Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Glioma-associated radiation retinopathy treated successfully with aflibercept
Authors Karagiannis D, Kontomichos L, Georgalas I , Peponis V, Antoniou E, Parikakis E
Received 11 February 2019
Accepted for publication 8 July 2019
Published 26 July 2019 Volume 2019:15 Pages 937—941
DOI https://doi.org/10.2147/TCRM.S204841
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
D Karagiannis,1 L Kontomichos,1 I Georgalas,2 V Peponis,1 E Antoniou,3 E Parikakis1
1Second Department of Ophthalmology, Opthalmiatreion Eye Hospital of Athens, Athens, Greece; 2First Department of Ophthalmology, National and Kapodistrian University of Athens, Athens, Greece; 3Moorfields Eye Hospital, London, UK
Abstract: Radiation retinopathy is a chronic, progressive retinal microangiopathy which can occur with variable latency after retina exposure to ionizing radiation used for cancer treatment. It can occur secondary to treatment of nasopharyngeal tumors, as well as intraocular tumors, such as uveal melanoma and retinoblastoma. Several treatment modalities have been reported including intravitreal corticosteroids, intravitreal anti-VEGFs and argon laser photocoagulation. Our purpose is to present a case report of bilateral radiation retinopathy with macular edema in one eye that was revealed 6 years after glioma therapy and treated successfully by using monotherapy of aflibercept. A 59-year-old male patient presented with gradually deteriorating visual acuity in his left eye for the past 12 months. Best corrected visual acuity in his right eye was 20/25 and in his left eye 20/100. Fundoscopy and fluorescein angiography revealed severe non-proliferative retinopathy in his right eye and proliferative retinopathy in his left eye with macular edema. Following complete work-up and due to his past medical history, he was diagnosed with radiation retinopathy. The patient received 6 intravitreal injections of aflibercept in a period of 9 months in order to treat macular edema and radiation retinopathy. According to the literature, there is minimal experience using aflibercept monotherapy to successfully treat macular edema due to radiation retinopathy. In addition, radiotherapy for glioma is a rather rare cause of radiation retinopathy compared to other more common causes, such as nasopharyngeal tumors, meningiomas, and uveal melanomas.
Keywords: aflibercept, macular edema, glioma, radiation retinopathy
Introduction
Radiation retinopathy is a progressive vascular clinical manifestation, potentially leading to remarkable deterioration in visual acuity or even blindness. It can occur secondary to treatment of nasopharyngeal tumors,1 meningiomas, as well as intraocular tumors, such as uveal melanoma2 and retinoblastoma. Latency period varies between 3 months and 8 years, with a peak at 1–1.5 years.3 The crucial upper limits of radiation (dose and daily fraction size) are ill-defined. The occurrence of radiation retinopathy is related to conditions such as hypertension, diabetes, and collagen vascular diseases.4 There is no consensus of treatment but current treatment strategies embrace intravitreal ranibizumab5,6 and bevacizumab.6,7 Other options include argon laser photocoagulation,8 intravitreal steroid injection9,10 or a combination of intravitreal anti-vascular endothelial growth factor (anti-VEGF) and focal laser photocoagulation.11 Our aim is to present a case report of macular edema due to radiation retinopathy that was revealed 6 years after the patient received ionizing radiotherapy for glioma treatment, and treated successfully by using aflibercept monotherapy. Aflibercept is a fully human, recombinant fusion protein designed to bind all isoforms of VEGF-A, as well as placental growth factor (PGF), thereby inhibiting the binding and activation of VEGF receptors. Vascular endothelial growth factor (VEGF) has emerged as a key target of treatment, with inhibitors of VEGF being shown to arrest the angiogenic process and avoid the visual damage typically associated with its presence in cases of wet age-related macular degeneration12 and diabetic macular edema.13
Materials and methods
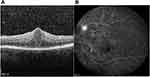
A 59-year-old-male presented to our outpatient department with decreased vision in his left eye (LE) over the last 12 months. ago. His previous ocular history was unremarkable. His best corrected visual acuity was 20/25 right eye (RE) (+1.75sph +0.50×155°) and 20/100 OS (+1.75sph). Slit -lamp examination of the anterior segment was insignificant. Intraocular pressure was 16 mmHg in both eyes. Fundoscopic examination disclosed multiple retinal hemorrhages and cotton wool spots in both eyes (more in the LE than in the RE) and macular edema OS. OCT of the LE showed cystoid macular oedema with central retinal thickness of 603 µm due to intraretinal fluid accumulation (Figure 1A). Fluorescein Angiography revealed capillary non-perfusion, microaneurysms and hemorrhages in both eyes. Moreover, in the left eye (OS), there was cystoid macular oedema and leakage of fluorescein due to retinal neovascularization (Figure 1B). In conclusion, the patient suffered from severe non-proliferative retinopathy OD and proliferative retinopathy OS. We requested complete blood count (CBC) along with blood glucose level and HbA1c. All of the above proved normal. The rest of the workup included ESR, CRP, ANA, ANCA and antiphospholipid antibodies. These were also all negative. Carotid ultrasound imaging was normal as well. His past medical history was unremarkable apart from glioma which was treated with external beam radiation 6 years ago.
Results
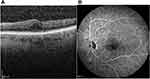
Based on the characteristic retinal findings and history of radiation in the past, a diagnosis of radiation retinopathy was made. It was decided to treat the patient with intravitreal aflibercept in his LE. One month after the first injection, macular oedema decreased remarkably. OCT of the LE disclosed a reduction of Central Foveal Thickness (CFT) from 603 μm to 420 μm. Apart from the regression of intraretinal fluid, visual acuity OS increased from 20/100 to 20/60. Nine months after the initiation of therapy, the patient has received 6 intravitreal injections of aflibercept. Fluorescein angiography of the left eye revealed complete regression of Neovascularization Elsewhere (NVE) (Figure 2B). His visual acuity is 20/50 OS and the intraretinal fluid in Optical Coherence Tomography (OCT) (Figure 2A) has almost resolved (CFT 320 μm) in comparison with the initial presentation before starting treatment. The patient is still under review although there is a remarkable response to aflibercept.
Discussion
Radiation retinopathy is a typically late, devastating complication of radiation therapy and remains a devastating cause of visual loss, however, early diagnosis and treatment shows good prognosis. The occurrence of radiation retinopathy is related to the total dose of radiation as well as to the daily fraction size.
A study by Parsons et al.14 showed that radiation retinopathy was not observed at doses below 45 Gy, but increased steadily in incidence at doses > or =45 Gy. In the range of doses between 45 and 55 Gy, there was an increased risk of injury among patients who received doses per fraction of > or =1.9 Gy.
Monroe AT et al.15 stated that The incidence of ipsilateral radiation retinopathy after treatment of nasal cavity/paranasal tumors is 20% at 5 and 10 years. Retinal dose and fractionation schedule are the strongest predictors of retinopathy. Hyperfractionated radiotherapy is associated with a significant reduction in the incidence of radiation retinopathy, especially when the retina receives more than 50 Gy.
Conditions such as hypertension, diabetes, and collagen vascular diseases could play a key role. Although the management of radiation-induced retinal damage remains challenging, meticulous examination, regular follow-up and proper treatment may help in the prevention of vision loss.
Until the emergence of anti-VEGF injections, focal or panretinal laser photocoagulation and intravitreal corticosteroids were the only available therapeutic modalities for the management of radiation retinopathy.8,10
According to a 10-year study by Finger PT et.al.6 continuous intravitreal anti-VEGF therapy with ranibizumab and bevacizumab in patients with radiation maculopathy was well-tolerated and preserved vision. In most cases, reduction or resolution of retinal hemorrhages, cotton-wool spots, and retinal edema were noted for up to 10 years.
A study by Tarmann et al.9 showed that intravitreal dexamethasone seems to be efficient in reducing retinal thickness in radiation induced maculopathy refractory to bevacizumab.
Pooprasert et al.16 presented a case in which aflibercept monotherapy was used to treat cystoid macular oedema in a patient with radiation retinopathy.
As stated in a study by Fallico et al.17 in which 9 patients were included, intravitreal aflibercept is an effective treatment for patients with radiation-induced macular edema, allowing functional and anatomical improvements to be achieved with a relatively low number of injections.
Loukianou et al.18 published a case report in which intravitreal aflibercept was used effectively in a patient with recalcitrant macular edema due to external beam radiotherapy for nasopharyngeal cancer.
In a prospective, randomized clinical trial (in press) by Murray et al.19 intravitreal aflibercept seems to limit visual loss associated with radiation maculopathy. Forty patients were enrolled with 1 year of follow-up.
In our patient, a 59-year-old male suffering from radiation retinopathy after radiation for glioma treatment, we applied aflibercept monotherapy to treat not only radiation induced maculopathy but also proliferative retinopathy. The patient showed almost complete resolution of macular edema and regression of neovascularization in a period of 9 months. It is known that aflibercept has favorable results in neovascularization from the Clarity study.20,21 The latter showed that patients receiving aflibercept for proliferative diabetic retinopathy had complete regression of neovascularization in 64% in week 52. Despite the good response the patient is still under regular follow-up.
Conclusion
Radiation retinopathy has been reported as a common cause secondary to treatment of nasopharyngeal tumors, and meningiomas. Different therapeutic modalities have been used to treat radiation retinopathy. According to the literature there is minimal experience using intravitreal aflibercept as a treatment for patients with macular edema and proliferative retinopathy due to radiation retinopathy. We report an interesting case of radiation retinopathy, after radiotherapy for glioma treatment, with macular edema treated successfully with intravitreal aflibercept. In addition, proliferative disease was regressed completely during the follow up period.
Ethics and consent
Institutional approval was required to publish the case details. We proceeded with the study after having received approval from the Ethical Committee of Ophthalmiatreion Eye Hospital of Athens.
The patient provided written informed consent for the case details and any accompanying images to be published.
Acknowledgment
No financial or grant support was received for this report.
Disclosure
The authors report no conflicts of interest in this study.
References
1. Takeda A, Shigematsu N, Suzuki S, et al. Late retinal complications of radiation therapy for nasal and paranasal malignancies: relationship between irradiated‐dose area and severity. Int J Radiat Oncol Biol Phys. 1999;44:599–605. doi:10.1016/S0360-3016(99)00057-7
2. Matet A, Daruich A, Zografos L. Radiation maculopathy after proton beam therapy for uveal melanoma: optical coherence tomography angiography alterations influencing visual acuity.invest. Ophthalmol Vis Sci. 2017;58(10):3851–3861. doi:10.1167/iovs.17-22324
3. Brown G, Shields JA, Sanborn G, Augsburger JJ, Savino PJ, Schatz NJ. Radiation retinopathy. Ophthalmology. 1982;89:1494–1501.
4. Seregard S, Pelayes DE, Singh AD. Radiation therapy: posterior segment complications. Dev Ophthalmol. 2013;52:114–123.
5. Finger PT, Chin KJ. High-dose (2.0 mg) intravitreal ranibizumab for recalcitrant radiation retinopathy. Eur J Ophthalmol. 2013;23:850–856. doi:10.5301/ejo.5000333
6. Finger PT, Chin KJ, Semenova EA. Intravitreal anti-VEGF therapy for macular radiation retinopathy: a 10-year study. Eur J Ophthalmol. 2016;26:60–66. doi:10.5301/ejo.5000670
7. Mashayekhi A, Rojanaporn D, AL-Dahmash S, Shields CL, Shields JA. Monthly intravitreal bevacizumab for macular edema after iodine-125 plaque radiotherapy of uveal melanoma. Eur J Ophthalmol. 2014;24:228–234. doi:10.5301/ejo.5000352
8. Kikyoun JL. Long term visual acuity results of treated and untreated radiation retinopathy (an AOS thesis). Trans Am Ophthalmol Soc. 2008;106:325–335.
9. Tarmann L, Langmann G, Mayer C, Weger M, Haas A, Wackernagel W. Ozurdex reduces the retinal thickness in radiation maculopathy refractory to bevacizumab. Acta Ophthalmol. 2014;92:e694–e696. doi:10.1111/aos.12424
10. Shields CL, Demirci H, Dai V, et al. Intravitreal triamcinolone acetonide for radiation maculopathy after plaque radiotherapy for choroidal melanoma. Retina. 2005;25:868–874.
11. Hurtikova K, Gerding H. Combined laser photocoagulation and anti-VEGF injection treatment in radiation retinopathy. KlinMonblAugenheilkd. 2017;234(4):515–519.
12. Ohr M, Kaiser PK. Intravitreal aflibercept injection for neovascular (wet) age-related macular degeneration. Expert Opin Pharmacother. 2012;13(4):585–591. doi:10.1517/14656566.2012.658368
13. Avitabile T, Azzolini C, Bandello F, et al. Aflibercept in the treatment of diabetic macular edema: a review and consensus paper. Eur J Ophthalmol. 2017;27(6):627–639. doi:10.5301/ejo.5001053
14. Parsons JT
15. Monroe AT
16. Pooprasert P, Young-Zvandasara T, Al-Bermani A. Radiation retinopathy treated successfully with aflibercept. BMJ Case Rep. 2017;2017. doi:10.1136/bcr-2017-220744
17. Fallico M, Reibaldi M, Avitabile T, et al. Intravitreal aflibercept for the treatment of radiation-induced macular edema after ruthenium 106 plaque radiotherapy for choroidal melanoma. Graefes Arch Clin Exp Ophthalmol. 2019;257(7):1547–1554. doi:10.1007/s00417-019-04347-6
18. Loukianou E, Loukianou G. Intravitreal aflibercept in recalcitrant radiation maculopathy due to external beam radiotherapy for nasopharyngeal cancer: a first case report. Case Rep Ophthalmol. 2017;8(1):87–90. doi:10.1159/000456535
19. Murray TG, Latiff A, Villegas VM, Gold AS. Aflibercept for Radiation Maculopathy Study: A Prospective Randomized Clinical Study. Ophthalmol Retina. 2019;3(7):561–566.
20. Sivaprasad S, Prevost AT, Bainbridge J, et al. Clinical efficacy and mechanistic evaluation of aflibercept for proliferative diabetic retinopathy (acronym CLARITY): a multicentre phase IIb randomised active-controlled clinical trial. BMJ Open. 2015;5(9):e008405. doi:10.1136/bmjopen-2015-008405
21. Sivaprasad S, Hykin P, Prevost AT, et al. Intravitreal Aflibercept Compared with Panretinal Photocoagulation for Proliferative Diabetic Retinopathy: The CLARITY Non-inferiority RCT. Southampton (UK): NIHR Journals Library; Oct 2018.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.