscentsci.9b01097
46. Roye Y, Bhattacharya R, Mou X, Zhou Y, Burt MA, Musah S. A personalized glomerulus chip engineered from stem cell-derived epithelium and vascular endothelium. Micromachines. 2021;12:967. doi:10.3390/mi12080967
47. Kanwar YS, Venkatachalam MA. Ultrastructure of glomerulus and juxtaglomerular apparatus. Compr Physiol. 2011:3–40. doi:10.1002/cphy.cp080101
48. Scott RP, Quaggin SE. The cell biology of renal filtration. J Cell Biol. 2015;209:199–210. doi:10.1083/jcb.201410017
49. Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol - Ren Physiol. 2009;296:F947–F956. doi:10.1152/ajprenal.90601.2008
50. Kawasaki Y, Hosoyamada Y, Miyaki T, et al. Three-dimensional architecture of glomerular endothelial cells revealed by FIB-SEM tomography. Front Cell Dev Biol. 2021;9. doi:10.3389/fcell.2021.653472
51. Miner JH. Renal basement membrane components. Kidney Int. 1999;56:2016–2024. doi:10.1046/j.1523-1755.1999.00785.x
52. Lennon R, Randles MJ, Humphries MJ. The importance of podocyte adhesion for a healthy glomerulus. Front Endocrinol. 2014;5. doi:10.3389/fendo.2014.00160
53. Ichimura K, Kakuta S, Kawasaki Y, et al. Morphological process of podocyte development revealed by block-face scanning electron microscopy. J Cell Sci. 2016:
54. Miyaki T, Kawasaki Y, Hosoyamada Y, et al. Three-dimensional imaging of podocyte ultrastructure using FE-SEM and FIB-SEM tomography. Cell Tissue Res. 2020;379:245–254. doi:10.1007/s00441-019-03118-3
55. Ruotsalainen V, Ljungberg P, Wartiovaara J, et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A. 1999;96:7962–7967. doi:10.1073/pnas.96.14.7962
56. Unnersjö-Jess D, Scott L, Blom H, Brismar H. Super-resolution stimulated emission depletion imaging of slit diaphragm proteins in optically cleared kidney tissue. Kidney Int. 2016;89:243–247. doi:10.1038/ki.2015.308
57. Artelt N, Siegerist F, Ritter AM, et al. Comparative analysis of podocyte foot process morphology in three species by 3D super-resolution microscopy. Front Med. 2018;5:292. doi:10.3389/fmed.2018.00292
58. Myers BD, Guasch A. Selectivity of the glomerular filtration barrier in healthy and nephrotic humans. Am J Nephrol. 1993;13:311–317. doi:10.1159/000168645
59. Grahammer F, Wigge C, Schell C, et al. A flexible, multilayered protein scaffold maintains the slit in between glomerular podocytes. JCI Insight. 2016;1:9. doi:10.1172/jci.insight.86177
60. Lawrence MG, Altenburg MK, Sanford R, et al. Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc Natl Acad Sci U S A. 2017;114:2958–2963. doi:10.1073/pnas.1616457114
61. Schlöndorff D, Wyatt CM, Campbell KN. Revisiting the determinants of the glomerular filtration barrier: what goes round must come round. Kidney Int. 2017;92:533–536. doi:10.1016/j.kint.2017.06.003
62. Andreucci VE, Herrera-Acosta J, Rector FC, Seldin DW. Effective glomerular filtration pressure and single nephron filtration rate during hydropenia, elevated ureteral pressure, and acute volume expansion with isotonic saline. J Clin Invest. 1971;50:2230–2234. doi:10.1172/JCI106719
63. Benzing T, Salant D. Insights into glomerular filtration and albuminuria. N Engl J Med. 2021;384:1437–1446. doi:10.1056/NEJMra1808786
64. Pollak MR, Quaggin SE, Hoenig MP, Dworkin LD. The glomerulus: the sphere of influence. Clin J Am Soc Nephrol. 2014;9:1461–1469. doi:10.2215/CJN.09400913
65. Brenner BM, Bohrer MP, Baylis C, Deen WM. Determinants of glomerular permselectivity: insights derived from observations in vivo. Kidney Int. 1977;12:229–237. doi:10.1038/ki.1977.107
66. Vehaskari VM, Root ER, Germuth FG, Robson AM. Glomerular charge and urinary protein excretion: effects of systemic and intrarenal polycation infusion in the rat. Kidney Int. 1982;22:127–135. doi:10.1038/ki.1982.144
67. Holthöfer H. Molecular architecture of the glomerular slit diaphragm: lessons learnt for a better understanding of disease pathogenesis. Nephrol Dial Transplant. 2007;22:2124–2128. doi:10.1093/ndt/gfm344
68. Martin CE, Jones N. Nephrin signaling in the podocyte: an updated view of signal regulation at the slit diaphragm and beyond. Front Endocrinol. 2018;9. doi:10.3389/fendo.2018.00302
69. Butt L, Unnersjö-Jess D, Höhne M, et al. A molecular mechanism explaining albuminuria in kidney disease. Nat Metab. 2020;2:461–474. doi:10.1038/s42255-020-0204-y
70. Haas M, Rastaldi MP, Fervenza FC. Histologic classification of glomerular diseases: clinicopathologic correlations, limitations exposed by validation studies, and suggestions for modification. Kidney Int. 2014;85:779–793. doi:10.1038/ki.2013.375
71. Hebert LA, Parikh S, Prosek J, Nadasdy T, Rovin BH. Differential diagnosis of glomerular disease: a systematic and inclusive approach. Am J Nephrol. 2013;38:253–266. doi:10.1159/000354390
72. Sethi S, Fervenza FC. Standardized classification and reporting of glomerulonephritis. Nephrol Dial Transplant. 2019;34:193–199. doi:10.1093/ndt/gfy220
73. Kim P, Kwon KW, Park MC, Lee SH, Kim SM, Suh KY. Correspondence and requests for materials should be addressed. 2008.
74. Gale BK, Jafek AR, Lambert CJ, et al. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions. 2018;3:60. doi:10.3390/inventions3030060
75. Deguchi S, Tsuda M, Kosugi K, et al. Usability of polydimethylsiloxane-based microfluidic devices in pharmaceutical research using human hepatocytes. ACS Biomater Sci Eng. 2021;7:3648–3657. doi:10.1021/acsbiomaterials.1c00642
76. Zhang J, Wei X, Zeng R, Xu F, Li X. Stem cell culture and differentiation in microfluidic devices toward organ-on-a-chip. Future Sci OA. 2017;3:FSO187. doi:10.4155/fsoa-2016-0091
77. Wu Q, Liu J, Wang X, et al. Organ-on-a-chip: recent breakthroughs and future prospects. Biomed Eng OnLine. 2020;19:9. doi:10.1186/s12938-020-0752-0
78. Quirós-Solano WF, Gaio N, Stassen OMJA, et al. Microfabricated tuneable and transferable porous PDMS membranes for Organs-on-Chips. Sci Rep. 2018;8:13524. doi:10.1038/s41598-018-31912-6
79. Apel P, Blonskaya I, Dmitriev S, Orelovitch O, Sartowska B. Structure of polycarbonate track-etch membranes: origin of the “paradoxical” pore shape. J Membr Sci. 2006;282:393–400. doi:10.1016/j.memsci.2006.05.045
80. Kim MY, Li DJ, Pham LK, Wong BG, Hui EE. Microfabrication of high-resolution porous membranes for cell culture. J Membr Sci. 2014;452:460–469. doi:10.1016/j.memsci.2013.11.034
81. Ikeda K, Nagata S, Okitsu T, Takeuchi S. Cell fiber-based three-dimensional culture system for highly efficient expansion of human induced pluripotent stem cells. Sci Rep. 2017;7:2850. doi:10.1038/s41598-017-03246-2
82. Kruegel J, Rubel D, Gross O. Alport syndrome—insights from basic and clinical research. Nat Rev Nephrol. 2013;9:170–178. doi:10.1038/nrneph.2012.259
83. Nozu K, Nakanishi K, Abe Y, et al. A review of clinical characteristics and genetic backgrounds in Alport syndrome. Clin Exp Nephrol. 2019;23:158–168. doi:10.1007/s10157-018-1629-4
84. Chittiprol S, Chen P, Petrovic-Djergovic D, Eichler T, Ransom RF. Marker expression, behaviors, and responses vary in different lines of conditionally immortalized cultured podocytes. Am J Physiol - Ren Physiol. 2011;301:F660–F671. doi:10.1152/ajprenal.00234.2011
85. Agarwal S, Sudhini YR, Reiser J, Altintas MM. From infancy to fancy: a glimpse into the evolutionary journey of podocytes in culture. Kidney360. 2021;2:385–397. doi:10.34067/KID.0006492020
86. Ciampi O, Iacone R, Longaretti L, et al. Generation of functional podocytes from human induced pluripotent stem cells. Stem Cell Res. 2016;17:130–139. doi:10.1016/j.scr.2016.06.001
87. Yoshimura Y, Taguchi A, Tanigawa S, et al. Manipulation of nephron-patterning signals enables selective induction of podocytes from human pluripotent stem cells. J Am Soc Nephrol. 2019;30:304–321. doi:10.1681/ASN.2018070747
88. Bejoy J, Qian ES, Woodard LE. Accelerated protocol for the differentiation of podocytes from human pluripotent stem cells. STAR Protoc. 2021;2:100898. doi:10.1016/j.xpro.2021.100898
89. Sacco SD, Lemley KV, Sedrakyan S, et al. A novel source of cultured podocytes. PLoS One. 2013;8(12):e81812. doi:10.1371/journal.pone.0081812
90. Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi:10.1016/j.semcancer.2005.05.004
91. Li Y, Meng H, Liu Y, Lee BP. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. Sci World J. 2015;2015:1–10. doi:10.1155/2015/685690
92. Antoine EE, Vlachos PP, Rylander MN. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Eng Part B Rev. 2014;20:683–696. doi:10.1089/ten.teb.2014.0086
93. Yoshida F, Yuzawa Y, Shigematsu H, et al. Nephrotic syndrome with massive accumulation of type I and type III collagen in the glomeruli. Intern Med. 1993;32:171–176. doi:10.2169/internalmedicine.32.171
94. Nagata M, Nakayama K, Terada Y, Hoshi S, Watanabe T. Cell cycle regulation and differentiation in the human podocyte lineage. Am J Pathol. 1998;153:1511–1520. doi:10.1016/S0002-9440(10)65739-2
95. McCaffrey JC, Webb NJ, Poolman TM, et al. Glucocorticoid therapy regulates podocyte motility by inhibition of Rac1. Sci Rep. 2017;7:6725. doi:10.1038/s41598-017-06810-y
96. Yaoita E, Yoshida Y, Nameta M, Takimoto H, Fujinaka H. Induction of interdigitating cell processes in podocyte culture. Kidney Int. 2018;93:519–524. doi:10.1016/j.kint.2017.06.031
97. Doi K, Kimura H, Wada T, et al. A novel method for successful induction of interdigitating process formation in conditionally immortalized podocytes from mice, rats, and humans. Biochem Biophys Res Commun. 2021;570:47–52. doi:10.1016/j.bbrc.2021.07.029
98. Yaoita E, Nameta M, Yoshida Y, Fujinaka H. Dynamic changes of podocytes caused by fibroblast growth factor 2 in culture. Cell Tissue Res. 2021;386:117–126. doi:10.1007/s00441-021-03511-x
99. Kikkawa Y, Virtanen I, Miner JH. Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin α5 in the glomerular basement membrane. J Cell Biol. 2003;161:187–196. doi:10.1083/jcb.200211121
100. Vaughan MR, Quaggin SE. How do mesangial and endothelial cells form the glomerular tuft? J Am Soc Nephrol. 2008;19:24–33. doi:10.1681/ASN.2007040471
101. Scindia YM, Deshmukh US, Bagavant H. Mesangial pathology in glomerular disease: targets for therapeutic intervention. Adv Drug Deliv Rev. 2010;62:1337–1343. doi:10.1016/j.addr.2010.08.011
102. Ballesteros Hernando J, Ramos Gómez M, Díaz Lantada A. Modeling living cells within microfluidic systems using cellular automata models. Sci Rep. 2019;9:14886. doi:10.1038/s41598-019-51494-1
103. Sung JH, Wang Y, Shuler ML. Strategies for using mathematical modeling approaches to design and interpret multi-organ microphysiological systems (MPS). APL Bioeng. 2019;3:021501. doi:10.1063/1.5097675
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.