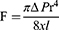Back to Journals » Clinical Ophthalmology » Volume 18
Glaucoma Surgery and Ocular Blood Flow in Colour Doppler Imaging: Is There a Link?
Authors Zarzecki M , Obuchowska I , Ustymowicz A , Konopińska J
Received 24 September 2023
Accepted for publication 13 December 2023
Published 6 January 2024 Volume 2024:18 Pages 49—60
DOI https://doi.org/10.2147/OPTH.S441805
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mateusz Zarzecki,1 Iwona Obuchowska,1 Andrzej Ustymowicz,2 Joanna Konopińska1
1Department of Ophthalmology, Medical University of Bialystok, Bialystok, Poland; 2Department of Radiology, Medical University of Bialystok, Bialystok, Poland
Correspondence: Joanna Konopińska, Department of Ophthalmology, Medical University of Bialystok, Bialystok, Poland, Tel +48-857468372 ; +48-600471666, Fax +48-857468604, Email [email protected]
Abstract: Glaucoma is a common cause of blindness worldwide. This disease is characterised by increased intraocular pressure (IOP) and the concomitant disruption of ocular haemodynamic. Several studies have demonstrated that trabeculectomy is associated with changes in extraocular blood flow. In this study, we reviewed the available evidence on the use of colour Doppler imaging to evaluate and manage patients with open-angle glaucoma. We present the detailed anatomy of ocular blood flow to provide a background for the research findings. We also discuss the physiological foundations of ocular blood flow and detailed flow characteristics of specific extraocular vessels. Finally, we reviewed published studies that analysed the effects of glaucoma surgery on the blood flow parameters of the eye.
Keywords: glaucoma, colour doppler, trabeculectomy, deep sclerectomy
Introduction
Glaucoma comprises a group of ocular diseases with a multifaceted aetiology; however, the common feature is progressive, irreversible optic nerve injury that eventually leads to vision loss.1 According to the World Health Organisation, glaucoma is the second leading cause of blindness worldwide.2 It is estimated that glaucoma affects 80 million individuals worldwide and approximately 11 million patients experience complete blindness. Owing to the aging population, the number of patients with glaucoma has been increasing steadily and is projected to reach 112 million by 2040.3 The pathogenesis of this disease involves the loss of retinal ganglion cells and their axons, leading to optic disc alterations and visual field loss. The major quantifiable risk factor for glaucoma is an increase in intraocular pressure (IOP), resulting from an imbalance between the production of aqueous humour and its drainage from the anterior chamber of the eye. Reducing IOP is the only established treatment option that may inhibit or slow the progression of vision loss.4 The progression of glaucoma is associated with the gradual development of structural and functional anomalies within the optic nerve, resulting in peripheral visual field loss, which significantly deteriorates quality of life during advanced stages of the disease.1 The pathogenesis of glaucoma is complex, multifactorial and poorly understood. Although increased IOP is believed to directly contribute to mechanical damage to the optic nerve head, only one-third of patients with glaucoma present with increased IOP during the early stages of the disease.5 Furthermore, visual field loss progresses in some patients despite achieving IOP control. According to the “vascular theory” of glaucoma, the loss of retinal ganglion cells results from an inadequate blood supply. Reduced ocular blood flow may be associated with vascular constriction and autoregulatory disorders. This hypothesis is supported by the co-occurrence of glaucoma with vascular diseases such as arterial hypertension and diabetes mellitus. Potential contributing factors include mechanical stress, overproduction of reactive oxygen species (ROS), and neurotrophin deficiency.6 An increase in IOP causes compression of blood vessels, which may impair blood flow in the retina. If the IOP is normal, retinal ischaemia may result from systemic vascular deregulation observed in many diseases, including arterial hypertension or hypotension, nocturnal hypotonia, heart rate disorders, Raynaud syndrome, and migraine. Reduced ocular blood flow has been identified as an IOP-independent risk factor for glaucoma progression because it leads to oxygen and nutrient deficiencies, resulting in disorders of cellular energy metabolism and accumulation of ROS.7,8 In the normal retina, ROS are generated during metabolic processes and are scavenged by antioxidant enzymes and chemical antioxidants. However, in glaucomatous eyes, ROS is generated in excess of the neutralising potential of the antioxidant system. This leads to oxidative stress in retinal ganglion cells, resulting in structural damage to proteins and nucleic acids, oxidation of lipids, hyaluronic acid depolymerisation, and eventually, cell apoptosis.9 Although blood flow anomalies associated with glaucoma have been examined using various techniques, it is still unclear whether they are the cause or a consequence of the disease.
Doppler imaging is one of the most widespread, safe, non-invasive, and affordable methods used in medical centres worldwide. Doppler ultrasonography is considered a potentially useful tool for the initial diagnosis and further evaluation of extraocular blood flow anomalies associated with glaucoma. Therefore, it is recommended to monitor treatment outcomes. Despite the abundance of published data regarding the haemodynamic of extraocular blood flow in patients with glaucoma,10–13 little is known about the effects of glaucoma surgery, especially the most recent modality (minimally invasive glaucoma surgery), on blood perfusion in the eye and orbit. Owing to the scarcity of published data regarding the effects of non-pharmacological glaucoma therapies on ocular blood flow parameters determined using colour Doppler imaging (CDI), we evaluated the applicability of this imaging method for monitoring treatment outcomes.
Anatomy of Retinal and Optic Nerve Blood Flow
Understanding the role of vascular mechanisms in glaucomatous optic neuropathy is impossible without knowledge of the anatomy and physiology of the optic nerve blood flow. The primary function of the ocular vascular bed is to adequately nourish various components of the eye to meet their energy demands. The energy demands of the retina and optic nerve are among the highest in the human body.14 Ocular and optic nerve blood flow systems originate from the internal carotid arteries. The extracranial ramification of the ophthalmic artery (OA) is the primary source of blood supply to the eyes. The OA also supplies other orbital structures such as the sphenoidal sinus, ethmoid cells, nasal septum, and superior nasal concha. The artery enters the orbit via the lacrimal canal together with the optic nerve and runs within its inferolateral portion. The OA has multiple branches within the orbit, with the principal branch being the central retinal artery (CRA), which delivers blood to the internal layers of the retina and the centrally located fibres of the optic nerve. The CRA penetrates the optic nerve approximately 0.5 to 1.5 cm from the posterior ocular wall and leaves the nerve at the fundus, within the optic disc.15 At the fundus, the CRA ramifies into the following radially spreading terminal branches: the two temporal branches (superior and inferior) and the two nasal branches (superior and inferior). These arteries then undergo further ramifications to form capillary networks.16,17 Capillaries are grouped into the following four plexuses: the peripapillary capillary plexus, which runs parallel to the axons of the neuroretina; the superficial capillary plexus, which is located within the ganglion cell layer; the intermediate capillary plexus, which is formed between the inner plexiform and inner nuclear layers; and the deep capillary plexus, which runs between the inner and outer plexiform layer.18
OA has further ramifications in the anterior and posterior ciliary arteries, with the latter divided into the long posterior ciliary arteries providing blood to the anterior segment of the choroid and short posterior ciliary arteries (SPCAs) supplying the choroid and outer retinal layers, including the photoreceptors. The SPCAs form two bundles, the lateral and medial bundles, on both sides of the optic nerve, which penetrate the sclera at the posterior pole and reach the choroid. SPCAs can also be divided into perioptic SPCAs, which enter the eye immediately next to the optic nerve, and distal SPCAs, which penetrate the sclera more distally than the optic nerve. The perioptic branches deliver blood to the optic nerve, while the distal branches supply blood to the choroid.15 The arteries within the choroid form three layers. The outermost layer, called Haller’s layer, contains large vessels that originate directly from SPCAs. The intermediate layer, called Sattler’s layer, consists of small- and medium-sized arteries. The smallest capillaries form the choriocapillaris layer.
The vasculature of the optic nerve originates from both the CRA branches and SPCAs and varies depending on the nerve segment. The superficial layer of nerve fibres at the fundus is supplied by minute arterioles and retrograde ramifications of the CRA branches. The prelaminar and lamina regions of the nerve receive blood directly from the SPCAs as well as via the peripapillary arterial circle of Zinn-Haller. A circle located within the sclera of the peripapillary area is typically ellipsoidal in shape, although it may be round or absent. It is formed by arterial anastomoses derived from the lateral and medial bundles of ciliary vessels.19 Because SPCAs are terminal branches, a relatively poorly vascularised zone called the watershed zone is formed at the periphery of the supply area. Whenever blood flow within the ciliary vessels of either the medial or lateral bundle is reduced, the watershed zone is at a high risk of ischaemia. Angiographic imaging allows accurate localisation of the watershed within the posterior pole of the eyeball. In 86% of cases, the watershed is located within the optic disc and extends to the entire disc or is limited to its temporal fragments.20
The segment of the optic nerve behind the lamina cribrosa is supplied with blood from two principal sources: the CRA and the vascular system derived from the meningeal arteries. The latter system forms an anastomotic network located within the pia mater of the optic nerve and is supplied by retrograde branches ramifying from the peripapillary arterial circle of Zinn-Haller and SPCAs passing through that area. The vascular network of the pia mater forms the peripheral concentric vascular system of the optic nerve. The CRA, which delivers blood to the central area of the nerve, forms the axial eccentric vascular system of the optic nerve.15,20 In both the systems, the vessels communicate via an anastomotic network. Venous blood from the entire optic nerve drains into the central retinal vein (CRV) and its branches in the prelaminar area, and into the choroidal veins of the peripapillary region.20
Physiological Foundations of Ocular Blood Flow
Blood flow within the vessels correlates directly with perfusion pressure (PP) and inversely with vascular resistance (VR).21 Ocular blood flow is modulated not only by systemic blood pressure (BP), but also by IOP. Thus, ocular perfusion pressure (OPP) can be described using the following formula:
Furthermore, the mean BP can be derived from the following formula:
In the OPP formula, “2/3” reflects the reduction of BP between the brachial artery, where the systemic pressure is measured with a sphygmomanometer while the patient is in a seated position, and the ophthalmic artery, where IOP is determined using tonometry. Additionally, the fact that the arteries delivering blood to the eye are more anatomically distant from the heart than the brachial artery should be considered.22
In addition to the PP, blood flow is influenced by the VR, which is modulated by a plethora of parameters. This relationship is described by the Hagen-Poiseuille equation. According to that equation, the intensity of laminar flow (F) is modulated by blood viscosity ( ), pressure divergence between arteries and veins (
), pressure divergence between arteries and veins ( ), vessel length (
), vessel length ( ), and vessel diameter (
), and vessel diameter ( ).
).
By reversing this statement, the factors that influence the changes in VR can be identified. Considering the modifiable elements of the formula, an increase in flow resistance was promoted by the higher viscosity of the blood, lower pressure in the arterial bed, higher pressure in the venous bed, and shorter radius of the vessel. The fact that vascular diameter in the formula increased to the fourth power implies that this factor is the most important determinant of blood flow and flow resistance; however, it has the highest variability.20
Colour Doppler Imaging
CDI was used to examine ocular and orbital blood flow. This validated modern diagnostic modality is superior to other imaging techniques because it visualises the actual dynamics of blood flow within specific eye vessels.
CDI was first used in clinical practice in 1957 to visualise large vessels, such as the carotid arteries and heart.20 Since then, CDI techniques have progressed substantially and have been introduced into routine clinical practice. The first study on the applicability of Doppler imaging in ophthalmology was published in 1989.23 The authors discussed specific aspects of extraocular blood flow imaging and the ultrasonographic characteristics of the principal blood vessels supplying the orbit.
Doppler ultrasonography is based on a phenomenon discovered in 1842 by Austrian mathematician Christian Andreas Doppler during his research on electromagnetic waves. The Doppler effect is the apparent change in the frequency of a wave relative to an observer moving in relation to the wave source. The Doppler effect is utilised in medicine to assess blood flow through arteries and veins based on changes in the frequency of the ultrasound reflected by moving erythrocytes. This allowed the velocity and direction (from/to the ultrasonographic probe) of the flow to be determined. The frequency of the wave dispersed by the erythrocytes differs from that of the wave emitted by the probe; this slight difference is referred to as the Doppler shift. This shift, also known as Doppler frequency, is proportional to the velocity of moving erythrocytes.24
Examination Technique and Colour Doppler Imaging Measurements Used in Ophthalmology
When passing through tissues, the ultrasound beam is attenuated, and its amplitude decreases proportionally with the penetration depth of the beam. In soft tissues, the attenuation coefficient increases linearly with wave frequency and average of 0.5 to 0.7 dB per 1 cm. The most appropriate frequency for the measurement of blood flow within the ocular and orbital vessels located at a depth of 4 to 10 cm was found to be 7.5 to 10 MHz.25 Optimally, the examination should be conducted with the patient in a supine position with the head tilted at 30°. The patients were asked to close their eyes and look straight ahead. Subsequently, a special ultrasound gel was applied to their eyelids. Before the examination, the signal gain should be set to a level suitable for measuring low flow velocities.26 An appropriate calibration of the pulse repetition frequency eliminated the problem of aliasing. The latter refers to a bias in low-flow velocity measurements caused by the excessively low sampling frequency of the Doppler echo, that is, an excessively low pulse repetition frequency. The choice of the correct sample volume guarantees that the waveform provides all the information about the flow within a given vessel. Depending on the device, the minimum size of the Doppler sample is 1.2 mm × 1.2 mm or 1.5 mm × 1.5 mm. Organs with detectable blood flow were presented as collections of colour pixels, whereas no-flow tissues were visualised using standard greyscale. Doppler signals can be encoded based on various criteria. The colour of a pixel often reflects the frequency (velocity) and orientation (direction) of the flow. Under these circumstances, the colour intensity depends on the flow velocity. The colour map of Doppler imaging is usually set such that the flow toward the probe is shown in red, and the opposite flow is depicted in blue.
The extraocular vessels routinely examined include the OA, CRA, SPCAs, and CRV. In some cases, the evaluation of flow within the ocular and orbital vessels is preceded by the determination of the haemodynamic parameters of the internal carotid artery as the source of the entire ocular blood flow. This allows the detection of potential abnormalities in this vessel that might affect the flow through the extraocular arteries. The OA was visualised as the largest arterial vessel in the orbit and is depicted in red. During the OA examination, the Doppler gate was set 10–25 mm behind the eyeball. The CRA is a small artery (depicted in red) in the central optic nerve. Its identification may be facilitated by simultaneous visualisation of the flow in the CRV (depicted in blue). Both vessels were examined 5–10 mm behind the posterior wall of the eyeball. SPCAs can be found in the temporal and nasal portions of the optic nerve because the red points are located in close proximity thereof.22 Owing to the large number of ciliary arteries, the analysis included only the strongest signals received from these vessels. The flow parameters within the CRA were measured at Doppler angles of 0–20° to obtain the maximum values of the Doppler shift. For the OA and SPCAs, the Doppler angle was set at 10–45°.
Analysis of the blood flow spectrum within specific vessels includes the measurement of flow velocities, that is, peak systolic velocity (PSV), end diastolic velocity (EDV), mean flow velocity (MFV), and resistance index (RI). Although all velocities are expressed in cm/s, RI has no units and ranges from 0 to 1, corresponding to no resistance and a very high VR, respectively.16 From a clinical perspective, the PSV, ESV, and RI are the most critical parameters. The PSV was measured at peak systole, whereas the EDV was measured at end-diastole. The mean velocity was calculated automatically by the device based on Doppler spectrum recording. The mean flow velocity and transverse cross-section of the vessel are, in turn, used to determine the flow volume. The RI, which is a measure of VR, was calculated using the following formula:  . An additional parameter determined during the examination is the pulsatility index. The pulsatility index is used to evaluate vessels in which retrograde flow can occur and is obtained using the following formula:
. An additional parameter determined during the examination is the pulsatility index. The pulsatility index is used to evaluate vessels in which retrograde flow can occur and is obtained using the following formula:  10
10
Characteristics of Blood Flow Within the Ocular Vessels
Ophthalmic Artery
Blood flow velocities within the OA were the highest among all extraocular vessels, and the measurements were highly reproducible. The flow velocity within the OA can be modulated by age, BP, cigarette smoking, and body position during examination. The OA is mostly contorted in the posterior part of the orbit and has many ramifications that can influence blood flow. Thus, the most accurate flow velocity measurement can be obtained from a straight segment of the OA in the nasal part of the orbit.22 (Figure 1).
 |
Figure 1 Blood flow spectrum of ophthalmic artery in Colour Doppler Imaging. |
Central Retinal Artery and Central Retinal Vein
The CRA and CRV are easy to identify and examine because they run in a straight line in the central portion of the optic nerve. They are small vessels with low flow velocities. Owing to its autoregulatory potential, retinal circulation is less dependent on BP and body position; however, it is more dependent on IOP. Excessively strong eyeball compression with the probe during the examination may increase the IOP and cause bias in the CRA flow measurement. Blood flow through the CRV is characterised as nonpulsatile (Figure 2).
 |
Figure 2 Blood flow spectrum of central retinal artery and central retinal vein in Colour Doppler Imaging. |
Short Posterior Ciliary Arteries
Since the SPCAs are multiple vessels, they were examined as bundles in the vicinity of the lamina cribrosa on both sides of the optic nerve. The number of SPCAs was highly variable; hence, it was impossible to determine the number of vessels examined simultaneously. Owing to the variation in the number and course of SPCAs, the reproducibility of flow measurements within these vessels was lower than that in other extraocular vessels. The flow velocity in SPCAs is generally lower and less IOP-dependent than that in the CRA.22 (Figure 3).
 |
Figure 3 Blood flow spectrum of short posterior ciliary artery in Colour Doppler Imaging. |
Surgical Treatment of Glaucoma
IOP is the most important established risk factor for the occurrence and progression of open-angle glaucoma, and its reduction remains the only evidence-based intervention that slows or halts disease progression. The initial steps in glaucoma treatment usually include pharmacotherapy and lifestyle modifications. Eye drops are typically used as first-line treatment for glaucoma. Topical agents decrease the IOP by reducing aqueous humour production or improving drainage. Additional treatment modalities can be considered if the initial therapeutic approach is insufficient to control the IOP and prevent further optic nerve injury. Alternative treatment options include laser procedures such as selective laser trabeculoplasty and minimally invasive glaucoma surgeries such as iStent,27 ab-interno canaloplasty,28 or trabeculectomy with Kahook-Dual Blade.29 The treatment choice depends on the type and severity of glaucoma, patient health, and response to previous therapies.30
The results of the Collaborative Initial Glaucoma Treatment Study confirmed that surgery, when implemented as first-line treatment, provides better IOP control than a conservative approach.31 The demand for minimally invasive surgical interventions suitable for application during the early stages of glaucomatous optic neuropathy has stimulated research on operational techniques that could improve quality of life through effective control of IOP with minimal risk of postoperative complications.
Trabeculectomy is still considered the gold standard for glaucoma surgery because it reduces IOP by 47.73%–65.48%.30,32 Although this bleb-dependent filtering procedure has high success rates, it poses a high risk of severe intra-and postoperative complications and requires prolonged recovery. Therefore, a trabeculectomy is more likely to be performed in patients with advanced glaucoma. Implementing non-invasive or minimally invasive procedures is a milestone in the surgical treatment of glaucoma, and these treatments are frequently offered to patients during the early and intermediate stages of the disease.33
Nonpenetrating surgical procedures, during which the aqueous humour can be filtered without removing the entire thickness of the trabecular meshwork, also aim to achieve IOP control and are considered safer than trabeculectomy. Surgical correction of the natural drainage pathway for the aqueous humour, including Schlemm’s canal, to restore normal function and IOP control without penetrating the intraocular space as an alternative to penetrating methods has stimulated the interest of researchers dealing with open-angle glaucoma.34
Although pharmacotherapy slows the progression of visual field loss, better IOP control can be achieved with filtration surgery. Surgical intervention may result in greater IOP reduction and fewer fluctuations than pharmacotherapy; as such, it is more likely to prevent future visual field loss in the future35 (see also AGIS Investigators 2000). Since blood flow impairment within the optic nerve can be an underlying mechanism of glaucoma, we reviewed published studies that analysed the effects of surgical intervention on ocular blood flow in patients with this disease.
In addition to IOP reduction, the effect of glaucoma surgery on ocular blood flow has important clinical implications. Trabeculectomy is frequently performed in patients with glaucoma in whom IOP cannot be sufficiently controlled with pharmacotherapy. Given the substantial decrease in IOP after trabeculectomy, a considerable increase in OPP may be expected in patients who have undergone surgery. However, to the best of our knowledge, only a few published studies have analysed the effects of glaucoma surgery on ocular and orbital haemodynamics. The aforementioned studies included patients who underwent conventional glaucoma surgeries such as trabeculectomy and deep sclerectomy. However, these studies did not analyse the problems associated with minimally invasive glaucoma surgeries.
Trabeculectomy
Research on the effects of glaucoma surgery on the haemodynamic parameters of extraocular vessels has been performed since the last decade of the 20th century. Trabeculectomy was the primary surgical intervention for glaucoma and remained the gold standard for the management of narrow-angle glaucoma. Trible et al36 conducted the first study to analyse the effects of glaucoma surgery on blood flow within the optic nerve. In 1993, these authors demonstrated for the first time that trabeculectomy with the resultant IOP reduction markedly improved the haemodynamic parameters of the extraocular vessels of patients with chronic glaucoma, namely the OA, CRA, and nasal and temporal SPCAs.
The study conducted by Trible et al36 documented significant favourable changes in the Doppler parameters of all analysed vessels. Patients were examined 2, 5, and 14 weeks after trabeculectomy. At all time points during the study, significant increases in PSV, MFV, and EDV were observed in the CRA group, along with a decrease in RI. In the nasal and temporal SPCAs, significant increases in MSV and EDV with a simultaneous decrease in RI were observed during each follow-up visit. Increases in the PSV, MSV, and EDV were also observed in the OA group; however, only some of these changes were statistically significant. The latter observation was not surprising because the OA, which is the largest of the examined vessels and is located at the longest distance from the posterior ocular wall, is naturally less susceptible to the effect of glaucoma surgery. The first study to analyse the effect of trabeculectomy on ocular haemodynamics had some drawbacks, including differences in the number of patients available during consecutive follow-up visits and inhomogeneity of the group in terms of postoperative pharmacotherapy. However, these potential limitations do not jeopardise the value of this study and provide a new perspective in the field of glaucoma research.
Based solely on decreases in the RIs of the CRA and SPCAs, Carenini et al37 claimed that trabeculectomy improved the ocular blood flow in patients with primary open-angle glaucoma (POAG). Using the non-operated fellow eye as a reference, the authors observed a 32% improvement in the haemodynamic parameters. Their study showed no significant changes in the haemodynamic parameters of the OA 2.5 months after the procedure. Nevertheless, treatment was associated with a significant increase in blood flow velocity and a concomitant decrease in the RIs of the CRA and SPCAs.
Owing to their good design, well-defined inclusion and exclusion criteria, and meticulous collection of follow-up data, other studies38,39 have provided more reproducible results than those previously mentioned. The authors examined patients in whom POAG was not accompanied by other ophthalmic comorbidities, such as diabetes mellitus, arterial hypertension, aortic stenosis, or cardiovascular diseases, and who did not receive systemic pharmacotherapies with potential effects on blood circulation. This study demonstrated a significant increase in the EDV and a decrease in the RI of all analysed extraocular vessels (OA, CRA, and SPCAs) at 1 month and 3 months after trabeculectomy. Other parameters analysed in that study were the corneal surface temperature (CST) and ocular surface temperature (OST), which were measured using thermography with an infrared detector. CST and OST are determined based on the amount of heat radiated from the body surface, and hence constitute a measure of local blood flow. This study showed that the CST and OST increased significantly after trabeculectomy. In all the examined vessels, significant inverse correlations were observed between the OST and RI, and a significant positive correlation was found between the OST and EDV of the OA and SPCAs. These findings highlight the role of the vascular component as an independent risk factor for glaucoma and suggest the CST and OST as adjunct tests for detecting and monitoring diseases.
Another study40 also confirmed that trabeculectomy decreased IOP in patients with POAG, which was associated with a significant persistent change in ocular blood flow. Their work, which was the first published study with an extended follow-up, showed a significant increase in the EDV and a decrease in the RIs of the CRA and nasal and temporal SPCAs that persisted for up to 1 year after trabeculectomy.
The aforementioned studies analysed the effect of trabeculectomy on ocular blood flow in patients with POAG or open-angle glaucoma, without considering other types of diseases. Januleviciene et al41 were the first to compare patients with POAG and those with pseudoexfoliative glaucoma (PXG). Their study revealed interesting differences between the two groups. One month after intervention, changes in extraocular blood flow were more evident in patients with POAG, whereas the effect of surgical treatment was weaker in those with PXG. Although significant increases in the PSV and EVD for the CRA, along with an increase in the EDV and decrease in the RI of the temporal SPCAs, were observed in the POAG group, the only significant change found in the PXG group was an increase in the PSV of the CRA and temporal SPCAs. None of the groups showed significant changes in the OA haemodynamics. Notably, the changes reported in this study were less evident than those reported in other studies. One limitation of this study was the short follow-up duration of one month. The results of other studies with longer follow-up periods imply that the beneficial effects of trabeculectomy on the haemodynamic parameters of various vessels vary and may weaken over time. In the case of RI, the most considerable difference between the preoperative and postoperative values was observed six months after trabeculectomy. The difference decreased slightly by month 12; however, this difference was statistically significant.41 In contrast, changes in the PSV and EDV appeared to be more persistent and remained at similar levels throughout the 12-month follow-up period.
Januleviciene et al41 also analysed the serum levels of antiphospholipid antibodies (IgG) in patients with PXG. These antibodies target phospholipids and phospholipid–protein complexes, which are the primary components of the plasma membrane. Abnormal plasma membrane function may impair the autoregulation of ocular blood flow. Increased levels of antiphospholipid antibodies are generally associated with a higher risk of vascular disease and glaucoma progression. Januleviciene et al41 observed that in a group of patients with PXG, haemodynamic parameters improved after trabeculectomy alone in those with normal serum levels of antiphospholipid antibodies.
The primary therapeutic objective of glaucoma surgery is a persistent, life-long reduction in IOP, resulting in an improvement in the CDI parameters of the ocular vessels. Thus, the follow-up duration seems to be a critical factor in monitoring surgical outcomes. Kuerten et al42 were the first to monitor the extraocular haemodynamics of patients with POAG for up to three years after trabeculectomy. Similar to most of the previously mentioned studies, one study did not document significant changes in the CDI parameters of OA after trabeculectomy. Moreover, the study did not show any improvement in the EDV in any of the examined vessels. However, a significant increase in EDV with a concomitant decrease in RI has been documented for CRA, and nasal and temporal SPCAs. No significant fluctuations in the CDI parameters were observed throughout the 3-year follow-up period, implying that the beneficial effects of trabeculectomy on blood flow within the optic nerve persisted. The study also demonstrated a significant improvement in OPP, a parameter known to depend on both BP and IOP, after trabeculectomy. Three years after the intervention, OPP exceeded its baseline level by approximately 38%; however, an increase of up to 50% was observed earlier during the follow-up period. The improvement in OPP was directly associated with a decrease in IOP, with the latter reduced by an average of 50% (from 25 to 12–13 mmHg).42 This observation implies that the improvement in the haemodynamic parameters of the extraocular vessels may occur secondary to IOP reduction and the resultant increase in OPP. A similar conclusion was also reached by Karadağ et al,26 who analysed the haemodynamics of the temporal and nasal SPCAs of patients with POAG 1 month after trabeculectomy. The study showed a significant improvement in all analysed parameters (increases in PSV, EDV, and MFV, along with a decrease in RI) for temporal SPCAs, and all parameters except PSV for nasal SPCAs. The authors also analysed the correlations of the CDI parameters of SPCAs with IOP and ocular pulse amplitude (OPA), with the latter calculated as the difference between systolic and diastolic IOP. They found a positive correlation between IOP and RI, and an inverse correlation between IOP and EDV. Additionally, a strong positive correlation between RI and OPA has been demonstrated in nasal SPCAs.26
OPA is a measure of the volumetric variation in choroidal blood flow during the cardiac cycle. In clinical practice, the OPA primarily serves as a direct marker of choroidal perfusion and is a measure of ocular blood flow during a heartbeat. The ocular blood flow volume is approximately 650 to 750 µm per minute, with the primary source of blood being choroid (85–90%), followed by the retina (2–5%). Thus, the OPA is considered a marker of blood flow within the entire eyeball. Maintaining adequate blood flow within eye tissues, especially the optic nerve, requires appropriate PP in the ocular vessels. As PP is the difference between BP and IOP, the blood flow difference during each heartbeat must exceed the IOP to maintain adequate ocular perfusion.
The results published by Cantor43 contradicted the conclusions of previous studies. Their study showed no statistically significant changes in any Doppler parameters of the OA, CRA, or SPCAs in patients with POAG during a 12-month follow-up period after trabeculectomy. According to the authors, the autoregulatory mechanisms of the eye facilitate adjustment to chronically elevated IOP during the early stages of glaucoma, thus explaining the lack of significant changes in ocular haemodynamics after trabeculectomy.
Deep Sclerectomy
The effect of deep sclerectomy on blood flow parameters within the extraocular vessels has been the subject of only two published studies. A study by Özsoy et al44 showed that deep sclerectomy improved ocular haemodynamics in patients with glaucoma within six months of intervention. During follow-up visits scheduled at 2, 12, and 24 weeks after the procedure, the EDVs of the OA, CRA, and SPCAs were significantly higher than those at baseline, whereas the RIs of the CRA and SPCAs were significantly lower. The authors postulated that these changes might have occurred secondary to a postoperative decrease in IOP and an increase in OPP.
A comparative analysis of the effects of trabeculectomy and deep sclerectomy on extraocular haemodynamics suggested that both procedures may improve ocular blood flow to a similar degree, causing an increase in EDV and a decrease in the RIs of the OA, CRA, and SPCAs.38 Surprisingly, Galambos et al reported contradictory results regarding the effect of deep sclerectomy on extraocular haemodynamics, and found no improvement in CDI parameters after the intervention. However, it must be stressed that those authors solely analysed PSV and EDV, and the latter parameter increased, albeit not significantly, four weeks after the intervention. Moreover, the mean decrease in IOP during that study was only 3 mmHg, implying that the effect of deep sclerectomy on IOP might have been too weak to affect the blood flow within the eye.
Laser Treatment
Compared with surgery, laser treatment is a markedly less invasive method for IOP reduction. Chiou et al45 analysed the effect of laser iridotomy on ocular haemodynamics in patients with acute glaucoma episodes. We examined 15 patients with acute glaucoma who had undergone laser iridotomy. The treatment contributed to a significant decrease in the RI of the temporal and nasal SPCAs; however, the changes were markedly less pronounced than those reported after surgical treatment. However, it must be emphasised that the study patients presented with a very high baseline IOP (>30 mmHg) because of an acute episode of angle closure. With such an elevated IOP, OPP, and hence ocular blood flow, is substantially decreased. Thus, the question remains why a considerable reduction in IOP after laser iridotomy does not exert a more pronounced effect on ocular blood flow. Unfortunately, to the best of our knowledge, the aforementioned study is the only one that analysed the effect of laser treatment on extraocular haemodynamics, and the interpretation of such sparse evidence poses a substantial risk of bias.
Discussion
Interpretation of Doppler Findings of the Ocular Vessels
The interpretation of the CDI parameters discussed herein is challenging and requires an understanding of the mechanisms that determine blood flow within the ocular vessels. A principal question that needs to be addressed is whether an increase in flow velocity and concomitant decrease in RI, an indirect measure of resistance in peripheral vessels, can be interpreted as an improvement in blood flow, defined as the volume of blood flowing through the eyeball over a certain period. Notably, CDI does not provide information regarding flow volume. The latter parameter can be obtained using the following formula: volumetric flow = mean velocity – area of the lumen. Consequently, an increase in the flow velocity alone may not be sufficient to increase the volumetric flow if the vessel lumen is not sufficiently large. The interpretation of CDI findings is more accurate if the flow velocities are analysed in conjunction with the RI values. The RI provides information on the VR downstream of the Doppler gate.45 Thus, measurement of the CDI parameters of the CRA and SPCAs at the sites mentioned above provides information about the VR between the measurement point and the posterior wall of the eyeball.
The analysis of blood flow spectra within the extraocular vessels before and after surgery, that is, during periods of increased and decreased IOP, respectively, led to the conclusion that an increase in IOP is associated with the compression of minute precapillary arterioles, which, in turn, is reflected by an increase in resistance in those vessels.14 An increase in the VR leads to reduced perfusion. However, an increase in IOP causes a decrease in PP, another factor that reduces blood flow, in addition to peripheral VR. Additionally, an inverse relationship exists between RI and EDV, with an increase in the former reflected by a decrease in the latter. This explains why in most of the studies discussed herein, a decrease in RI was associated with an increase in EDV, whereas PSV did not change significantly.
Most studies reviewed herein did not show significant changes in the CDI parameters of OA. This vessel is located relatively far from the posterior wall of the eyeball, and changes in ocular IOP primarily affect the ocular blood flow dynamics. Moreover, the OA is not a terminal artery, and it produces multiple branches that distribute blood. As the CDI parameters provided information regarding the flow downstream of the measurement site, their values were also influenced by the flow within the OA branches. It must be stressed that the CRA and SPCAs, which are the most susceptible to IOP fluctuations, are only part of the OA blood distribution system, and their influence on the flow within that system is not substantial.15 In addition to the CRA and SPCAs, the OA has long posterior and anterior ciliary branches, none of which are dependent on IOP.
In this review, we summarise the evidence supporting the concept that glaucoma surgery improves ocular blood flow. Most cross-sectional studies have demonstrated a relationship between improved blood flow and reduced resistance within the ocular vessels, namely, increases in PSV, EDV, and MFV, a decrease in the RI of temporal SPCAs, and similar changes in all parameters except PSV for nasal SPCAs. The consistency of these findings could be further verified through a meta-analysis or another type of complex analysis. Studies with longer follow-up periods may explain the temporal relationship between these changes and the occurrence or progression of glaucoma. Given the high variability of the methodologies used during the studies and the analysed parameters as well as the discrepancies in published findings, it is necessary to obtain more evidence by performing prospective long-term observations.
Data Sharing Statement
All data are included in the manuscript.
Further Reading
2000. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration, The AGIS investigators. Am J Ophthalmol. 130(4):429–440. doi:10.1016/s0002-9394(00)00538-9.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Omodaka K, Kikawa T, Kabakura S, et al. Clinical characteristics of glaucoma patients with various risk factors. BMC Ophthalmol. 2022;22(1):373. Pubmed:36123604. doi:10.1186/s12886-022-02587-5
2. Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020;12(11):e11686. doi:10.7759/cureus.11686
3. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013
4. Chung HW, Park JH, Yoo C, Kim YY. Effects of trabecular meshwork width and Schlemm’s canal area on intraocular pressure reduction in glaucoma patients. Korean J Ophthalmol. 2021;35(4):311–317. doi:10.3341/kjo.2021.0007
5. Chan KKW, Tang F, Tham CCY, Young AL, Cheung CY. Retinal vasculature in glaucoma: a review. BMJ Open Ophthalmol. 2017;1(1):e000032. doi:10.1136/bmjophth-2016-000032
6. Zhi Z, Cepurna WO, Johnson EC, Morrison JC, Wang RK. Impact of intraocular pressure on changes of blood flow in the retina, choroid, and optic nerve head in rats investigated by optical microangiography. Biomed Opt Express. 2012;3(9):2220–2233. doi:10.1364/BOE.3.002220
7. Fan N, Wang P, Tang L, Liu X. Ocular blood flow and normal tension glaucoma. BioMed Res Int. 2015;2015:308505. doi:10.1155/2015/308505
8. Mallick J, Devi L, Malik PK, Mallick J. Update on normal tension glaucoma. J Ophthalmic Vis Res. 2016;11(2):204–208. doi:10.4103/2008-322X.183914
9. Qu J, Wang D, Grosskreutz CL. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp Eye Res. 2010;91(1):48–53. doi:10.1016/j.exer.2010.04.002
10. Banou L, Dastiridou A, Giannoukas A, Kouvelos G, Baros C, Androudi S. The role of color Doppler imaging in the diagnosis of glaucoma: a review of the literature. Diagnostics. 2023;13(4):588. doi:10.3390/diagnostics13040588
11. Meng N, Zhang P, Huang H, et al. Color Doppler imaging analysis of retrobulbar blood flow velocities in primary open-angle glaucomatous eyes: a meta-analysis. PLoS One. 2013;8(5):e62723. doi:10.1371/journal.pone.0062723
12. Ehrlich R, Harris A, Siesky BA, et al. Repeatability of retrobulbar blood flow velocity measured using color Doppler imaging in the Indianapolis glaucoma progression study. J Glaucoma. 2011;20(9):540–547. doi:10.1097/IJG.0b013e3181f46606
13. Xu S, Huang S, Lin Z, Liu W, Zhong Y. Color Doppler imaging analysis of ocular blood flow velocities in normal tension glaucoma patients: a meta-analysis. J Ophthalmol. 2015;2015:919610. doi:10.1155/2015/919610
14. Harris A, Guidoboni G, Siesky B, et al. Ocular blood flow as a clinical observation: value, limitations and data analysis. Prog Retin Eye Res. 2020:100841. doi:10.1016/j.preteyeres.2020.100841
15. Obuchowska I, Mariak Z. Podstawy anatomii i fizjologii ukrwienia newru wzrokowego [Anatomical and physiological essentials of the blood supply of the optic nerve]. Klin Oczna. 2006;108:243–246. Polish.
16. Behar-Cohen F, Gelizé E, Jonet L, Lassiaz P. Anatomia siatkówki [Anatomy of the retina]. Med Sci. 2020;36(6–7):594–599. French. doi:10.1051/medsci/2020094
17. Onda E, Cioffi GA, Bacon DR, Van Buskirk EM. Microvasculature of the human optic nerve. Am J Ophthalmol. 1995;120(1):92–102. doi:10.1016/s0002-9394(14)73763-8
18. Hwang TS, Zhang M, Bhavsar K, et al. Visualization of 3 distinct retinal plexuses by projection-resolved optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol. 2016;134(12):1411–1419. doi:10.1001/jamaophthalmol.2016.4272
19. Hayreh SS. Posterior ciliary artery circulation in health and disease: the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2004;45(3):749–57; 748. doi:10.1167/iovs.03-0469
20. Hayreh SS. The blood supply of the optic nerve head and the evaluation of it – myth and reality. Prog Retin Eye Res. 2001;20(5):563–593. doi:10.1016/s1350-9462(01)00004-0
21. Hayreh SS. The 1994 von Sallman lecture. The optic nerve head circulation in health and disease. Exp Eye Res. 1995;61(3):259–272. doi:10.1016/s0014-4835(05)80121-6
22. Williamson TH, Harris A. Color Doppler ultrasound imaging of the eye and orbit. Surv Ophthalmol. 1996;40(4):255–267. doi:10.1016/s0039-6257(96)82001-7
23. Erickson SJ, Hendrix LE, Massaro BM, et al. Color Doppler flow imaging of the normal and abnormal orbit. Radiology. 1989;173(2):511–516. doi:10.1148/radiology.173.2.2678264
24. Oglat AA, Matjafri MZ, Suardi N, Oqlat MA, Abdelrahman MA, Oqlat AA. A review of medical Doppler ultrasonography of blood flow in general and especially in common carotid artery. J Med Ultrasound. 2018;26(1):3–13. doi:10.4103/JMU.JMU_11_17
25. Modrzejewska M. Guidelines for ultrasound examination in ophthalmology. Part III: color Doppler ultrasonography. J Ultrason. 2019;19(77):128–136. doi:10.15557/JoU.2019.0019
26. Tranquart F, Bergès O, Koskas P, et al. Color Doppler imaging of orbital vessels: personal experience and literature review. J Clin Ultrasound. 2003;31(5):258–273. doi:10.1002/jcu.10169
27. Konopińska J, Kozera M, Kraśnicki P, Mariak Z, Rękas M. The effectiveness of first-generation iStent microbypass implantation depends on initial intraocular pressure: 24-month follow-up-prospective clinical trial. J Ophthalmol. 2020;2020:8164703. doi:10.1155/2020/8164703
28. Konopińska J, Lewczuk K, Jabłońska J, Mariak Z, Rękas M. Microinvasive glaucoma surgery: a review of Schlemm’s canal-based procedures. Clin Ophthalmol. 2021;15:1109–1118. doi:10.2147/OPTH.S293702
29. Lewczuk K, Jabłońska J, Konopińska J, Mariak Z, Rękas M. Schlemm’s canal: the outflow “vessel”. Acta Ophthalmol. 2022;100(4):e881–e890. doi:10.1111/aos.15027
30. Gedde SJ, Feuer WJ, Shi W, et al. Treatment outcomes in the primary tube versus trabeculectomy study after 1 year of follow-up. Ophthalmology. 2018;125(5):650–663. doi:10.1016/j.ophtha.2018.02.003
31. Musch DC, Gillespie BW, Lichter PR, Niziol LM, Janz NK; CIGTS Study Investigators. Visual field progression in the collaborative initial glaucoma treatment study the impact of treatment and other baseline factors. Ophthalmology. 2009;116(2):200–207. doi:10.1016/j.ophtha.2008.08.051
32. Aktas Z, Korkmaz S, Hasanreisoglu M, Onol M, Hasanreisoglu B. Trabeculectomy with large area Mitomycin-C application as a first-line treatment in advanced glaucoma: retrospective review. Int J Ophthalmol. 2014;7(1):104–109. doi:10.3980/j.issn.2222-3959.2014.01.19
33. Ahmed IIK, Fea A, Au L, et al. A prospective randomized trial comparing hydrus and iStent microinvasive glaucoma surgery implants for standalone treatment of open-angle glaucoma: the COMPARE study. Ophthalmology. 2020;127(1):52–61. doi:10.1016/j.ophtha.2019.04.034
34. Rękas M, Byszewska A, Petz K, Wierzbowska J, Jünemann A. Canaloplasty versus non-penetrating deep sclerectomy – a prospective, randomised study of the safety and efficacy of combined cataract and glaucoma surgery; 12-month follow-up. Graefes Arch Clin Exp Ophthalmol. 2015;253(4):591–599. doi:10.1007/s00417-015-2931-4
35. Konopińska J, Deniziak M, Saeed E, et al. Prospective randomized study comparing combined phaco-ExPress and phacotrabeculectomy in open angle glaucoma treatment: 12-month follow-up. J Ophthalmol. 2015;2015:720109. doi:10.1155/2015/720109
36. Trible JR, Sergott RC, Spaeth GL, et al. Trabeculectomy is associated with retrobulbar hemodynamic changes. A color Doppler analysis. Ophthalmology. 1994;101(2):340–351. doi:10.1016/s0161-6420(13)31332-3
37. Boles Carenini A, Brogliatti B, Sibour G, Bellone A, Valli A. Evaluation of the choroidal and retinal blood flows by means of the pOBF system and the Eco-Color-Doppler in glaucomatous patients after trabeculectomy surgery. Acta Ophthalmol Scand Suppl. 1997;224:41–42. doi:10.1111/j.1600-0420.1997.tb00471.x
38. Galassi F, Giambene B, Corvi A, Falaschi G, Menchini U. Retrobulbar hemodynamics and corneal surface temperature in glaucoma surgery. Int Ophthalmol. 2008;28(6):399–405. doi:10.1007/s10792-007-9160-8
39. Galassi F, Giambene B, Corvi A, Falaschi G. Evaluation of ocular surface temperature and retrobulbar haemodynamics by infrared thermography and colour Doppler imaging in patients with glaucoma. Br J Ophthalmol. 2007;91(7):878–881. doi:10.1136/bjo.2007.114397
40. Yamazaki Y, Hayamizu F. Effect of trabeculectomy on retrobulbar circulation and visual field progression in patients with primary open-angle glaucoma. Clin Ophthalmol. 2012;6:1539–1545. doi:10.2147/OPTH.S36331
41. Januleviciene I, Siaudvytyte L, Diliene V, Barsauskaite R, Siesky B, Harris A. Effect of trabeculectomy on ocular hemodynamic parameters in pseudoexfoliative and primary open-angle glaucoma patients. J Glaucoma. 2015;24(5):e52–e56. doi:10.1097/IJG.0000000000000055
42. Kuerten D, Fuest M, Koch EC, Remky A, Plange N. Long term effect of trabeculectomy on retrobulbar haemodynamics in glaucoma. Ophthalmic Physiol Opt. 2015;35(2):194–200. doi:10.1111/opo.12188
43. Cantor LB. The effect of trabeculectomy on ocular hemodynamics. Trans Am Ophthalmol Soc. 2001;99:241–252.
44. Özsoy A, Sarıcaoğlu MS, Çavuşoğlu M. The effect of deep sclerectomy on ocular blood flow: a 6-month clinical trial. Turk J Med Sci. 2016;46(6):1773–1778. doi:10.3906/sag-1506-122
45. Chiou HJ, Chou YH, Liu CJ, et al. Evaluation of ocular arterial changes in glaucoma with color Doppler ultrasonography. J Ultrasound Med. 1999;18(4):295–302. doi:10.7863/jum.1999.18.4.295
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.