Back to Journals » Eye and Brain » Volume 15
GGT1 Suppresses the Development of Ferroptosis and Autophagy in Mouse Retinal Ganglion Cell Through Targeting GCLC
Authors Xu G, Wang J, Zhang Y, Chen Z, Deng R
Received 7 August 2023
Accepted for publication 3 November 2023
Published 21 November 2023 Volume 2023:15 Pages 139—151
DOI https://doi.org/10.2147/EB.S434280
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Margaret Wong-Riley
Guihua Xu, Juanjuan Wang, Yiting Zhang, Zilin Chen, Ruidong Deng
Eye Department, Huizhou Municipal Central Hospital, Huizhou, Guangdong, People’s Republic of China
Correspondence: Ruidong Deng; Zilin Chen, Email [email protected]; [email protected]
Background: Glaucoma is a neurodegenerative disorder characterized with optic nerve injury and the loss of retinal ganglion cells (RGCs). Ferroptosis has been proved to be associated with the degradation of RGCs. The aim of this study is to elucidate the relationship between ferroptosis and glaucoma pathogenesis, and unveil the underlying mechanism.
Methods: Methyl thiazolyl tetrazolium (MTT) assay was used to evaluate the proliferation of RGCs. The accumulation of cellular iron was measured by Iron assay kit, and the level of reactive oxygen species (ROS) was detected by fluorescence probe. The mitochondrial morphology and autophagosomes were analysed by using transmission electron microscopy (TEM). The contents of glutathione (GSH) and malondialdehyde (MDA) were tested by a GSH assay kit and an MDA detection kit, respectively. The expression of autophagy-related proteins was detected by Western blotting.
Results: A serious cell damage, aberrant iron homeostasis, and oxidative stress was shown in RGC-5 after oxygen-glucose deprivation/reoxygenation (OGD/R) treatment and gamma-Glutamyl transpeptidase 1 (GGT1) knockdown, but these effects were significantly alleviated by overexpression of GGT1 or ferroptosis inhibitors. The TEM and immunofluorescent results indicated that mitochondria impairment and autophagosome accumulation in OGD/R-treated cells was improved after GGT1 overexpression, while the phenomenon in GGT1-silenced cells was aggravated. Furthermore, we found that GGT1 can interact with glutamate cysteine ligase catalytic subunit (GCLC) to inhibit autophagy and ferroptosis in RGC-5 cells.
Conclusion: GGT1 represses autophagy in RGC-5 cells by targeting GCLC, which further restrains the development of ferroptosis in cells.
Keywords: glaucoma, gamma-glutamyl transpeptidase 1, ferroptosis, autophagy, glutamate cysteine ligase catalytic subunit
Introduction
Glaucoma is a chronic progressive disorder of the optic neuropathy characterized by progressive impairment of visual field and structural damages in the optic nerve head, which can cause irreversible preventable blindness if untreated.1 In 2020, the number of global blindness in patients over 50 years old with glaucoma reached to 3.6 million.2 The main risk factors for glaucoma includes advanced age, elevated intraocular pressure (IOP), high myopia, and a positive family history of glaucoma.3–5 Retinal ganglion cells (RGCs) are crucial neurons for the functional maintenance of the visual system, and the increasing loss of RGCs contributes to visual field defects and other disturbances, such as low contrast sensitivity, color perception and reading difficulties.6,7 IOP is the main cause of apoptosis of RGCs and oxidative stress, ultimately resulting in the pathophysiological changes of glaucoma.8 Current strategies, including topical treatment, laser therapy, and glaucoma surgery, inhibit glaucoma progression by reducing IOP.9 At diagnosis, however, IOP is normal in some cases of glaucoma,10 which has led to plenty of explorations to identify new targets for glaucoma therapy.
Gamma-Glutamyl transpeptidase is a kind of membrane-bound extracellular enzyme that can break down glutathione (GSH) and provide cysteine, which is required for the survival of tumor cells to avoid excessive oxidative stress and ferroptosis.11 There are 13 genes identified in GGT family, among which GGT1 was reported to be significantly associated with human cancers.12–14 Inhibition of GGT1 facilitates cystine deprivation-induced ferroptosis in glioblastoma cells at a high cell density.15 Moreover, ridonin can block out the gamma-glutamyl cycle via inhibiting GGT1 activity, and further induce ferroptosis in TE1 cells.16 Abnormal iron homeostasis and RGC cell ferroptosis were reported to be two important features in glaucoma progression.17 Inhibition of ferroptosis promotes RGCs survival in experimental glaucoma and other optical neuropathies.18,19 Although the studies of GGT1 on tumor cells are well known, the role of GGT1 in RGCs has not been reported yet. Here, investigations are performed to uncover whether GGT1 is involved in glaucoma pathogenesis.
In this study, we analyzed GGT1 expression in retinal ganglion cells of DBA/2J mice with spontaneously chronic high intraocular pressure glaucoma, and compared it with data on wildtype mice. Furthermore, we explore the role of GGT1 in OGD/R-induced RGC-5 cells through evaluating proliferation, and the progression of ferroptosis and autophagy in cells.
Materials and Methods
Acquisition of Gene Expression Omnibus (GEO) Sequencing Data
R (R Programming Language) package was used to analyze a GEO series GSE202012, which was obtained from the GEO database on National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov/geo).
Cell Culture and OGD/R Treatment
Retinal ganglion cells (RGC-5) were purchased from Jennio Biotech (Guangzhou, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA), 1% streptomycin and penicillin. Cells were maintained in a humidified incubator at 37°C with 5% carbon dioxide. To establish an OGD/R model, RGC-5 cells were cultured in glucose-free DMEM (Gibco) and incubated in an anoxic environment (95% N2, 5% CO2) for 2 h.
Cell Transfection and Short Interfering RNA (siRNA)
Adenovirus with mCherry-EGFP-LC3((Genomeditech, Shanghai, China)) were applied to transfect RGC-5 cells. Then, autophagic flux was determined using a ZEISS fluorescence microscope, and the images were analysed by ImageJ software (National Institutes of Health, Bethesda, MD, USA). For genes silencing experiments, siRNA oligonucleotides against GGT1 or GCLC, and negative controls were synthesized by Sangon biotechnology (Shanghai, China), which were subsequently transfected into RGC-5 cells. The sequences of siRNA oligonucleotides are as follow: GGT1: F: 5′- ACTGCGGGACGGTGGCTCTG-3’; 5′- CTGCTTGGCATCCGCGGCCA-3′. GCLC: F: 5′-GCTTCTCCTCACTCCCAATTA-3′; R:5′-GCTTCGCGCCGTAGTCTTA-3′.
Methyl Thiazolyl Tetrazolium (MTT) Assay
Cells were seeded in a 96-well plate at a density of 5 × 103 cells/well. After 24 h, twenty microliters of 5 mg/mL MTT (Sigma Aldrich, St Louis, USA) was added to the supernatant and incubated for 4 h. Subsequently, 100 μL of DMSO solution was added to dissolve formazan crystals. The OD values were detected at 490 nm in a microplate reader (Molecular Devices, Sunnyvale, USA).
Iron Parameters
Iron levels in cell cytoplasm (total iron, ferrous iron, and ferric iron) were measured using an Iron Assay Kit (Sigma, USA). Perl’s staining was used to detect the ferric iron distribution. FerroOrange (Dojindo, Beijing, China) was used to target ferrous iron in living RGC-5 cytoplasm. Specifically, RGC-5 cells were incubated with serum-free medium containing 1 μM FerroOrange at 37°C for 30 min, followed by detection using a confocal laser scanning microscope (Leica, Frankfurt, Germany).
Reactive Oxygen Species (ROS) Measurement
To detect intracellular ROS accumulation, cells were stained with 10 μmol/L 2,7-dichlorodihydrofluorescein diacetate (Sigma-Aldrich) in serum free medium for 30 minutes at 37°C, and photographed using a fluorescence microscope (Olympus, Tokyo, Japan).
Immunofluorescence Assay
Cells were plated in a standard six-well format containing a glass coverslip. After different treatments, cells were rinsed with PBS, fixed with 4% paraformaldehyde at room temperature for 30 min, and permeabilized with 0.3% Triton X-100 in PBS for 15 min. Then, cells were blocked with 5% BSA in PBS for 1 h before incubating with the primary antibodies at 4°C overnight. After incubation of fluorescent secondary antibodies, the coverslips were mounted onto microscope slides. Images were analysed using the ZEISS fluorescence microscope and ImageJ software (National Institutes of Health).
Estimation of Malondialdehyde (MDA) and GSH Assays
MDA and GSH levels were tested using an MDA detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and a GSH assay kit (Beyotime) respectively.
RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
Total RNA was extracted using TRIzol reagents (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. The cDNA was synthesized by using MMLV reverse transcriptase (Promega, Madison, USA). The quantitative PCR was performed with a GoTaq® qPCR Master Mix kit (Promega) on an ABI 7900HT PCR System (Applied Biosystems, Foster City, USA). The relative expression was quantified by the 2−ΔΔCT method, using β-actin as an internal standard control. The primers are as follow: F: 5’-AGAGCTACGAGCTGCCTGAC-3’; R: 5’-AGCACTGTGTTGGCGTACAG-3’.
Western Blotting Analysis
RGC-5 cells were digested and lysed in RIPA buffer (Beyotime, Shanghai, China), and then the total protein was detected using a BCA protein assay kit (Beyotime). Equal amounts of protein were separated by 10% SDS-PAGE and transferred into PVDF membranes (Millipore, Schwalbach, Germany). After being blocked with TBST buffer containing 5% non-fat milk, the membranes were incubated with primary antibodies (1:1000), including anti-GGT1 antibody (Abcam), anti-LC-3 antibody (Abcam), anti- Beclin antibody (Abcam), and anti-p62 antibody (Abcam), at 4°C overnight. Anti-β-actin antibody (1:3000; Cell signaling Technology) acted as an internal control. Subsequently, the membrane was hybridized with an HRP-conjugated secondary antibody (1:5000; Cell signaling Technology). The signals were determined by chemiluminescence.
Co-Immunoprecipitation (CoIP) Assay
After 6 h treatment of MG132, cells were lysed with IP lysis buffer (Servicebio). The lysates were incubated with GGT1 antibody overnight at 4°C. Immunocomplexes were captured using Protein-A/G MagBeads (Yeason, Shanghai, China) for 3h at 4°C, and eluted with cold PBS supplemented with protease inhibitor cocktail. The protein complexes were harvested and immunoblotted with antibodies.
Transmission Electron Microscopy (TEM)
Cell pellets were fixed with precooled 2.5% glutaraldehyde for 24 h. Then, the pellets postfixed in 2% aqueous osmium tetroxide for 24 h at 4°C. After dehydration in ethanol solutions with propylene oxide, the fixed cells were embedded in Epon 812 (Merck, USA), followed by incubation for 48h at 60°C. Ultrathin sections (80 nm) were trapped onto 200-mesh copper grids for TEM imaging following a standard protocol. The cellular structure was observed under a HT7700 transmission electron microscope (Hitachi, Tokyo, Japan).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7 software. The Student’s t-test was performed to analyse comparisons between the two groups. The differences between multiple groups were performed by ordinary one-way ANOVA with Tukey’s multiple comparisons test. All data are shown as means ± standard deviation (SD). Statistically significant differences were indicated as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
GGT1 Expression in RGC-5 Was Decreased in OGD/R-Induced RGC-5
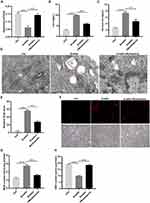
The RNA sequencing data of GEO database (GSE202012) was re-mined to analyze the differential expression of GGT1 gene between chronic ocular hypertension mice (OHT) and normal mice. As shown in Figure 1A, GGT1 expression is significantly reduced in OHT mice, suggesting that GGT1 may play an important role in glaucoma progression. Oxidative stress and iron homeostasis have been reported to participate in glaucoma pathology.20,21 To investigate the role of GGT1 in RGC injury, we established an in vitro OGD/R-induced cell model. The results showed that OGD/R treatment affected cell viability, and broken iron homeostasis in RGC-5 cells (Figure 1B–D). TEM images showed that the mitochondrial size and cristae had become smaller and thinner in OGD/R-treated cells than controls (Figure 1E). Consistently, an obvious accumulation of ferrous iron was observed in OGD/R-treated cells (Figure 1F). Compared with control group, ROS generation and MDA level were increased, while GSH level was decreased in OGD/R-induced cells (Figure 1G–I). Furthermore, we found that the RNA and protein levels of GGT1 in OGD/R-treated cells were lower than those in controls (Figure 1J and K), indicating that GGT1 might be involved in OGD/R-induced ferroptosis.
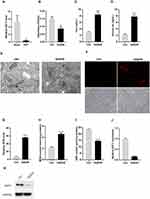 |
Figure 1 GGT1 expression in RGC-5 was decreased in OGD/R-induced RGC-5 (A). The difference of GGT1 gene expression between chronic ocular hypertension (OHT) mice and normal mice. (B) MTT assay showing the viability of RGC-5 cells with or without OGD/R induction. (C and D) The concentration of iron and ferric iron in RGC-5 cells after control or OGD/R treatment. (E) TEM images of RGC-5 cells after OGD/R treatment (the red arrow reflects the outer membrane of mitochondrion). (F) Ferrorange probe targeted the intracellular ferrous iron produced in RGC-5 cells. (G) The ROS level in RGC-5 cells in two groups (control, OGD/R) was detected. (H and I) The GSH and MDA levels in RGC-5 cells were detected. (J) qRT-PCR analysis examined the mRNA level of GGT1 in RGC-5. (K) Western blot analysis of GGT1 expression in RGC-5 cells after treated with OGD/R or untreated for 48 hours, full-length blots are presented in Supplementary Figure 1. **p < 0.01, ***p < 0.001, ****p < 0.0001. |
GGT1 Restrained OGD/R-Induced Ferroptosis in RGC-5
To further verify the relationship between GGT1 and iron homeostasis, GGT1 was overexpressed or silenced in RGC-5 cells before the treatment of OGD/R. As expected, GGT1 overexpression promoted cell proliferation of OGD-R-treated cells, while silencing its expression increased cell injury, and these effects could be reversed by ferroptosis inhibitor Fer-1 (Figure 2A and B). Upregulation of GGT1 significantly decreased both total iron and ferrous iron levels in RGC-5 cells (Figure 2C and D). Consistently, GGT1-silenced RGC-5 cells had a higher ferrous iron accumulation in cytoplasm than GGT1-overexpressed cells (Figure 2G). As indicated in Figure 2E, the mitochondrial volume shrunk and the cristae even disappeared in OGD/R-induced and GGT1-silenced cells, but those changes were improved after GGT1 overexpression or fer-1 treatment, which suggested that GGT1 alleviated mitochondrial impairment in the ferroptosis progress of cells. Moreover, an increase in ROS intensity and MDA level, and a reduction in GSH, was witnessed after GGT1 downregulation compared to the OGD/R-treated group (Figure 2F, H and I). These findings indicated that GGT1 inhibited ferroptosis in OGD/R-induced RGC-5 cells.
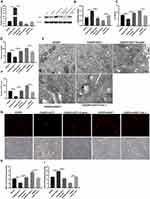 |
Figure 2 GGT1 restrained OGD/R-induced ferroptosis in RGC-5. (A) The expression of GGT in RGC-5 cells after overexpressing or silencing GGT1 and treated with Erastin or fer-1, full-length blots are presented in Supplementary Figure 1. (B) Cell proliferation was tested in RGC-5 cells. (C and D) The iron and ferric iron levels was determined. (E) TEM images showing that the mitochondrial structure was observed (the red arrow reflects the outer membrane of mitochondrion). (F) The production of ROS was examined in RGC-5 cells. (G) The intracellular ferrorange fluorescent intensity was detected in RGC-5 cells. (H and I) The production of MDA and GSH was examined in RGC-5 cells. ***p < 0.001, ****p < 0.0001. |
GGT1 Suppressed OGD/R-Induced Autophagy in RGC-5
We further found that the number of autophagosomes in OGD/R-induced RGC-5 cells was reduced after GGT1 overexpression, but the number was increased when GGT1 knockdown (Figure 3A). To explore whether GGT1 has effects on autophagy development, the expression of autophagy-associated markers, including LC-3, Beclin-1, and p62 was detected. As shown in Figure 3B, a decreased expression of LC-3 and Beclin-1 was observed in RGC-5 cells after GGT1 upregulation, whereas the expression in GGT1-silenced cells was elevated. The expression of p62, however, was not significantly changed between GGT1-overexpressed and GGT1-silenced cells (Figure 3C). In addition, we used LC3 antibody to target extracellular autophagy spots, and the result showed that LC3 expression was remarkably downregulated through GGT1 overexpression (Figure 3C). After silencing GGT1, the number of yellow-labelled autophagosomes and red-labelled autophagolysosomes was significantly increased (Figure 3D), which indicated that lysosomes are fused with autophagosome. Collectively, GGT1 has the inhibitory effect on the accumulation of autophagosome in OGD/R-induced cells.
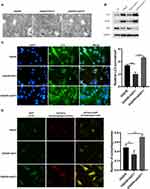 |
Figure 3 GGT1 suppressed OGD/R-induced autophagy in RGC-5. (A) TEM images showing that the mitochondrial structure was observed after overexpressing or silencing GGT1. (B) The expression of LC3, Beclin, and p62 in cells, full-length blots are presented in Supplementary Figure 1. (C) Immunofluorescence staining of LC3 in RGC-5 cells. (D) mCherry-EGFP-LC3-based immunofluorescence staining of autophagolysosomes (red) and autophagosomes (yellow) in RGC-5. **p < 0.01, ***p < 0.001, ****p < 0.0001. |
Wortmannin Suppressed Ferroptosis in RGC-5 Through Inhibition of Autophagy
Previous evidences have been reported that autophagy may facilitate ferroptosis.22 Hence, we further investigated whether the ferroptosis induced by OGD/R was autophagy associated. Here, we used wortmannin, a well-known autophagosome inhibitor, to pretreat RGC-5 cells. Intriguingly, wortmannin rescued the growth inhibition and aberrant iron homeostasis induced by erastin (Figure 4A–C). Meanwhile, a diminishing mitochondrial cristate observed in erastin-treated cells was partly reversed by wortmannin (Figure 4D). Furthermore, a reduction of ferrous iron accumulation was seen in wortmannin group compared with that in erastin group (Figure 4F). Indeed, wortmannin also increased the level of GSH, and decreased the content of ROS and MDA in erastin-treated cells (Figure 4E, 4G and H). The data demonstrated that wortmannin alleviated erastin-induced ferroptosis in RGC-5 cells by inhibiting autophagy.
GGT1 Targeting GCLC Inhibited Ferroptosis in RGC-5
To uncover the inhibitory mechanism of GGT1 in glaucoma, we searched a bioinformatic website (https://string-db.org) and predicted that there might be an interaction between GGT1 and GCLC. First, we confirmed that GGT1 can interact with GCLC (Figure 5A and B). Thereafter, depletion of GCLC affected cell viability and iron homeostasis in GGT1-overexpressed RGC-5 cells (Figure 5C and D). Morphologically, electronic microscopy showed that more autophagosome accumulation, and reduced size and cristae of mitochondria were shown in GCLC-silenced cells (Figure 5E). Immunofluorescence staining indicated that the ferrorange intensity was enhanced in RGC-5 cells after GCLC knockdown (Figure 5G). Compared with GGT1 group, the level of GSH in cells was reduced, while the MDA and the ROS levels were increased by silencing GCLC (Figure 5F, H and I).
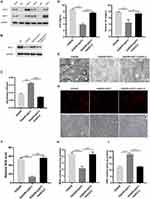 |
Figure 5 GGT1 targeting GCLC inhibited ferroptosis in RGC-5. (A) CO-Immunoprecipitation to assess the interaction between GGT1 and GCLC, full-length blots are presented in Supplementary Figure 1. (B) Western blot analysis of GCLC in cells after GGT1 overexpression and GCLC knockdown, full-length blots are presented in Supplementary Figure 1. (C) Cell viability fo RGC-5 was evaluated using MTT assay. (D) The iron and ferrous ion levels in RGC-5 cells after GGT1 overexpression and GCLC knockdown. (E) The mitochondrial structure in cells was visualized by TEM assay. (F) The ROS level was examined in cells after GGT1 overexpression and GCLC knockdown. (G) The ferrorange intensity was detected using immunofluorescence assay. (H and I) MDA and GSH levels in cells was tested.**p < 0.01, ***p < 0.001, ****p < 0.0001. |
GGT1 Targeting GCLC Inhibited Autophagy in RGC-5
We also found that the mitochondrion became smaller and the cristae was thinner in cells after GCLC knockdown (Figure 6A). The expression of autophagy-related markers, such as LC-3, Beclin-1 and p62 in GCLC-silenced group was similar to that in OGD/R group, while LC-3 and Beclin-1 proteins expression was significantly decreased after overexpressing GGT1 (Figure 6B). Notably, upregulation of GGT1 blocked LC-3 expression and autophagy flux, but depletion of GCLC restore this progression (Figure 6C and D). Together, these results revealed that GGT1 inhibited the autophagy development by interacting with GCLC, and then suppressed ferroptosis in OGD/R-induced cells.
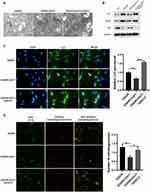 |
Figure 6 GGT1 targeting GCLC inhibited autophagy in RGC-5. (A) TEM images showing the mitochondria structure in cells after GGT1 overexpression and GCLC knockdown, full-length blots are presented in Supplementary Figure 1. (B) The proteins expression was detected using Western blot assay. (C) Immunofluorescence staining of LC3 in cells was visualized. (D) mCherry-EGFP-LC3-based immunofluorescence staining of autophagolysosomes (red) and autophagosomes (yellow) in RGC-5 cells. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. |
Discussion
Ferroptosis is a specific form of programmed cell death with iron-dependent properties, which is different from other types of death and requires three key factors, including accumulation of redox active iron, depletion of GSH, and lipid peroxidation.23 Some evidences have been reported that ferroptosis might be related to the loss of RGCs in the retina, but the mechanism underlying glaucoma pathogenesis and optic nerve damage is not clear.23–25 More recently, three potential ferroptosis pathways in glaucoma were proposed, which still need to be verified though.18 Herein, OGD/R treatment was used to induce cell injury in RGC-5. A lower cell viability and abnormal iron homeostasis were observed in OGD/R-treated RGC-5. Normally, the level of iron parameters keeps at a stable level, and excess iron is toxic and cause cell death.26 Thus, investigating the possible mechanism of aberrant iron metabolism in RGCs after OGD/R treatment is critical to develop the strategy for glaucoma therapy. Furthermore, we found that GGT1 was downregulated in OGD/R-induced RGC-5, and overexpression of GGT1 decreased cell damage and cellular ferrous accumulation. Iron-mediated lipid ROS production and GSH depletion are important for the induction of ferroptosis.27 Cysteine deprivation resulting from GSH depletion contributes to mitochondrial membrane potential hyperpolarization and lipid peroxide accumulation.28 Hence, we detected the content of GSH and MDA in OGD/R-induced RGC-5 cells after upregulation or depletion of GGT1, and the results showed that GGT1 increased the synthesis of GSH and lowered the level of MDA in cells.
Moreover, the TEM imaging indicated that mitochondria impairment was improved in GGT1-overexpressed RGC-5 cells, and the number of autophagosome was also reduced. Autophagy plays an important role in RGC survival in both in vivo and in vitro models. In glaucoma rat and mouse models, RGC loss was associated with autophagy and apoptosis pathways.29,30 MicroRNAs (miRNAs), miR-708 and miR-335-3P, repressed RGC apoptosis via inhibition of autophagy.16 Ferroptosis is recognized as an autophagy-dependent cell death since autophagy can cause the degradation of ferritin.31 Here, erastin-induced ferroptosis in cells was partly reversed by the autophagy inhibitor wortmannin, suggesting that inhibition of autophagy is a promising pathway to restrain ferroptosis. The expression of autophagy-associated proteins was decreased, while the protein level of p62 was increased after GGT1 overexpression, which indicates that the inhibitory effects of GGT1 on ferroptosis in RGC-5 cells was probably achieved by blocking autophagy development. Additionally, we found that GGT1 can interact with GCLC in RGC-5 cells. GCLC plays an important role in the regulation of glutamate and has a glutathione-independent mechanism in the inhibition of ferroptosis.32 GCLC knockdown facilitated ferroptosis progression and inhibited cell proliferation and invasion.32,33 In this study, we further confirmed that the decreased autophagy and ferroptosis in GGT1-overexpressed cells was thwarted by silencing GCLC. However, we still have some shortcomings in improving the overall aspects of glaucoma, such as the association of high IOP with autophagy and iron death, and we look forward to further studies in vivo.
Conclusion
In conclusion, GGT1 suppressed the OGD/R-induced cell injury, ferroptosis, and autophagy in RGC-5 cells. This was associated with the combination of GGT1 and GCLC. Our findings indicated that GGT1 may serve as a promising candidate for glaucoma therapy.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by Guangdong Basic and Applied Basic Research Foundation (2018A030310059).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stein JD, Khawaja AP, Weizer JS. Glaucoma in adults—screening, diagnosis, and management: a review. JAMA. 2021;325(2):164–174. doi:10.1001/jama.2020.21899
2. Steinmetz JD, Bourne RR, Briant PS, et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Global Health. 2021;9:e144–e160.
3. Ramdas WD, Wolfs RC, Hofman A, et al. Ocular perfusion pressure and the incidence of glaucoma: real effect or artifact?: the Rotterdam study. Investig Ophthalmol Vis Sci. 2011;52(9):6875–6881. doi:10.1167/iovs.11-7376
4. Ekström C. Risk factors for incident open‐angle glaucoma: a population‐based 20‐year follow‐up study. Acta Ophthalmol. 2012;90:316–321.
5. Le A, Mukesh BN, McCarty CA, Taylor HR. Risk Factors Associated with the Incidence of Open-Angle Glaucoma: the Visual Impairment Project. Investig Ophthalmol Vis Sci. 2003;44:3783–3789.
6. Vrabec JP, Levin LA. The neurobiology of cell death in glaucoma. Eye. 2007;21(S1):S11–S14. doi:10.1038/sj.eye.6702880
7. Erb C. Funktionelle Störungen im zeitlichen Verlauf der Glaukomerkrankung. Der Ophthalmologe. 2015;112(5):402–409. doi:10.1007/s00347-015-0005-y
8. Guo L, Moss SE, Alexander RA, et al. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Investig Ophthalmol Vis Sci. 2005;46(1):175–182. doi:10.1167/iovs.04-0832
9. Schuster AK, Erb C, Hoffmann EM, Dietlein T, Njdäi P. The diagnosis and treatment of glaucoma. Dtsch Arztebl Int. 2020;117(13):225. doi:10.3238/arztebl.2020.0225
10. Sáles CS, Lee RY, Agadzi AK, Hee MR, Singh K, SCJJog L. Open-angle glaucoma in Filipino and white Americans: a comparative study. J Glaucoma. 2014;23(4):246–253. doi:10.1097/IJG.0b013e318279b3e2
11. Zhang H, Forman HJ, Choi J. Gamma-glutamyl transpeptidase in glutathione biosynthesis. Methods Enzymol. 2005;401:468–483. doi:10.1016/S0076-6879(05)01028-1
12. Heisterkamp N, Groffen J, Warburton D, Sneddon TP. The human gamma-glutamyltransferase gene family. Hum Genet. 2008;123(4):321–332. doi:10.1007/s00439-008-0487-7
13. Brand H, Diergaarde B, O’Connell MR, Whitcomb DC, Rejp B. Variation in the Gamma-Glutamyltransferase 1 (GGT1) gene and risk of chronic pancreatitis. Pancreas. 2013;42(5):836. doi:10.1097/MPA.0b013e318279f720
14. Batsios G, Najac C, Cao P, et al. In vivo detection of γ-glutamyl-transferase up-regulation in glioma using hyperpolarized γ-glutamyl-[1-13C] glycine. Sci Rep. 2020;10:1–10.
15. Hayashima K, Bc KH. Expression of gamma-glutamyltransferase 1 in glioblastoma cells confers resistance to cystine deprivation–induced ferroptosis. J Biol Chem. 2022;298(3). doi:10.1016/j.jbc.2022.101703
16. Zhang J, Wang N, Zhou Y, et al. Oridonin induces ferroptosis by inhibiting gamma‐glutamyl cycle in TE1 cells. Phytother Res. 2021;35(1):494–503. doi:10.1002/ptr.6829
17. Yao F, Peng J, Zhang E, et al. Pathologically high intraocular pressure disturbs normal iron homeostasis and leads to retinal ganglion cell ferroptosis in glaucoma. Cell Death Differ. 2023;30(1):69–81. doi:10.1038/s41418-022-01046-4
18. Yang M, So K-F, Lam W-C, Lo ACY. Ferroptosis and glaucoma: implications in retinal ganglion cell damage and optic nerve survival. Neural Regen Res. 2023;18(3):545. doi:10.4103/1673-5374.350196
19. Guo M, Zhu Y, Shi Y, et al. Inhibition of ferroptosis promotes retina ganglion cell survival in experimental optic neuropathies. Redox Biol. 2022;58:102541. doi:10.1016/j.redox.2022.102541
20. Yaz YA, Yıldırım N, Yaz Y, Tekin N, İnal M, Şahin FM. Role of oxidative stress in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Turk J Ophthalmol 2019;49:61.
21. Yao F, Peng J, Zhang E, et al. Pathologically high intraocular pressure disturbs normal iron homeostasis and leads to retinal ganglion cell ferroptosis in glaucoma. Cell Death Diff 2022;30(1):69–81.
22. Xie Y, Li J, Kang R, Tang D. Interplay Between Lipid Metabolism and Autophagy. Front Cell Dev Biol. 2020;8:431.
23. Bertrand RL. Iron accumulation, glutathione depletion, and lipid peroxidation must occur simultaneously during ferroptosis and are mutually amplifying events. Med Hypotheses. 2017;101:69–74. doi:10.1016/j.mehy.2017.02.017
24. Kaminska A, Romano GL, Rejdak R, et al. Influence of trace elements on neurodegenerative diseases of the eye-the glaucoma model. Int J Mol Sci. 2021;22(9):4323. doi:10.3390/ijms22094323
25. Vernazza S, Oddone F, Tirendi S, Bassi AM. Risk factors for retinal ganglion cell distress in glaucoma and neuroprotective potential intervention. Int J Mol Sci. 2021;22(15):7994. doi:10.3390/ijms22157994
26. Camaschella C, Nai A, Silvestri LJH. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105(2):260. doi:10.3324/haematol.2019.232124
27. Xie Y, Hou W, Song X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369–379. doi:10.1038/cdd.2015.158
28. Gao M, Yi J, Zhu J, et al. Role of mitochondria in ferroptosis. Mol Cell. 2019;73(2):354–363. e353. doi:10.1016/j.molcel.2018.10.042
29. Lee SH, Shim KS, Kim CY, Park TK. Characterization of the role of autophagy in retinal ganglion cell survival over time using a rat model of chronic ocular hypertension. Sci Rep. 2021;11(1):5767. doi:10.1038/s41598-021-85181-x
30. Zhang M-L, Zhao G-L, Hou Y, et al. Rac1 conditional deletion attenuates retinal ganglion cell apoptosis by accelerating autophagic flux in a mouse model of chronic ocular hypertension. Cell Death Dis. 2020;11(9):734. doi:10.1038/s41419-020-02951-7
31. Hou W, Xie Y, Song X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–1428. doi:10.1080/15548627.2016.1187366
32. Kang YP, Mockabee-Macias A, Jiang C, et al. Non-canonical Glutamate-Cysteine Ligase Activity Protects against Ferroptosis. Cell Metab. 2021;33(1):e177. doi:10.1016/j.cmet.2020.12.007
33. Luo L, Zhang Z, Weng Y, Zeng J. Ferroptosis-related gene GCLC is a novel prognostic molecular and correlates with immune infiltrates in lung adenocarcinoma. Cells. 2022;11(21):3371. doi:10.3390/cells11213371
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.