Back to Journals » Clinical Interventions in Aging » Volume 13
Ginkgo biloba extract EGb 761® alleviates neurosensory symptoms in patients with dementia: a meta-analysis of treatment effects on tinnitus and dizziness in randomized, placebo-controlled trials
Authors Spiegel R, Kalla R, Mantokoudis G, Maire R, Mueller H, Hoerr R , Ihl R
Received 23 November 2017
Accepted for publication 20 April 2018
Published 13 June 2018 Volume 2018:13 Pages 1121—1127
DOI https://doi.org/10.2147/CIA.S157877
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Walker
Rainer Spiegel,1 Roger Kalla,2 Georgios Mantokoudis,3 Raphael Maire,4 Heiko Mueller,5 Robert Hoerr,5 Ralf Ihl6
1Division of Internal Medicine, Basel University Hospital, University of Basel, Basel, Switzerland; 2Department of Neurology, Inselspital, Bern University Hospital, Bern, Switzerland; 3Department of Otorhinolaryngology, Head and Neck Surgery, Inselspital, Bern University Hospital, Bern, Switzerland; 4Lausanne University Hospital, Lausanne, Switzerland; 5Clinical Research Department, Dr Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany; 6Department of Psychiatry, University of Duesseldorf, Alexian Research Center Krefeld, Krefeld, Germany
Background: Tinnitus and dizziness are frequent in old age and often seen as concomitant symptoms in patients with dementia. In earlier clinical trials, Ginkgo biloba extract EGb 761® was found to alleviate tinnitus and dizziness in elderly patients. Consequently, a meta-analysis was conducted to evaluate the effects of EGb 761® at a daily dose of 240 mg on tinnitus and dizziness associated with dementia.
Methods: Randomized, placebo-controlled clinical trials of G. biloba extract EGb 761® identified by a systematic database search were included in a meta-analysis if they met all of the following selection criteria: 1) diagnosis of dementia according to generally accepted criteria, 2) treatment period of at least 20 weeks, 3) outcome measures covering at least two of the three conventional domains of assessment, 4) presence and severity of dizziness and tinnitus were assessed, and 5) assessment was done before and after randomized treatment.
Results: Five trials that met the inclusion criteria were included in the meta-analysis. The risk of bias was judged as low, with Jadad scores of 3 and 5. In all trials, 11-point box scales were used to assess the severity of tinnitus and dizziness. Overall, EGb 761® was superior to placebo, with weighted mean differences for change from baseline, calculated in meta-analyses using random effects models, of -1.06 (95% CI: -1.77, -0.36) for tinnitus (p = 0.003) and -0.77 (95% CI: -1.44, -0.09) for dizziness (p = 0.03).
Conclusion: Our findings support the notion that EGb 761® is also effective in alleviating concomitant neurosensory symptoms in patients with dementia.
Keywords: neurodegenerative disorders, gait, unsteadiness, inner ear, hearing, review
Introduction
Neurosensory symptoms, such as tinnitus and dizziness, are frequently observed in elderly people and even more so in patients with dementia. Five-year and 10-year incidence rates of 18.0% and 12.7% were reported for tinnitus from the Blue Mountains Hearing Study and the Beaver Dam Epidemiology of Hearing Loss Study, respectively.1,2 Epidemiological studies have found an increase in the prevalence of tinnitus as a function of age.3 Jahn et al reported 1-year prevalence rates for significant dizziness of 20% in persons older than 60 years, 30% in those older than 70 years, and 50% in those older than 80 years.4 In elderly patients with dementia, we found prevalence rates between 13% and 52% for tinnitus and between 14.2% and 77.5% for dizziness in five clinical trials.5–9 Age-related hearing loss is likely to account for higher rates of tinnitus in the elderly and may even contribute to cognitive decline and the development of Alzheimer’s disease (AD) and other dementias.10–13 In a case-control study of elderly patients with neurological disorders, those with dementia had a particularly high rate of falls (60% in 1 year), which may indicate a higher prevalence of dizziness and impaired equilibrium control.14
Given the high comorbidity of tinnitus and dizziness in dementia and the findings from earlier studies in which Ginkgo biloba extract EGb 761® (Dr Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany) alleviated tinnitus and dizziness or vertigo,15,16 measures of tinnitus and dizziness were included in recent clinical trials of EGb 761® in patients with dementia.
When considering why G. biloba extract EGb 761® may alleviate tinnitus, dizziness, or vertigo in patients with dementia, the pathomechanisms underlying these symptoms should be taken into account. Neurons of the central vestibular and auditory systems, cochlear hair cells, and vestibular sensory cells have a high energy demand in order to maintain and continuously restore their transmembrane electrical potential. Impaired mitochondrial function and impaired perfusion are thought to contribute to both cochlear and vestibular dysfunction and sensory cell degeneration.17 EGb 761® improves inner ear and cerebral blood flow by decreasing blood viscosity; it also improves mitochondrial function and energy metabolism, which altogether may play a role in improving inner ear and brain function in elderly people with dementia who often have vascular disorders and compromised mitochondrial function.18–20 The antiapoptotic and neuroprotective properties of EGb 761® may inhibit aging-related loss of cochlear and vestibular sensory cells,21–24 which may play a role in tinnitus and vertigo.25,26 Coping with the distress of tinnitus as well as compensating for vestibular dysfunction involves both learning and neuroplasticity. EGb 761® enhances neuroplasticity, improves learning, and accelerates vestibular compensation.18,27,28 Tinnitus is likely to cause distress and anxiety, while dizziness often causes unsteadiness and fear of falling. Due to anxiolytic effects and by attenuating the activation of the stress axis, EGb 761® may decrease the distress in both conditions.18,29,30 By improving the speed of information processing, it may improve gait and reduce unsteadiness.18
Here, we present a meta-analysis of the trials that used rating scales for the assessment of presence and severity of tinnitus and dizziness. The question addressed by this meta-analysis was whether, taking into account all available evidence, EGb 761® treatment was superior to placebo in alleviating tinnitus or dizziness or both in patients with dementia who had one or both of these neurosensory symptoms at pre-treatment examination.
Materials and methods
In 2014, Gauthier and Schlaefke published a systematic review and meta-analysis of randomized, placebo-controlled, double-blind clinical trials of G. biloba extract EGb 761® in patients with mild to moderate dementia (AD, vascular dementia [VaD], mixed dementia, ie, AD with cerebrovascular disease [CVD]).31 The search strategy is described in detail in their original paper.31 Our aim was to provide an update on studies until October 2017. We did not identify any further relevant studies. Briefly, PubMed, including and excluding MedLine (from beginning to October 2017), EMBASE (from January 2006 to October 2017), and PASCAL (from beginning to end of 2015, no further update of PASCAL existed beyond this date) were searched using the following search terms (with * characterizing a wildcard, and the items AND and OR being used as Boolean functions): (ginkg* OR gingk*) AND clinical trial[pt] for PubMed including MedLine, ((ginkg* OR gingk*) NOT medline[sb]) AND (clinical* OR trial OR randomized) for PubMed excluding Medline, (GINKGO OR GINGKO) AND (HUMAN/CT OR HOMME/CTFR) for PASCAL, and (ginkgo or gingko) AND CT=(CLINICAL TRIAL; CLINICAL STUDY; DOUBLE BLIND PROCEDURE) AND py>2005 for EMBASE. The papers retrieved were assessed for eligibility by two scientists independently and trials were selected for the review, if 1) the diagnoses were established in accordance with generally accepted diagnostic criteria, 2) the treatment periods were at least 20 weeks, and 3) outcome measures covered at least two of the three conventional domains (cognition, global judgment, activities of daily living). For the current meta-analysis, we applied two additional inclusion criteria, requiring that 4) the presence and severity of tinnitus or dizziness or both were assessed and 5) assessment was done before the start and after the end of randomized treatment. Five of the seven trials included in the meta-analysis by Gauthier and Schlaefke met these additional criteria.5–9 All trials were sponsored by Dr Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany. The risk of bias was low for all five trials, with Jadad scores of 3 and 5 (Table 1).32
  | Table 1 Study characteristics and demographics (mean ± standard deviation for age) |
In all five trials, 11-point box scales were used to assess the presence and severity of tinnitus and dizziness. The 11-point box scale is a type of visual analog scale (VAS) consisting of 11 adjacent boxes which contain ascending numbers from 0 to 10 and descriptors for the extremes only, thus providing a distinct number of possible responses in a single dimension. Such types of 11-point VASs are often used as measures for pain,33,34 and have also been found useful for the assessment of other unpleasant and distressing symptoms, such as dizziness35 or tinnitus.33,36,37 In the studies reviewed here, 11-point box scales for tinnitus and dizziness were administered with the extreme ends indicating “no tinnitus at all” (0) and “extremely severe tinnitus” (10) or “no dizziness at all” (0) and “extremely severe dizziness” (10).
One study enrolled patients exclusively with AD,5 while all other studies also accepted patients with VaD or AD with CVD.6–9 In one study, two different doses of EGb 761® (240 mg or 120 mg) and placebo were compared;5 however, the patients treated with 120 mg of EGb 761® were excluded from the present meta-analysis. In all other studies, patients received either 240 mg of EGb 761® or placebo.
For each study, EGb 761® and placebo treatment were compared with regard to mean differences between baseline and end of treatment on both 11-point box scales for tinnitus and dizziness. These calculations, conducted with SAS version 9.3, were based on individual patient data, which were provided by Dr Willmar Schwabe GmbH & Co. KG. Based on the study-specific mean differences, weighted mean differences (and corresponding forest plots) were calculated using meta-analytical models to compare EGb 761® and placebo treatments by Review Manager (version 5). Due to the somewhat different inclusion criteria and design of the trial conducted by Schneider et al5 compared to the other four trials, which were similarly designed, a random effects model was chosen for the analysis and a fixed effects model as a sensitivity analysis. Only data from patients who had tinnitus or dizziness at baseline (baseline scores >0 on the respective 11-point box scale) were included in the respective meta-analyses. Missing data were replaced by the last-observation-carried-forward method.
Results
In the five studies, a total of 1,972 patients, aged 50–98 years, were randomly assigned to receive EGb 761® at a daily dose of 240 mg or placebo. The treatment periods were 22–26 weeks. The full analysis comprised a total of 1,942 patients, of whom 904 were diagnosed with probable AD, 374 had probable VaD, and 664 had mixed dementia. Study characteristics and demographics are provided in Table 1. Dementia-related baseline scores and outcomes are provided in detail by Gauthier and Schlaefke.31
Altogether, 773 patients had tinnitus at baseline (EGb 761®, 372; placebo, 401). In the individual studies, prevalence of tinnitus ranged between 13% and 52%; the average severity ratings varied between ~2.7 and 4.0. The baseline scores for the 11-point box scale related to tinnitus are provided in Table 2.
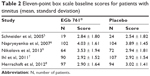  | Table 2 Eleven-point box scale baseline scores for patients with tinnitus (mean, standard deviation) |
A total of 1,040 patients reported dizziness at baseline (EGb 761®, 528; placebo, 512). Prevalence in the individual studies ranged from 13% to 77%; the average severity ratings varied between 2.5 and 4.3. The baseline scores for the 11-point box scale related to dizziness are shown in Table 3.
  | Table 3 Eleven-point box scale baseline scores for patients with dizziness (mean, standard deviation) |
A mean reduction in tinnitus severity for the EGb 761®-treated patients compared to placebo was observed in all five trials. The difference in favor of EGb 761® was statistically meaningful in four trials. Overall, there was a weighted mean difference of −1.06 (95% CI: −1.77, −0.36) favoring EGb 761® at p = 0.003 (Figure 1). This was similar to the result of the sensitivity analysis by the fixed effects model: −0.97 (95% CI: −1.16, −0.78; p < 0.001). Considering that the average severity of tinnitus was between 2.7 and 4.0 at baseline,5,6 the weighted mean difference corresponds to an improvement over placebo effects by 27%–40% of baseline severity in the single studies.
  | Figure 1 Meta-analysis of changes in tinnitus severity across five clinical trials using the 11-point box scale (weighted mean differences [95% CI] from random effects model). |
A greater reduction in dizziness was observed for the EGb 761®-treated patients in four of the five trials comprising 96% of all patients included in the meta-analysis. The differences in favor of EGb 761® were statistically meaningful in three trials comprising 80% of all patients. Overall, there was a weighted mean difference of −0.77 (95% CI: −1.44, −0.09) favoring EGb 761® at p = 0.03 (Figure 2) and similar to the fixed effects model calculated as a sensitivity analysis: −0.98 (95% CI: −1.15, –0.81; p < 0.001). The average severity of dizziness at baseline was between 2.5 and 4.3.5,6 Therefore, the weighted mean difference corresponds to an improvement over placebo effects of between 18% and 31% of baseline severity in the four larger trials.
  | Figure 2 Meta-analysis of changes in dizziness severity across five clinical trials using the 11-point box scale (weighted mean differences [95% CI] from random effects model). |
Discussion
In our meta-analysis, we included five randomized, placebo-controlled clinical trials of G. biloba extract EGb 761® in patients with mild to moderate dementia. Using 11-point box scales to assess the presence and severity of tinnitus and dizziness, we found that a considerable proportion of the patients enrolled for their diagnoses of dementia, and not for tinnitus or dizziness, had such neurosensory symptoms. On average, these symptoms were mild to moderate at baseline. Overall, we found EGb 761® to be clearly superior to placebo in alleviating both tinnitus and dizziness. This is in line with the results of earlier trials in patients with tinnitus or vertigo,15,16 in whom these neurosensory symptoms were the main complaints. It is also in line with conclusions from systematic reviews that found EGb 761®, but not other G. biloba extracts, to be effective in the treatment of tinnitus and vertigo.15,16 EGb 761® is a defined extract of G. biloba leaves that is obtained by a proprietary multi-step extraction procedure during which pharmacodynamically active molecules are enriched and harmful compounds are removed. EGb 761® is adjusted to 22.0%–27.0% ginkgo flavonoids calculated as ginkgo flavone glycosides and 2.0%–7.0% terpene lactones consisting of 2.8%–3.4% ginkgolides A, B, C and 2.6%–3.2% bilobalide and contains less than 5 ppm ginkgolic acids. High batch-to-batch consistency, which is a prerequisite for the generalization of study results to daily clinical practice, has been demonstrated. Extracts that result from different productive processes are inherently different in composition, and in pharmacodynamic and clinical activity.18 Our literature search did not identify any randomized, placebo-controlled clinical trials of any other Ginkgo extract in patients with dementia in which effects on tinnitus or dizziness were evaluated.
The clinical relevance of the effects is difficult to assess. Reductions in tinnitus severity by 27%–40% over and above the placebo effect may represent clinically relevant effect sizes. The effects on dizziness are less pronounced, but may still be clinically relevant in the given population of elderly people with ischemic and neurodegenerative CNS changes, who are particularly prone to falling. When evaluating clinical relevance in this context, the fact that the patients had mild to moderate dementia has to be kept in mind. Tinnitus may contribute to cognitive decline in dementia.12,13 Both tinnitus and dizziness cause anxiety, fear (fear of enduring annoyance and fear of falling, respectively), and distress.1,4,38,39 Tinnitus often leads to sleep disturbances,1,39 thus adding to the burden of night-time disturbances frequently experienced by patients with dementia.40 Stress-induced activation of the hypothalamic–pituitary–adrenal axis (the so-called stress axis) tends to further compromise cognitive functioning in patients with cognitive impairment.41 Focusing on and being distracted by tinnitus may additionally impair attention and processing speed, ie, the cognitive abilities already compromised in patients with dementia.39 Dizziness and fear of falling often lead to reduced physical activity, decreased participation in social life, disability, and frailty.42 Physical activity is not only necessary to maintain muscle mass and postural control,43,44 it is also closely correlated with cognitive performance in elderly people with and without cognitive impairment.45–47 Moreover, dizziness is not only associated with fear of falling, it also increases the risk of falling and the incidence of falling-related injuries, such as hip fractures.4 However, hospitalization and major surgery increase the risk of confusion and delirium and may accelerate cognitive decline in patients with dementia.48
Our meta-analysis has some limitations related to the studies included. First, the symptom ratings were done by patients with dementia, which may cast doubts on their reliability. However, patients with mild to moderate dementia are still able to understand questions about ringing in the ears and dizziness, when asked in simple language. They may have problems remembering the intensity of tinnitus or dizziness at an earlier point in time, but the 11-point box scales ask about the current severity, and not about changes. Rating errors due to cognitive problems may possibly increase scatter, but does not build a consistent pattern of treatment benefits across the studies as found in the present meta-analysis.
Second, no examinations of auditory or vestibular functions were performed, and disabilities related to tinnitus and dizziness were not assessed within the studies. As a consequence, it is not known to what extent alleviation of tinnitus and/or dizziness may have contributed to the improvement in everyday functioning and quality of life. After all the examinations required by current guidelines for clinical trials in dementia have been performed, the patients are usually too exhausted to undergo further examinations during the same visit. Investigators were free to perform all examinations required for medical reasons, but any findings not related to the studies were not documented in the case record forms. As a result, to what extent dizziness was related to vestibular vertigo or to non-vestibular disorders remains unknown. Yet, the dizziness-related problems in patients with dementia are largely the same. It is possible, however, that different proportions of patients with vestibular vertigo and non-vestibular dizziness contributed to the heterogeneity of the outcomes related to dizziness.
Third, average baseline scores for tinnitus and dizziness were around 3, ie, about one-third of the range of the scales, in most trials. As a consequence, the relative effect sizes may lead to an overestimation of their clinical relevance. On the other hand, floor effects cannot be excluded, so that the real therapeutic potential of the drug could be underestimated.
In general, patients complaining of tinnitus or dizziness should be examined for causes and contributors that are amenable to specific treatments, irrespective of whether or not they have dementia. Assessment of auditory or vestibular function might provide additional information about the underlying causes. However, in many patients the causes of neurosensory symptoms remain unclear, and even if known, there are often no causal or cause-specific treatments. As outlined earlier, EGb 761® interferes with a number of mechanisms that seem to contribute to dysfunction and degenerative processes in the inner ear and cerebral structures.
Conclusion
G. biloba extract EGb 761®, at daily doses of 240 mg, alleviated both tinnitus and dizziness in patients with dementia, ie, in patients who are particularly vulnerable to such disturbances. This should be taken into account when choosing an appropriate treatment for patients with dementia and neurosensory symptoms.
Acknowledgment
H Mueller and R Hoerr made their contribution during working hours paid by Dr Willmar Schwabe GmbH & Co. KG. There was no other funding for this work. Rainer Spiegel and Roger Kalla share first authorship.
Author contributions
R Spiegel and R Kalla contributed equally to the manuscript. All authors contributed to the interpretation of the data and the intellectual content of the manuscript and approved the final version to be published. H Mueller performed the meta-analyses.
Disclosure
R Maire received a speaker honorarium from Schwabe Pharma AG, Kuessnacht, Switzerland; R Kalla and G Mantokoudis were supported by the Swiss National Science Foundation (Grant #320030-173081); R Ihl received speaker honoraria from Dr Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany; H Mueller and R Hoerr are full-time employees of Dr Willmar Schwabe GmbH & Co. KG receiving fixed salaries. R Spiegel reports no conflicts of interest in this work.
References
Gopinath B, McMahon CM, Rochtchina E, Karpa MJ, Mitchell P. Incidence, persistence, and progression of tinnitus symptoms in older adults: the Blue Mountains Hearing Study. Ear Hear. 2010;31:407–412. | ||
Nondahl DM, Cruickshanks KJ, Wiley TL, et al. The 10-year incidence of tinnitus among older adults. Int J Audiol. 2010;49:580–585. | ||
McCormack A, Edmondson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70–79. | ||
Jahn K, Kressig RW, Bridenbaugh SA, Brandt T, Schniepp R. Dizziness and unstable gait in old age. Dtsch Arztebl Int. 2015;1112:387–393. | ||
Schneider LS, DeKosky ST, Farlow MR, Tariot PN, Hoerr R, Kieser M. A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer’s type. Curr Alzheimer Res. 2005;2:541–551. | ||
Napryeyenko O, Borzenko I; for GINDEM-NP Study Group. Ginkgo biloba special extract in dementia with neuropsychiatric features. A randomised, placebo-controlled, double-blind clinical trial. Arzneimittelforschung. 2007;57:4–11. | ||
Ihl R, Bachinskaya N, Korczyn AD, et al. Efficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:1186–1194. | ||
Herrschaft H, Nacu A, Likhachev S, Sholomov I, Hoerr R, Schlaefke S. Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: a randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J Psychiatr Res. 2012;46:716–723. | ||
Nikolova G, Yancheva S, Raychev I, Hoerr R; for PLAGIN Study Group. Ginkgo biloba extract in dementia: a 22-week randomised, placebo-controlled, double-blind trial. Bulgarian Neurol. 2013;14:139–143. | ||
Su P, Hsu CC, Lin HC, et al. Age-related hearing loss and dementia: a 10-year national population-based study. Eur Arch Otorhinolaryngol. 2017;274:2327–2334. | ||
Lin FR, Metter J, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68:214–220. | ||
Taljaard DS, Olaithe M, Brennan-Jones CG, Eikelboom RH, Bucks RS. The relationship between hearing impairment and cognitive function: a meta-analysis in adults. Clin Otolaryngol. 2016;41:718–729. | ||
Hardy CJD, Marshall CR, Golden HL, et al. Hearing and dementia. J Neurol. 2016;263:2339–2354. | ||
Homann B, Plaschg A, Grundner M, et al. The impact of neurological disorders on the risk for falls in the community dwelling elderly: a case-controlled study. BMJ Open. 2013;3:e003367. | ||
von Boetticher A. Ginkgo biloba extract in the treatment of tinnitus: a systematic review. Neuropsychiatr Dis Treat. 2011;7:441–447. | ||
Hamann KF. Ginkgo-Spezialextrakt bei Schwindel. Ein systematischer Review randomisierter, doppelblinder, placebokontrollierter klinischer Prüfungen [Special Ginkgo extract in cases of vertigo: a systematic review of randomised, double-blind, placebo-controlled clinical examination]. HNO. 2007;55:258–263. German. | ||
Yamasoba T, Lin FR, Someya S, Kashio A, Sakamoto T, Kondo K. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear Res. 2013;303:30–38. | ||
Lang F, Hoerr R, Noeldner M, Koch E. Ginkgo biloba extract EGb 761®: from an ancient Asian plant to a modern European herbal medicinal product. In: Wagner H, Ulrich-Merzenich G, editors. Evidence and Rational Based Research on Chinese Drugs. Wien: Springer; 2013:431–470. | ||
Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136:2697–2706. | ||
Eckert A, Keil U, Scherping I, Hauptmann S, Müller WE. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba extract EGb 761. Ann N Y Acad Sci. 2005;1056:474–485. | ||
Luo Y, Smith JV, Paramasivam V, et al. Inhibition of amyloid-β aggregation and caspase-3 activation by the Ginkgo biloba extract EGb 761. Proc Natl Acad Sci U S A. 2002;99:12197–12202. | ||
Massieu L, Morán J, Christen Y. Effect of Ginkgo biloba (EGb 761) on staurosporine-induced neuronal death and caspase activity in cortical cultured neurons. Brain Res. 2004;1002:76–85. | ||
Schindowski K, Leutner S, Kressmann S, Eckert A, Müller WE. Age-related increase of oxidative stress-induced apoptosis in mice. Prevention by Ginkgo biloba extract (EGb 761). J Neural Transm. 2001;108:969–978. | ||
Yang TH, Young YH, Liu SH. EGb 761 (Ginkgo biloba) protects cochlear hair cells against ototoxicity induced by gentamicin via reducing reactive oxygen species and nitric oxide-related apoptosis. J Nutr Biochem. 2011;22:886–894. | ||
Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12:711–717. | ||
Walther LE, Westhofen M. Presbyvertigo – aging of otoconia and vestibular sensory cells. J Vestib Res. 2007;17:89–92. | ||
Müller WE, Heiser J, Leuner K. Effects of the standardized Ginkgo biloba extract EGb 761® on neuroplasticity. Int Psychogeriatr. 2012;24(Suppl 1):S21–S24. | ||
Tighilet B, Lacour M. Pharmacological activity of the Ginkgo biloba extract (EGb 761) on equilibrium function recovery in the unilateral vestibular neurectomized cat. J Vestibular Res. 1995;5:187–200. | ||
Woelk H, Arnoldt KH, Kieser M, Hoerr R. Ginkgo biloba special extract EGb 761® in generalized anxiety disorder and adjustment disorder with anxious mood: a randomized, double-blind, placebo-controlled trial. J Psychiatr Res. 2007;41:472–480. | ||
Jezova D, Duncko R, Lassanova M, Kriska M, Moncek F. Reduction of rise in blood pressure and cortisol release during stress by Ginkgo biloba extract (EGb 761) in healthy volunteers. J Physiol Pharmacol. 2002;53:337–348. | ||
Gauthier S, Schlaefke S. Efficacy and tolerability o Ginkgo biloba extract EGb 761® in dementia: a systematic review and meta-analysis of randomized placebo-controlled trials. Clin Interv Aging. 2014;9:2065–2077. | ||
Jadad AR, Moore A, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. | ||
Adamchic I, Langguth B, Hauptmann C, Tass PA. Psychometric evaluation of visual analog scale for the assessment of chronic tinnitus. Am J Audiol. 2012;21:215–225. | ||
Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain. Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S240–S252. | ||
Bittar R, Alves NGP, Bertoldo C, Brugnera C, Oiticica J. Efficacy of carbon microcoils in relieving cervicogenic dizziness. Int Arch Otorhinolaryngol. 2017;21:4–7. | ||
Williams M, Hauptmann C, Patel N. Acoustic CR neuromodulation therapy for subjective tonal tinnitus: a review of clinical outcomes in an independent audiology practice setting. Front Neurol. 2015;6:54. | ||
Lim AW, Kim TS, Kang WS, Song CI, Baek S, Chung JW. Effect of a 4-week treatment with cilostazol in patients with chronic tinnitus: a randomized, prospective, placebo-controlled, double-blind pilot study. J Int Adv Otol. 2016;12:170–176. | ||
Zirke N, Seydel C, Arsoy D, et al. Analysis of mental disorders in tinnitus patients performed with Composite International Diagnostic Interview. Qual Life Res. 2013;22:2095–2104. | ||
Minen MT, Camprodon J, Nehme R, Chemali Z. The neuropsychiatry of tinnitus: a circuit-based approach to the causes and treatments available. J Neurol Neurosurg Psychiatry. 2014;85:1138–1144. | ||
Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment. Results from the Cardiovascular Health Study. JAMA. 2002;288:1475–1483. | ||
Paul S, Jeon WK, Bizon J, Han JS. Interaction of basal forebrain cholinergic neurons with the glucocorticoid system in stress regulation and cognitive impairment. Front Aging Neurosci. 2015;7:43. | ||
Mueller M, Strobl R, Jahn K, Linkohr B, Peters A, Grill E. Burden of disability attributable to vertigo and dizziness in the aged: results from the KORA-Age study. Eur J Public Health. 2013;24:802–807. | ||
Blankevoort CG, van Heuvelen MJG, Boersma F, Luning H, de Jong J, Scherder EJA. Review of effects of physical activity on strength, balance, mobility and ADL performance in elderly subjects with dementia. Dement Geriatr Cogn Disord. 2010;30:392–402. | ||
deAndrade LP, Gobbi LT, Coelho FG, Christofoletti G, Costa JL, Stella F. Benefits of multimodal exercise intervention for postural control and frontal cognitive functions in individuals with Alzheimer’s disease: a controlled trial. J Am Geriatr Soc. 2013;61:1919–1926. | ||
Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14:510. | ||
Wirth M, Haase CM, Villeneuve S, Vogel J, Jagust WJ. Neuroprotective pathways: lifestyle activity, brain pathology and cognition in cognitively normal older adults. Neurobiol Aging. 2014;35:1873–1882. | ||
Gajewski PD, Falkenstein M. Physical activity and neurocognitive functioning in aging – a condensed updated review. Eur Rev Aging Phys Act. 2016;13:1. | ||
Silverstein JH, Deiner SG. Perioperative delirium and its relationship to dementia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:108–115. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
