Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Giant Ulcer with Necrosis on Right Vulva, Groin, and Thigh-- A Case of Necrotizing Fasciitis Associated with Erythematous Pemphigus
Authors Wan M, Xu X, Zhao X, You X, Zhang G, Long H, He P, Long J, Zhu J
Received 8 November 2023
Accepted for publication 6 January 2024
Published 13 January 2024 Volume 2024:17 Pages 103—110
DOI https://doi.org/10.2147/CCID.S443374
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Miao Wan,1,* Xiangrong Xu,2,* Xiaojiao Zhao,1 Xia You,1 Guiying Zhang,3 Hai Long,3 Ping He,1 Jian Long,1 Jianjian Zhu1
1Department of Dermatovenerology of the First People’s Hospital of Changde City, Changde Hospital Affiliated to Xiangya School of Medicine of Central South University, Changde, 415000, People’s Republic of China; 2Department of Plastic Surgery of the First People’s Hospital of Changde City, Changde Hospital Affiliated to Xiangya School of Medicine of Central South University, Changde, 415000, People’s Republic of China; 3Department of Dermatovenerology of the Second Xiangya Hospital of Central South University, Changsha, 410000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianjian Zhu, No. 388, Renmin East Road, Changde, Hunan Province, People’s Republic of China, Tel +15873658886, Email [email protected]
Abstract: Necrotizing fasciitis is a rare, severe, rapidly progressing disease with a high mortality rate. We report a case of a 72-year female with erythematous pemphigus who developed erythema, swelling and ulceration on right vulva, groin, and thigh. The early clinical manifestations of the patient were nonspecific and easily misdiagnosed as cellulitis. However, upon the occurrence of ulceration and necrosis, deep fungal infection, pyoderma gangrenosum or lymphoproliferative disorders were considered. The pathology suggested IgG4-related diseases, plasmacytoma et al. But at last, surgical exploration and postoperative pathology confirmed the diagnosis of necrotizing fasciitis. The patient recovered after multiple aggressive surgical debridement procedures and antibiotic therapy and the patient has been followed up for 2 years without recurrence. Clinicians should be vigilant about the possibility of necrotizing fasciitis in patients with erythema, pain, rapid ulceration of skin and soft tissue, particularly in immunocompromised individuals with long-term use of immunosuppressive agents. It is crucial for saving life by early multi-disciplinary consultation, prompt diagnosis, and aggressive treatment.
Keywords: debridement, infection, multidisciplinary consultation, necrotizing fasciitis, pemphigus
Introduction
Necrotizing fasciitis is a rare but severe soft tissue infection that typically involves the fascia and subcutaneous tissues. Local symptoms may include severe pain, erythema, swelling, skin discoloration, bullae formation, and subcutaneous emphysema. Systemic symptoms such as fever, nausea, and vomiting may also occur.1 The diagnosis of necrotizing fasciitis is challenging, easily leading to misdiagnosis in the early stages, such as cellulitis. Since early debridement and antibiotics are particularly important in the management of necrotizing fasciitis, it is associated with difficult treatment and high mortality rates.2 It is commonly associated with conditions such as diabetes mellitus, smoking/alcohol abuse, liver cirrhosis, HIV infection, malignancy, prolonged use of corticosteroids, and chronic renal failure. We successfully treated a case of necrotizing fasciitis associated with erythematous pemphigus on long-term immunosuppressants by aggressive surgical intervention and administration of antibiotics.
Case Description
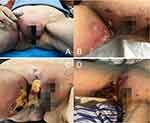
The patient, a 72-year-old female, was admitted to our hospital in 2021, with erythema, swelling, and pain in the right vulva, groin, and thigh, for 2 months, with ulceration for 20 days. She had a history of erythematous pemphigus for over 4 years, which was stable under long-term oral administration of methylprednisolone 8mg once daily. Since the day before 2 months ago, the patient had experienced itching in the right vulva with no obvious cause, followed by the development of erythema, swelling, and stiffness (Figure 1A). She had sought medical attention at a local hospital, where she received treatment with penicillin and cephalosporins for infection. However, there was no significant improvement, and the skin rash progressively worsened (Figure 1B), accompanied by severe pain but no fever or chills.
About a month later, the patient visited the Second Xiangya Hospital of Central South University, where a pelvic computed tomography revealed edema in the subcutaneous soft tissues of the right vulva, groin, and thigh, as well as avascular necrosis of the right femoral head. The histopathology showed edema in superficial dermis, multifocal necrosis in the subcutaneous adipose tissue with neutrophil, plasma cells and lymphocytes infiltration, periodic acid-Schiff (PAS) and silver staining for fungi were negative. Further immunohistochemical analysis showed positive staining for CD138, CD38, CD20, IgG, IgG4, MUM1, Kappa and Lambda, partial positive staining for CD3, CD30, CD5, negative staining for CD56 and PAX-5, approximately 40% IgG4/IgG ratio, 35% in hot spots for Ki-67, and focal positive staining for EBER1/2 by in situ hybridization, suggesting a high possibility of IgG4-related disease (Figures 2 and 3). and the possibility of skin involvement with plasmacytoma cannot be completely ruled out, further investigation by B-cell gene rearrangement was recommended.
The patient did not perform further examinations or treatments after discharge for her poor compliance, then the ulceration gradually expanded (Figure 1C), accompanied by severe pain. Therefore, she was admitted to our department and the primary diagnosis considered the cause of the skin lesions with infection, immune, or tumor-related. Further relevant laboratory tests were performed, revealing white blood cell count of 11.43×109/L (3.5–9.5×109/L), neutrophil percentage of 80% (40–75%), red blood cell count, hemoglobin level, platelet count, C-reactive protein level, ferritin level, total protein level, albumin level, glucose level, glycated hemoglobin level, creatine kinase level and creatine kinase isoenzyme level were roughly within the normal range. And others were positive anti-nuclear antibody with titer of 1:100 (nuclear membrane type), rheumatoid factor level of 35.90IU/L (0–25IU/L). Renal function, electrolytes, blood glucose, and blood lipids, extractable nuclear antibody spectrum, anti-streptolysin, Complement 3 and 4, immunoglobulin(Ig)G, A, M, G4, and serum protein electrophoresis (negative for M protein) were all normal. Serum procalcitonin, Epstein-Barr virus, human cytomegalovirus, 1, 3-beta-D glucan and glactomannan test, syphilis serology, human immunodeficiency virus antibodies, hepatitis B surface antigen, hepatitis C antibodies, and tuberculin skin test were all negative. Pummonary computed tomography revealed pneumonia and bilateral pleurisy.
After receiving intravenous cefmetazole 1.0g twice daily for anti-inflammatory treatment for 6 days, the ulcer showed no significant improvement and the area further expanded (Figure 1D). A consultation with Plastic Surgery Department considered necrotizing fasciitis, and suggested surgery therapy, So the patient was transferred to the Plastic Surgery Department. Escherichia coli was detected by microbial culture, and the antibiotic was changed to intravenous cefoperazone/sulbactam sodium (1g every 12 hours during 8 days). Three days after transferred, under general anesthesia, the patient underwent extended excision of the ulcers in the right vulva, groin, and thigh (rapid pathological examination), and wound closure with negative pressure drainage (Figure 4). The rapid pathological examination indicated a significant amount of inflammatory cells and granulation tissue proliferation. The postoperative pathology showed extensive fat and septal tissue necrosis, liquidation and neutrophil infiltration in the necrotic area (Figure 5). Considering the medical history, clinical presentations and surgical exploration, necrotizing fasciitis was diagnosed. The patient continued to receive anti-infective treatment and respectively underwent further extended debridement and closure with negative pressure drainage of the ulcers in right vulva, groin, and thigh on the day 6 and 12 after the first surgery. After improvement of the infection, the patient underwent further extended debridement, skin grafting and closure with negative pressure drainage of the ulcers in vulva, groin, and thigh on the day 8 after the third surgery, through anti-infective treatment (intravenous ceftriaxone/sulbactam sodium 2.0g once daily during 13 days, and intravenous ceftazidime 2.0g twice daily during 14 days) and dressing changes, she was discharged with much improvement after a month (Figure 6). Until now, the patient has been followed up for 2 years without recurrence.
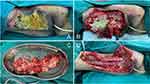 |
Figure 4 First Surgical Procedure: (A) Extensive fat necrosis with local necrosis; (B) liquefied fat and fascial necrosis; (C) Excised necrotic fat tissue; (D) Debridement deep into the muscle layer. |
 |
Figure 5 Histopathological Findings after debridement: Lobular panniculitis, fat and fascial necrosis, liquefaction, presence of neutrophils in the necrotic area ((A). 10×10; (B). 10×20; (C). 10×40). |
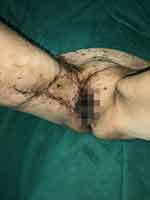 |
Figure 6 Postoperative Recovery after Skin Grafting and debridement. |
Discussion
We report a case of necrotizing fasciitis associated with erythematous pemphigus in the right vulva, groin, and thigh. After 2 months of treatment, the patient was finally diagnosed with type III necrotizing fasciitis based on typical symptoms (skin erythema, pain, ulcers, and necrosis), bacterial culture results (Gram-negative Escherichia coli), operative exploration (fat and fascial necrosis), and postoperative histopathology showing lobular panniculitis, extensive fat and septal tissue necrosis, liquefaction, neutrophilic abscesses. Type III necrotizing fasciitis has a high mortality rate. However, after four times of wound debridement and closure with negative pressure drainage therapy, one time of skin grafting procedure, along with potent broad-spectrum antibiotic therapy, the patient eventually recovered and was discharged. Two years after the surgery, there was no recurrence during follow-up. Our case involved a patient with erythematous pemphigus who had been using corticosteroids for a long time, which may be a potential predisposing factor. However, the dosage of long-term use of steroids was minimal. Jaouad Yousfi2 previously reported a case of necrotizing fasciitis combined with autoimmune disease. And we are only a case report study, the strength of evidence is weak, so whether necrotizing fasciitis in this case is related to erythematous pemphigus itself remains to be studied.
Necrotizing fasciitis is a rare, severe, and rapidly progressing disease,3 with an incidence rate of 0.004% to 0.001%, but the global incidence is increasing.4 Necrotizing fasciitis can occur as a complication of any trauma or injury such as surgical wounds, blunt trauma, puncture wounds, burns, lacerations, and insect bites. Patients often have comorbidities such as diabetes, smoking/alcohol abuse, liver cirrhosis, HIV infection, malignancies, long-term glucocorticoid therapy, and chronic renal failure.5,6 Early symptoms mainly include local erythema, swelling, and pain, which can be easily misdiagnosed as cellulitis. As the condition progresses, there may be further reddening and hardening, accompanied by increased temperature and other signs of inflammation. It may be accompanied by bloody blisters and even necrosis with a foul odor, liquefaction and necrosis of subcutaneous tissue, with severe systemic toxic symptoms, persistent high fever, shock, and multiple organ failure.7,8
Necrotizing fasciitis is typically a mixed bacterial infection and can be classified into four types based on different types of bacterial infections. Type I is the most common (55–90%), characterized by a polymicrobial infection involving aerobic and anaerobic organisms, usually associated with disruption of mucosal or skin integrity. Type II is a monomicrobial infection caused by Streptococcus pyogenes. Type III is mainly caused by monomicrobial infections with Clostridium, gram-negative bacteria, or Vibrio species. This type is characterized by multiple organ failure occurring within 24 hours, with a high mortality rate of 35–44% even with optimal treatment. Type IV is caused by fungal infections, mostly with Candida species or Aspergillus.9–11 Due to the insidious onset and rapid progression of necrotizing fasciitis, delayed diagnosis is a key factor for the high mortality rate of the disease. Therefore, early diagnosis is particularly important, which relies on the physician’s accurate assessment of clinical manifestations, and surgical exploration of necrotic fascial tissue can confirm the diagnosis.12 Ultrasound revealing the presence of gas in the fascial layer is one of the specific signs of necrotizing fasciitis. Computed tomography can show fascial and intermuscular fluid accumulation, which is of certain significance for early diagnosis. Magnetic resonance imaging demonstrated thickening of the fascia and involvement of multiple compartments, which could provide a more accurate indication of necrotizing fasciitis. Therefore, imaging examinations are powerful adjunctive tools for early diagnosis.13
The preferred treatment for necrotizing fasciitis is rapid debridement and the use of broad-spectrum antibiotics. AlMarshad et al14 and Dhawan et al15 suggest that hyperbaric oxygen therapy and low-dose radiation therapy are effective adjunctive treatments for necrotizing fasciitis through inhibiting the growth of anaerobic bacteria, accelerating the healing process and enhancing immune response. However, even with prompt and adequate treatment, patients with necrotizing fasciitis still face a high risk of limb amputation and multiple organ dysfunction leading to death.16,17
We report a case of necrotizing fasciitis in a patient with erythematous pemphigus. In the early stage, the patient presented with erythema, swelling, and stiffness, which can be easily misdiagnosed as cellulitis. Cellulitis is an acute suppurative inflammation involving shallower layers including the dermis and subcutaneous tissue, and systemic toxic symptoms are milder compared to necrotizing fasciitis. In this case, antibiotic treatment was ineffective and multiple superficial ulcers appeared locally. After skin biopsy, the histopathology and immunohistochemistry indicated IgG4-related disease and plasma cell myeloma. Firstly, cutaneous plasmacytoma is rare and represents as B-cell lymphoma, characterized by clonal proliferation of plasma cells. Clinically, it presents as painless, solitary or multiple skin-colored or purplish-red subcutaneous nodules, with few surface ulcerations. The histopathology mainly involved diffuse or nodular infiltration of plasma cells, with varying degrees of cellular atypia. Immunohistochemistry staining shows positive for CD79a, CD38, and CD138, while CD20 and T lymphocyte surface markers are negative.18–20 Its typical clinical symptoms and histopathology and immunohistochemistry differentiate it from necrotizing fasciitis. Secondly, IgG4-related disease is a newly recognized disease first described in 2003, which is characterized by elevated serum levels of IgG4 and infiltration of IgG4-positive cells in multiple organs and tissues. Its cutaneous manifestations vary including erythema, subcutaneous nodules, masses, and purpura, with serum IgG4 levels >1.35 g/L. Histopathology showed significant infiltration of plasma cells and lymphocytes, as well as fibrosis. The ratio of IgG4-positive to IgG-positive plasma cells is >40%, and there are more than 10 IgG4-positive plasma cells per high-power field.21,22 For this patient, histopathology and immunohistochemistry showed significant infiltration of plasma cells, positive staining for IgG and focal positive staining for IgG4. However, the serum IgG4 level was normal, which differed from necrotizing fasciitis.
The rapidly progressing ulceration reminded us of deep fungal diseases such as mucormycosis, pyoderma gangrenosum, and Epstein-Barr virus (EBV)-positive lymphoproliferative disorders. Firstly, mucormycosis often occurs in immunocompromised patients, with the lungs involved mostly and less, common with skin. The diagnosis of mucormycosis usually relies on pathological findings confirmed by culture, which often yields false negatives. Therefore, clinical presentations with ulcers and necrosis, histopathology, smears and special staining can help differentiate it from necrotizing fasciitis.23–25 In our case, the special staining showed negative for periodic acid-Schiff (PAS) and silver staining, which provided insufficient diagnostic evidence of mucormycosis.
Secondly, pyoderma gangrenosum needs to be differentiated from this condition as well. Pyoderma gangrenosum is a non-infectious, ulcerative, neutrophilic dermatosis characterized by destructive ulcers caused by neutrophil dysfunction. It is often painful and presents as aseptic ulcers, commonly occurring on the lower limbs and trunk often associated with systemic diseases such as inflammatory bowel disease. The edges of the ulcers are usually violet. The pathological feature is purulent inflammation in the dermis. The diagnosis is mainly based on the Paracelsus scoring system, which considers clinical symptoms, pathology, pathogen, and the level of necrosis to distinguish it from necrotizing fasciitis.26–28 In this case, the microculture showed Escherichia coli, and the histopathology suggested fat and septal tissue necrosis with predominant plasma cells and lymphocytes infiltration, which is not consistent with pyoderma gangrenosum.
Lastly, EBV-related lymphoproliferative diseases can present with multiple or solitary ulcers usually accompanied by systemic symptoms such as lymphadenopathy, fever, night sweats, and weight loss. Serum EB virus is usually positive. Histopathology shows small to medium-sized tumor cells with mild to moderate pleomorphism infiltrating the dermis and/or subcutaneous tissue, with evidence of central vascular infiltration. Most tumor cells express T-cell markers, while a small portion expresses NK-cell markers. T-cell receptor gene rearrangement is positive, and tumor cells are positive for Epstein-Barr-encoded small RNA (EBER) by in situ hybridization. Differential diagnosis is mainly based on pathology compared to necrotizing fasciitis.29 In this case, the patient had been taking steroids for a long time, possibly leading to immunosuppression. The initial immunohistochemical results showed focal positivity for EBER1/2, but serum EB virus was negative, providing insufficient diagnostic evidence.
Furthermore, based on subsequent surgical exploration and long-term follow-up of the patient, the diagnosis of mucormycosis, IgG4-related diseases, pyoderma gangrenosum, plasma cell tumors, and EBV-related lymphoproliferative diseases were not supported. The patient did not have high fever, obvious elevation of white blood cells and systemic toxic symptoms, unlike other cases of necrotizing fasciitis, in which erythema and edema typically spread more extensively and rapidly to sepsis and lead to multi-organ failure. This may be related to the older age and long-term use of steroids resulting in immunosuppression. The histopathology and immunohistochemistry showed many plasma cells and lymphocytes in the subcutaneous fat and septal tissues which are rare features of necrotizing fasciitis. This may be due to the limited sampling of the biopsy not including the necrotic fascial layer. The histopathology after wound debridement showed extensive necrosis, liquefaction, neutrophilic abscesses, combined with the necrotic fascia during operation, the diagnosis of necrotizing fasciitis was confirmed.
Conclusions
Necrotizing fasciitis is a rare, severe, and rapidly progressing disease with a high mortality rate. And early stage is highly susceptible to misdiagnosis and delayed treatment. We report a case of necrotizing fasciitis associated with erythematous pemphigus in the right vulva, groin, and thigh. The patient recovered after multiple aggressive debridement surgeries and antibiotic therapy. Clinicians should be vigilant about necrotizing fasciitis and surgical consultation for multi-disciplinary treatment to achieve early diagnosis, aggressive management, and life-saving interventions, when encountering patients with skin and soft tissue erythema, swelling, pain, and rapid ulceration, especially those with long-term use of immunosuppressive agents.
Consent Statement
Written informed consent was obtained from the patient to have the case details and associated images published. Our manuscript does not need any institutional approval to publish the case details.
Funding
This work is supported by Spreading Wings Program Research Fund of the first people’s hospital of Changde city (Grant ID:2023ZC04) and Changde City Science and Technology Innovation Guidance Project (Grant ID:2023ZD34).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Khamnuan P, Chongruksut W, Jearwattanakanok K, Patumanond J, Tantraworasin A. Necrotizing fasciitis: epidemiology and clinical predictors for amputation. Int J Gen Med. 2015;8:195–202. doi:10.2147/IJGM.S82999
2. Yousfi J, Oumlil S, Benjilali L, Essaadouni L. Necrotizing Fasciitis of the Breast Underlying an Autoimmune Disease. Eur J Case Rep Intern Med. 2021;8(4):002434. doi:10.12890/2021_002434
3. Wong C-H, Chang H-C, Pasupathy S, Khin L-W, Tan J-L, Low C-O. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003;85-A(8):1454–1460. doi:10.2106/00004623-200308000-00005
4. Jin L, Fan K, Liu S, Yu S. Necrotizing fasciitis of the jaw, neck and mediastinum caused by Klebsiella oxytoca and Streptococcus constellatus: a case report. Ann Palliat Med. 2021;10(7):8431–8436. doi:10.21037/apm-20-2427
5. Angoules AG, Kontakis G, Drakoulakis E, Vrentzos G, Granick MS, Giannoudis PV. Necrotising fasciitis of upper and lower limb: a systematic review. Injury. 2007;38(Suppl 5):S19–26. doi:10.1016/j.injury.2007.10.030
6. Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):e119–25. doi:10.1002/bjs.9371
7. Frank J, Barker JH, Marzi I. Necrotizing fasciitis of the extremities. Eur J Trauma Emerg Surg. 2008;34(3):229. doi:10.1007/s00068-008-8074-0
8. Herr M, Grabein B, Palm HG. Nekrotisierende Fasziitis. Der Unfallchirurg. 2011;114(3):197–216. doi:10.1007/s00113-010-1893-6
9. Misiakos EP, Bagias G, Patapis P, Sotiropoulos D, Kanavidis P. Current concepts in the management of necrotizing fasciitis. Front Surg. 2014;1:36. doi:10.3389/fsurg.2014.00036
10. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208(2):279–288. doi:10.1016/j.jamcollsurg.2008.10.032
11. Leiblein M, Marzi I, Sander AL, Barker JH, Ebert F, Frank J. Necrotizing fasciitis: treatment concepts and clinical results. Eur J Trauma Emerg Surg. 2018;44(2):279–290. doi:10.1007/s00068-017-0792-8
12. Stevens DL, Bryant AE, Goldstein EJ. Necrotizing Soft Tissue Infections. Infect Dis Clin North Am. 2021;35(1):135–155. doi:10.1016/j.idc.2020.10.004
13. Tso DK, Singh AK. Necrotizing fasciitis of the lower extremity: imaging pearls and pitfalls. Br J Radiol. 2018;91(1088):20180093. doi:10.1259/bjr.20180093
14. AlMarshad FA, Shah Mardan QNM, Mahabbat NA, et al. Skin preservation in the debridement of necrotizing fasciitis: a demon-strative case report. Plast Reconstr Surg Glob Open. 2022;10(4):e4227. doi:10.1097/GOX.0000000000004227
15. Dhawan G, Kapoor R, Dhamija A, et al. Necrotizing fasciitis: low-dose radiotherapy as a potential adjunct treatment. Dose Response. 2019;17(3):155932581987175. doi:10.1177/1559325819871757
16. Ozalay M, Ozkoc G, Akpinar S, Hersekli MA, Tandogan RN. Necrotizing soft-tissue infection of a limb: clinical presentation and factors related to mortality. Foot Ankle Int. 2006;27(8):598–605. doi:10.1177/107110070602700806
17. Wu PH, Wu KH, Hsiao CT, Wu SR, Chang CP. Utility of modified Laboratory Risk Indicator for Necrotizing Fasciitis (MLRINEC) score in distinguishing necrotizing from non-necrotizing soft tissue infections. World J Emerg Surg. 2021;16(1):26. doi:10.1186/s13017-021-00373-0
18. Muscardin LM, Pulsoni A, Cerroni L. Primary cutaneous plasmacytoma: report of a case with review of the literature. J Am Acad Dermatol. 2000;43(5 pt 2):962–965. doi:10.1067/mjd.2000.103997
19. Tuting T, Bork K. Primary plasmacytoma of the skin. J Am Acad Dermatol. 1996;34(2 pt 2):386–390. doi:10.1016/S0190-9622(07)80014-4
20. Kazakov DV, Belouscva IE, Muller B, et al. Primary cutaneous plasmacytoma: a clinicopathological study of two case with a long-term follow-up and review of the literature. J Cutan Pathol. 2002;29(4):244–248. doi:10.1034/j.1600-0560.2002.290408.x
21. Allace ZS, Stone JH. An update on IgG4-related disease. Curr Opin Rheumatol. 2015;27(1):83–90. doi:10.1097/BOR.0000000000000133
22. Tokura Y, Yagi H, Yanaguchi H, et al. IgG4-related skin disease. Br J Dermatol. 2014;171(5):959–967. doi:10.1111/bjd.13296
23. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi:10.1086/432579
24. Agarwal R, Kumar V, Gupta D. Pulmonary mucormycosis: two of a kind. Eur J Intern Med. 2006;17(1):63–65. doi:10.1016/j.ejim.2005.08.009
25. Greenberg RN, Scott LJ, Vaughn HH, Ribes JA. Zygomycosis (mucormycosis): emerging clinical importance and new treatments. Curr Opin Infect Dis. 2004;17(6):517–525. doi:10.1097/00001432-200412000-00003
26. Soto Vilches F, Vera-Kellet C. Pyoderma gangrenosum: classic and emerging therapies. Pioderma gangrenoso: terapias clásicas y emergentes. Med Clin. 2017;149(6):256–260. doi:10.1016/j.medcli.2017.04.013
27. Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14(3):225–233. doi:10.1080/1744666X.2018.1438269
28. Jockenhöfer F, Wollina U, Salva KA, Benson S, Dissemond J. The PARACELSUS score: a novel diagnostic tool for pyoderma gangrenosum. Br J Dermatol. 2019;180(3):615–620. doi:10.1111/bjd.16401
29. Eminger LA, Hall LD, Hesterman KS, Heymann WR. Epstein-Barr virus: dermatologic associations and implications: part II. Associated lymphoproliferative disorders and solid tumors. J Am Acad Dermatol. 2015;72(1):21–36. doi:10.1016/j.jaad.2014.07.035
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.