Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Genome-Wide Association Study of Alopecia Areata in Taiwan: The Conflict Between Individuals and Hair Follicles
Authors Yang JS, Liu TY, Chen YC, Tsai SC , Chiu YJ , Liao CC , Tsai FJ
Received 4 July 2023
Accepted for publication 14 September 2023
Published 21 September 2023 Volume 2023:16 Pages 2597—2612
DOI https://doi.org/10.2147/CCID.S428788
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jai-Sing Yang,1,* Ting-Yuan Liu,2,* Yu-Chia Chen,2 Shih-Chang Tsai,3 Yu-Jen Chiu,4,5 Chi-Chou Liao,2 Fuu-Jen Tsai6– 8
1Department of Medical Research, China Medical University Hospital, China Medical University, Taichung, 404327, Taiwan; 2Million-Person Precision Medicine Initiative, Department of Medical Research, China Medical University Hospital, Taichung, 404327, Taiwan; 3Department of Biological Science and Technology, China Medical University, Taichung, 406040, Taiwan; 4Division of Plastic and Reconstructive Surgery, Department of Surgery, Taipei Veterans General Hospital, Taipei, 112201, Taiwan; 5Department of Surgery, School of Medicine, National Yang Ming Chiao Tung University, Taipei, 112304, Taiwan; 6School of Chinese Medicine, College of Chinese Medicine, China Medical University, Taichung, 404333, Taiwan; 7China Medical University Children’s Hospital, Taichung, 404327, Taiwan; 8Department of Medical Genetics, China Medical University Hospital, Taichung, 404327, Taiwan
*These authors contributed equally to this work
Correspondence: Fuu-Jen Tsai, Department of Medical Genetics, China Medical University Hospital, No. 2, Yude Road, Taichung City, 404332, Taiwan, Tel +886 422052121, Email [email protected]
Purpose: Alopecia areata (AA) is one of the most prevalent autoimmune diseases affecting humans. Given that hair follicles are immune-privileged, autoimmunity can result in disfiguring hair loss. However, the genetic basis for AA in the Taiwanese population remains unknown.
Materials and Methods: A genome-wide association study was conducted using a cohort of 408 AA cases and 8167 controls. To link variants to gene relationships, we used 882 SNPs (P< 1E-05) within 74 genes that were associated with AA group to build the biological networks by IPA software. HLA diplotypes and haplotypes were analyzed using Attribute Bagging (HIBAG)-R package and chi-square analysis.
Results: Seven single nucleotide polymorphisms (SNPs) including LINC02006 (rs531166736, rs187306735), APC (rs112800832_C_CAT), SRP19 (rs139948960, rs144784670), EGFLAM (rs16903975) and LDLRAD3 (rs79874564) were closely associated with the AA phenotype (P< 5E-08). Examination of biological networks revealed that these genomic areas are associated with antigen presentation signaling, B cell and T cell development, Th1 and Th2 activation pathways, Notch signaling, crosstalk signaling between dendritic cells and natural killer cells, and phagosome maturation. Based on human leukocyte antigen (HLA) genotype analysis, four HLA genotypes (HLA-B*15:01-*40:01, HLA-DQA1*01:02-*03:03, HLA-DQA1*01:02, and HLA-DQB1*02:01) were found to be associated with AA (adjusted p-value< 0.05). HLA-DQA1*01:02 is the most significantly related gene in the Taiwanese population (adjusted p-value = 2.09E-05).
Conclusion: This study successfully identified susceptibility loci associated with AA in the Taiwanese population. These findings not only shed light on the origins of AA within the Taiwanese context but also contribute to a comprehensive understanding of the genetic factors influencing AA susceptibility.
Keywords: alopecia areata, genome-wide association study, network analysis, HLA genotypes
Introduction
Alopecia areata (AA) is one of Taiwan’s most common autoimmune hair diseases and incidence rate of AA is 0.22%.1–3 The main symptoms of AA are rapid, non-scarring hair loss that affects body hair, facial hair, eyelashes, and brows.1,2 In the United States, the prevalence of AA is estimated to be 0.21 per 1000 person-years, the average affected age is between 25 and 36 years, and there are no discernible gender disparities.4 In Britain, the results are similar; the prevalence rate is 0.26 per 1000 person-years, the average affected age is between 25 and 29 years, and there are no appreciable gender variations. The majority of AA patients are female, and the incidence rate of AA among non-white populations, particularly Asian populations, is much higher than for Western populations, at 3.32 per 1000 person-years.4 AA is likely to be correlated with a number of factors, including adverse drug reactions (ADRs) resulting from taking carbamazepine, phenytoin, or valproate, viral or bacterial infections, and psychological factors such as stress, smoking, and alcohol consumption.5–7 According to research, AA patients frequently experience psychological illnesses such as depression, anxiety, and social phobia.8,9 Moreover, an increased risk of AA is associated with polycystic ovarian syndrome, retinal illness, thyroid disease, and breast cancer.10–13 AA results from hyperactivity of the immune system that subsequently damages its exclusive zone, leading to aberrant inflammation and hair loss.14,15
There are several primary causes of AA lesions. These include significantly shorter hair cycles, localized micro trauma to the skin or hair, and aberrant expression of first-class major histocompatibility complex (MHC) molecules (HLA-A, HLA-B, and HLA-C) and second-class MHC molecules (HLA-DP, HLA-DQ, HLA-DR, HLA-DN, and HLA-DO).16–19 Hair bulbs are immune-privileged regions that immune cells (including CD4+T and CD8+T cells, mast cells, natural killer (NK) cells, and dendritic cells) can nonetheless infiltrate through. Disruption of the immune system occurs as follows: Interferon (IFN)-JAK-STAT signal transmission pathway-activated STAT protein activates T cells (CD8+NKG2D+T cells) through IL-2 receptor c (IL-2R/c complex) and IL-15 receptor/IL-15 activation, which in turn further activates T cells (CD8+NKG2D+T cells) and generates IFN-γ.20–23 IFN-γ and IFN-γ receptors induce the production of IL-15 by hair follicle epithelial cells. IFN-γ also induces the expression of MHC class I and MHC class II genes, as well as the activation of immune cells. In turn, lymphocytes attack the cells of the hair follicles. This causes disruption of the immune system, leading to hair loss.23–26
While AA does not appear to be contagious, it is frequently observed to occur within families.27–29 AA is mainly caused by genetic variants on the sixth chromosome, including HLA-DRB1, ULBP3, and RAET1L (ULBP6),28,30 but genetic variants on chromosomes 10, 16, and 18 also cause AA. To investigate linkage patterns among 102 affected individuals and 118 unaffected individuals, a comprehensive analysis of the genome was undertaken in a cohort of 20 families. Participants in the study came from both the United States and Israel. There have been several susceptibility loci identified on chromosomes 6, 10, 16, and 18.31 The markers loci D6S1009, D6S2427, D6S1270, D6S1003, and D6S128131 are located on chromosome 6. The chromosomal positioning of the marker loci D10S1239 and D10S248131 has been altered to be on chromosome 10. D16S753 and D18S976 are genetic markers located on the 16th and 18th chromosomes, respectively.31 There is a correlation between the gene variation site associated with the C3H-HeJ alopecia pattern in mice and the gene variation site of human leukocyte antigens (HLA).32 Collectively, this evidence suggests that AA is inherited and may run in families. The genetic basis underlying AA in Taiwan has not yet been definitively examined. Using the Taiwanese gene database of the China Medical University Hospital (CMUH), a genome-wide association study (GWAS) of gene variants in AA patients was carried out. Based on our research findings, the genetic diversity observed among Taiwanese individuals appears to exhibit distinct characteristics compared to other racial groups.
Materials and Methods
Study Design, Sample Sources, and Characteristics
The ethics committee of the China Medical University Hospital (CMUH) approved this study, categorized as the Precision Medicine Project (CMUHPMP) (IRB number: CMUH110-REC3-005 and CMUH111-REC1-176). Clinical information was gathered between 1992 and 2020 from the electronic medical records (EMRs) of CMUH using diagnostic codes of AA (International Classification of Diseases (ICD), Ninth Edition, Clinical Modification (ICD-9-CM) codes 704.01; Tenth Edition, Clinical Modification (ICD-10-CM) code L63).33,34 All cases included in this study have undergone validation by physicians specialized in the field of AA disorders. The exclusion criteria were patients with autoimmune diseases.35–37 Exclusion of the above cases left a dataset containing 8167 controls and 408 cases of AA. All results can be found at: https://my.locuszoom.org/gwas/398185/?token=15220589b5234108b60edff3dcc5eaee.
GWAS Analysis Using a SNP Array
GWAS analysis was performed using the TPMv1 customized SNP array (Thermo Fisher Scientific, Inc., Santa Clara, CA, USA), in accordance with the manufacturer’s instructions.33 The statistical study examined the relationship between the SNP array and AA risk using PLINK V.1.90 software. Using R studio, we generated Manhattan and quantile-quantile (QQ) plots with p-values.33
Network Analysis
The genome-wide significance level for network analysis was established at P<1E-05, and 882 SNPs gene loci were found to be associated with AA. We used core analysis in the IPA software to build a corresponding molecular network of 882 SNPs (Qiagen Sciences, Inc.). Using Fisher’s exact t-test, all accessible networks were determined to be statistically significant (P< 0.05).
Imputation and Prediction of HLA
HIBAG-R (HLA Genotype Imputation with Attribute Bagging) was used to calculate HLA types for each participant. Individuals can determine their haplotypes and diplotypes using HIBAG. Probabilities greater than 0.90 were retained. The haplotypes and diplotypes of HLA were then analyzed using chi-square statistics. The association between haplotypes, diplotypes, and the occurrence of AA was investigated using Bonferroni correction to account for multiple testing. Statistics were considered significant when the p-value was below 0.05.38,39
Results
GWAS Analysis of AA in a Taiwanese Population
A GWAS analysis was conducted using information from 408 Taiwanese cases and 8167 Taiwanese controls in Table 1. A total of 3311 men and 4856 women were in the control group, with an average age of 37.49±15.32 years. The average age of the 166 men and 242 women in the AA cohort was 37.49±15.57 years. It was not observed that there was a statistically significant difference in clinical characteristics based on gender, age, smoking, drinking, or chewing betel (Table 1). The outcomes of the GWAS investigation for associations using the Manhattan plot are displayed in Figure 1. For the complete genome, P<5E-08 was chosen as the significance threshold. The QQ plots were used in Figure 2 to examine the relationship between AA and controls using genome-wide association analysis. GWAS analysis revealed that AA at position 14,064,987 was strongly linked to 882 SNPs (Supplementary Table 1, P<1E-05). In Table 2, the top 15 SNPs associated with AA are listed. An association has been found between AA and six SNPs as well as a single nucleotide variant (SNV) on chromosomes 3 and 5. These six SNPs and one SNV include LINC02006 (rs531166736, rs187306735), APC (rs112800832_C_CAT), SRP19 (rs139948960, rs144784670), EGFLAM (rs16903975), and LDLRAD3 (rs79874564). Statistical significance is demonstrated by a p-value less than 5E-08. In addition, regional plots of rs397081 and rs9273067 show higher linkage disequilibrium than other SNPs (Figure 3). NOTCH4 (Figure 3A) and HLA-DQA1 (Figure 3B) variants were also highly correlated in the AA group (LD score r2>0.4). The results suggest that these loci share functionality, pathways, or disease relevance.
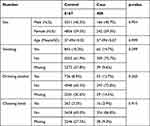 |
Table 1 The Features and Details of AA Research Participants |
 |
Table 2 The Genomic Locations on SNP in AA Disease (p<1E-07) |
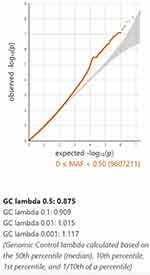 |
Figure 2 The relationship of genome-wide variations with AA as determined by quantile-quantile (QQ) plot analysis. The lambda (based on median chisq) was 0.875. |
Network Analysis of SNPs Associated with AA
An analysis of 882 SNPs (P<1E-05) within 74 genes associated with AA was conducted using IPA software, which examined their genome-wide importance (Supplementary Table 2). Our findings demonstrated that the ingenuity and canonical pathways resulting in AA included the antigen presentation pathway, Th1 and Th2 related pathway, PD-1/PD-L1 cancer immunotherapy pathway, macrophage-related pathway (MSP-RON signaling in macrophage pathway; macrophage classical activation signaling pathway; macrophage alternative activation signaling pathway), NOTCH signaling, IL-10 signaling, multiple sclerosis signaling pathway, crosstalk between dendritic cells and natural killer cells, leucine degradation I, calcium transport I, ketogenesis, embryonic stem cell pluripotency (Role of NANOG in mammalian embryonic stem cell pluripotency and human embryonic stem cell pluripotency), neuron inflammation signaling pathway, Cdc42 signaling, phagosome maturation, pathogen-induced cytokine storm signaling pathway, Wnt/β-catenin signaling, regulation of the epithelial-mesenchymal transition pathway (EMT), and graft-versus-host disease signaling (Table 3). Moreover, cellular immune response, humoral immune response, and cellular growth/proliferation and development, cytokine signaling and Pathogen-influenced signaling were ranked according to the results of the cross-analysis of the gene number with pathway (Figure 4). The antigen presentation pathway (Figure 5A) and Notch signaling target genes (Figure 5B) are shown in our results, respectively. Several HLA-related gene loci have been identified as major genomic locations of SNPs in AA: HLA-DQA1 (rs9272328, rs9273067), HLA-DRA (rs3129871, rs6911777, rs16822618), HLA-DRB1 (rs9270498, rs9270502, rs9270523), and Notch4-mediated T cells activation signaling (rs379464, rs2854048, rs367398, rs397081, rs3134928) of antigen presentation with Th1/Th2 activation. In Figure 6A, our GWAS findings showed that some associated genomic markers play a role in certain skin and hair conditions, including amyloidosis cutis dyschromia (genomic markers: GPNMB), autosomal dominant Hailey-Hailey disease (genomic markers: ATP2C1), skin pigmentation disorder (genomic markers: GPNMB, ANKRD12, MC1R), skin and hair hypopigmentation (genomic markers: MC1R), and gradual hair thinning (genomic markers: ABCE8). In addition, our GWAS findings also identified nine related genomic markers, including HLA-DRA, HLA-DRB1, HLA-DQA1, LIFR, ANKS1A, SEC11A, USP6NL, SLC16A9, and UBE4B, involved in the IFN-γ-JAK-STAT signal transmission pathway in AA (Figure 6B).
 |
Table 3 The Top 25 Canonical Network Analysis of GWAS Results in AA Disease |
 |
Figure 4 Network studies of 882 SNP gene locations that are associated with AA. Cross analysis of the number of genes and pathways (SNPs gene loci, P<1E-05). |
 |
Figure 5 Network analyses of the antigen presentation pathway target gene (A) and the gene that Notch signaling targets (B). IPA software analyzed network analysis (SNPs gene loci, P<1E-05). |
HLA Genotyping and Allele Frequency Analysis by HIBAG
Specific HLA alleles and variants have demonstrated an association with an increased susceptibility to AA.40–42 With the help of a high-resolution imputation system, we analyzed HLA genotypes and allele frequency associated with AA. Table 4 shows that the diplotypes HLA-B*15:01-*40:01, HLA-DQA1*01:02-*03:03 and the haplotypes HLA-DQA1*01:02 and HLA-DQB1*02:01 had a significant correlation with AA. Our results suggested that the HLA-DQA1*01:02 is the HLA allele most significantly associated with AA in the Taiwanese population (adjusted p-value = 2.09 E-05).
 |
Table 4 HLA Genotypes and Allele Frequency Significantly Associated with AA in a Taiwanese Population (Adjust p-value<0.05) |
Discussion
Several studies, including twin- and family linkage studies, suggest that AA has a genetic basis.4,36 However, there is yet no genetic evidence for the Taiwanese population in relation to AA. Our study identified six SNPs and one SNV across five loci in Taiwanese individuals that are significantly linked with AA. These six SNPs and one SNV include LINC02006 (rs531166736, rs187306735), APC (rs112800832_C_CAT), SRP19 (rs139948960, rs144784670), EGFLAM (rs16903975) and LDLRAD3 (rs79874564) (p<5E-08) (Table 2). The canonical network analysis revealed a substantial infiltration of aggressive immune cells, including T cells, mast cells, NK cells, and dendritic cells, into the immune-privileged region surrounding the hair bulb when genetic variant were identified at specific loci (p<1E-05). Notably, the HLA-DQA1, HLA-DRA, and HLA-DRB1 genes exhibited significant associations with these immune cell infiltrations in the Taiwanese population (Table 3). To initiate our analysis, we employed the HIBAG tool to investigate the HLA genotypes and allele frequencies associated with AA (Table 4). Our findings indicated that HLA-B*15:01-*40:01, HLA-DQA1*01:02-*03:03 HLA-DQA1*01:02 and HLA-DQB1*02:01 have been linked to AA, and HLA-DQA1*01:02 exhibited the strongest correlation in terms of connectivity in the Taiwanese population (adjusted p-value = 2.09E-05). Similar to previous studies, our findings suggest that HLA-DQA1, HLA-DRA, and HLA-DRB1 affect HLA-DQ and HLA-DR cell surface conformation and HLA-D type-peptide specificity.17,43 In addition, those results indicated that AA is caused by a HLA-D type gene alteration and disruption. Several Taiwanese individuals chew betel nuts, smoking, drinking alcohol as part of their daily routine, and the pathological mechanism of many disorders, including cancer, has been linked to this behavior.3,44 As shown in Table 1, AA is not associated with gender or lifestyle behaviors (smoking, drinking alcohol, consuming betel).
Furthermore, we compared our results with those of other GWAS, such as Petukhova et al 2010 study36 and Betz et al 2015 study37 (Table 5). Three types of variants: those that are common to all Taiwanese and other ethnic groups, those that are exclusive to Taiwanese, and those that are absent from Taiwanese. Lynn Petukhova et al demonstrated an association with genomic regions containing genes controlling the activation and proliferation of regulatory T cells, including cytotoxic T lymphocyte-associated antigen 4 (CTLA4), interleukin (IL)-2/IL-21, IL-2 receptor A (IL-2RA; CD25), and Eos (also known as Ikaros family zinc finger 4; IKZF4), as well as the human leukocyte antigen (HLA) region. PRDX5 and STX17 are genes expressed in hair follicles, and there was evidence of association for those regions as well.36 However, Taiwanese AA patients’ genetic susceptibility loci can be distinguished from those of patients from other countries. There are similarities between HLA-DRA (rs3129869, rs3129871, rs6911777, rs16822618; Foreign: rs9268657) and HLA-DRB1 (rs9270488, rs9270502, rs9270523, rs9270524). Many genetic susceptibility loci are associated with Taiwanese AA patients, while none are associated with AA patients from other countries. These include LINC02006 (rs531166736, rs187306735), APC (rs112800832_C_CAT, rs372900303, rs375616619), SRP19 (rs139948960, rs144784670), LDLRAD3 (rs79874564), NOTCH-4 (rs397081), LOC102467216 (rs376830017), and HLA-DQA1 (rs9273067). A notable finding is that AA does not appear to be associated with LINC02006 (rs531166736), SRP19 (rs139948960 and rs144784670), EGFLAM (rs16903975), and LDLRAD3 (rs79874564). To the best of our knowledge, the presence of SNPs unique to these five genes in Taiwanese AA patients is a novel finding in the field of AA research. Based on Betz et al findings, the cohorts had relatively small sample sizes. As our cohort size increases, the likelihood of identifying SNPs and enhancing statistical power increases. Individuals with diverse ethnic backgrounds share many risk loci. Several similar pathways have been identified among ancestral tribes, suggesting that diseases are influenced by similar biological mechanisms.37
 |
Table 5 AA Disease-Related Target Gene and SNP Location Analysis in Taiwan and International Studies |
Hennig et al found that LINC02006 (rs10935945 at 3q25.2) is strongly linked to colorectal cancer.45 The lncRNA coding gene LINC02006 contributes to cell proliferation, cell death, and cancer progression. Post-transcriptional modifications are influenced by lncRNAs through miRNA sponges and endogenous competitors.45 Further research is necessary to investigate the potential of LINC02006 (rs531166736) as a marker for assessing the risk of AA and generating a polygenic risk score (PRS). In an early GWAS study, it was discovered that EGFLAM polymorphisms were linked to developmental disorders and exhibited significant susceptibility to the influence of antidepressants.46 EGFLAM plays a crucial role in the formation of photoreceptor ribbon synapses, visual perception, and cell adhesion. Additionally, a Japanese exome-wide association study (EWAS) revealed a strong association between EGFLAM and authentic aortic aneurysm.47 Recently, novel biomarkers for glioblastoma (GBM) have been identified, with a focus on the EGFLAM protein.48 Additionally, there is evidence suggesting the involvement of LDLRAD3 in the etiology of Alzheimer’s disease.49 LDLRAD3 has been found to contribute to the increased synthesis of amyloid beta-peptide (A), which is associated with the proteolysis of amyloid precursor protein.49 Furthermore, it has been observed that LDLRAD3 interacts with host cell receptors during the entry of the Venezuelan equine encephalitis virus (VEEV) into the cell.50 GWAS data also have indicated an association between LDLRAD3 and the secretion of melatonin.51 However, it is important to note that the relationship between EGFLAM, LDLRAD3, and AA has not been firmly established. These findings require validation through additional research efforts.
Canonical network analysis identifies GWAS loci and target genes in cellular physiological processes, predicts unique disease mechanisms.52 As indicated in Table 3 and Supplementary Table 2, we verified that the GWAS target genes of patients with AA in Taiwan are involved in the information transmission route using canonical network analysis. Figure 4 shows the results of our canonical network study. The primary components of the conventional network included: (1) APC/WNT signaling pathway; (2) HLA-DQA1, HLA-DRA, and HLA-DRB1 implicated in antigen presentation; (3) Notch signaling pathway (Figure 5B). These findings were associated with hair disorders and skin-related disorders (Figure 6A) and the IFN-γ-JAK-STAT signaling pathway (Figure 6B). Our study offers compelling evidence that the pathophysiology of AA involves immune system activity. The primary role of the NOTCH-4 gene is to inhibit inflammatory response via IFN-γ, which then has an impact on the HLA-DR and HLA-DQ genes.17,53–55 IFN-γ-induced degeneration of hair follicle dermal papilla cells can be prevented by the APC/Wnt signaling pathway.56 The APC/Wnt signaling pathway is crucial for anagen re-entry and hair development. The Wnt-related transcription factor TCF7L2 (TCF4) is associated with AA. Activation of APC/Wnt/beta-catenin signaling can increase downstream cyclin D1 gene expression and reduce the production of inflammatory cytokines such as TGF-beta.57,58 Our SNP results (rs112800832_C_CAT, rs372900303 and rs375616619, Table 2) and canonical network analysis (Table 3) revealed that APC/Wnt signaling and its associated genes may have a significant impact on AA. This study also showed a significant correlation between AA and the Notch4 gene (rs397081, rs379464, rs2854049, rs2854048, rs367398, rs3134928). AlFadhli et al showed that RA and NOTCH-4 polymorphisms have a significant association.17,43 In keratinocytes, Notch signaling is involved in cell growth arrest and entry into differentiation.17,43 Our study indicates that AA is caused by a Notch gene alteration and disruption of Notch signaling.
High IFN secretion, which results in the collapse of hair follicles in immune-privileged areas, are also important factors that contribute to AA in addition to hereditary variability.59,60 IFN-γ-JAK-STAT signaling constitutes the majority of the IFN-induced signaling cascade.61,62 IFN-γ-stimulated STATs enter the nucleus, control gene expression, and influence cell development through the organization of JAK and JAK receptors, phosphorylation, and activation of downstream STAT proteins.24,61,62 IFN-γ-JAK-STAT signaling is crucial for the development of autoimmune disorders.63,64 IFN-γ, which is generated by CD8, NKG2D+/NK, and T cells, targets keratinocytes that make up the outer root sheath (ORS) of hair follicles in hair bulbs in AA patients. Moreover, keratinocytes release IL-15, which triggers the production of IFN-γ by NK and T cells and worsens AA progression.63,64 By interfering with the JAK/STAT signal transmission pathways, IFN-γ causes the expression of HLA-DR in the HHPDC of the hair follicle and further causes damage and death of dermal papilla cells.19,65 Recently, McDonagh et al demonstrated that IFN-γ induces overexpression of HLA class 1, HLA-DR, and ICAM-1 in hair follicles.66 In our preliminary results, we demonstrated that stimulation of human hair dermal papilla cells (HHPDCs) with IFN-γ caused a concentration-dependent decrease in cell survival. In NGS analyses, 686 genes were up-regulated by IFN and 494 genes were down-regulated by IFN, including genes related to IFN and genes associated with HLA subtypes (HLA-DQA1, HLA-DRA, and HLA-DRB1). These findings are in line with GWAS performed on patients with AA, which offer further evidence of AA’s pathogenicity.
Conclusion
Our research concluded that the pathogenic mechanism of autoimmune disorders underlying AA signaling is essential (Figure 7). The results of GWAS have uncovered for the first time numerous loci in Taiwan individuals that contribute to AA. We were able to define the signaling pathway in AA, thus corroborating the GWAS-derived genetic research findings. The next step will be to determine if functional validation will be necessary to distinguish pathogenic variants from non-pathogenic variants.
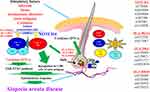 |
Figure 7 The molecular mechanisms involved in AA disease are depicted. Details are provided in the text. |
Data Sharing Statement
In the study, the original data are publicly available. All results can be found at: https://my.locuszoom.org/gwas/398185/?token=630b4f26538c47769997bee9319aec0f.
Ethics Approval and Informed Consent
The study protocol was approved by the Institutional Review Board of China Medical University Hospital and categorized as the Precision Medicine Project (CMUHPMP) (IRB number: CMUH110-REC3-005 and CMUH111-REC1-176). Patients have been granted access to their medical records by the CMUH IRB. The CMUH IRB also places considerable emphasis on ensuring patient confidentiality. De-identified genetic and clinical data were collected after obtaining informed consent from patients. We complied with the Helsinki Declaration in conducting our study.
Acknowledgments
We thank the Office of Research and Development, China Medical University (Taiwan) for providing Medical Research Core Facilities to perform the experiments and data analysis. We also thank Kuan-Wen Chen and Yao-Wei Jheng (GGA Corporation, Molecular Science and Digital Innovation Center, Taiwan) for their assistance and equipment.
Funding
This work was supported in part of the project (DMR-112-135 and DMR-112-140) from China Medical University Hospital, Taiwan. This work was also supported in part of the project (MOST 111-2314-B-075 −083 -MY2) from the Ministry of Science and Technology, Taiwan.
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Wei Y-H, Tai Y-H, Dai Y-X, Chang Y-T, Chen T-J, Chen M-H. Bidirectional association between alopecia areata and atopic dermatitis: a population-based cohort study in Taiwan. Clin Exp Allergy. 2020;50(12):1406–1414. doi:10.1111/cea.13729
2. Chiu YW, Chen YD, Hua TC, Wu CH, Liu HN, Chang YT. Comorbid autoimmune diseases in patients with pemphigus: a nationwide case-control study in Taiwan. Eur J Dermatol. 2017;27(4):375–381. doi:10.1684/ejd.2017.3060
3. Dai YX, Yeh FY, Shen YJ, et al. Cigarette smoking, alcohol consumption, and risk of alopecia areata: a population-based cohort study in Taiwan. Am J Clin Dermatol. 2020;21(6):901–911. doi:10.1007/s40257-020-00547-7
4. Harries M, Macbeth AE, Holmes S, et al. The epidemiology of alopecia areata: a population-based cohort study in UK primary care. Br J Dermatol. 2022;186(2):257–265. doi:10.1111/bjd.20628
5. Rajabi F, Drake LA, Senna MM, Rezaei N. Alopecia areata: a review of disease pathogenesis. Br J Dermatol. 2018;179(5):1033–1048. doi:10.1111/bjd.16808
6. Friedli A, Harms M. [Alopecia areata]. Ther Umsch. 2002;59(5):233–237. German. doi:10.1024/0040-5930.59.5.233
7. Redondo P, Vicente J, Espana A, Subira ML, De Felipe I, Quintanilla E. Photo-induced toxic epidermal necrolysis caused by clobazam. Br J Dermatol. 1996;135(6):999–1002. doi:10.1046/j.1365-2133.1996.d01-1111.x
8. Muntyanu A, Gabrielli S, Donovan J, et al. The burden of alopecia areata: a scoping review focusing on quality of life, mental health and work productivity. J Eur Acad Dermatol Venereol. 2023. doi:10.1111/jdv.18926
9. van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, Okkerse JME, Pasmans S. Anxiety, depression, and quality of life in children and adults with alopecia areata: a systematic review and meta-analysis. Front Med. 2022;9:1054898. doi:10.3389/fmed.2022.1054898
10. Ting H-C, Ma S-H, Tai Y-H, et al. Association between alopecia areata and retinal diseases: a nationwide population-based cohort study. J Am Acad Dermatol. 2022;87(4):771–778. doi:10.1016/j.jaad.2021.10.045
11. Dai Y-X, Tai Y-H, Chang Y-T, Chen T-J, Chen M-H. Bidirectional association between alopecia areata and thyroid diseases: a nationwide population-based cohort study. Arch Dermatol Res. 2021;313(5):339–346. doi:10.1007/s00403-020-02109-7
12. Lin WL, Lin WC, Jung SM, Yang CH, Hong HS. Breast cancer metastasized to the scalp mimicking alopecia areata: alopecia neoplastica. Breast J. 2007;13(1):94–95. doi:10.1111/j.1524-4741.2006.00372.x
13. Chang TH, Tai YH, Dai YX, Chang YT, Chen MH. Increased risk of alopecia areata among patients with polycystic ovary syndrome: a population-based cohort study. J Dermatol. 2021;48(2):242–244. doi:10.1111/1346-8138.15659
14. Ito T. Hair follicle is a target of stress hormone and autoimmune reactions. J Dermatol Sci. 2010;60(2):67–73. doi:10.1016/j.jdermsci.2010.09.006
15. Ito T, Meyer KC, Ito N, Paus R. Immune privilege and the skin. Curr Dir Autoimmun. 2008;10:27–52. doi:10.1159/000131412
16. Xiao FL, Yang S, Yan KL, et al. Association of HLA class I alleles with aloplecia areata in Chinese Hans. J Dermatol Sci. 2006;41(2):109–119. doi:10.1016/j.jdermsci.2005.07.008
17. Tazi-Ahnini R, Cork MJ, Wengraf D, et al. Notch4, a non-HLA gene in the MHC is strongly associated with the most severe form of alopecia areata. Hum Genet. 2003;112(4):400–403. doi:10.1007/s00439-002-0898-9
18. Akar A, Orkunoglu E, Sengul A, Ozata M, Gur AR. HLA class II alleles in patients with alopecia areata. Eur J Dermatol. 2002;12(3):236–239.
19. Konig A, Happle R, Hoffmann R. IFN-gamma-induced HLA-DR but not ICAM-1 expression on cultured dermal papilla cells is downregulated by TNF-alpha. Arch Dermatol Res. 1997;289(8):466–470. doi:10.1007/s004030050222
20. de Jong A, Jabbari A, Dai Z, et al. High-throughput T cell receptor sequencing identifies clonally expanded CD8+ T cell populations in alopecia areata. JCI Insight. 2018;3(19). doi:10.1172/jci.insight.121949
21. Ghraieb A, Keren A, Ginzburg A, et al. iNKT cells ameliorate human autoimmunity: lessons from alopecia areata. J Autoimmun. 2018;91:61–72. doi:10.1016/j.jaut.2018.04.001
22. Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol. 2022;13:955035. doi:10.3389/fimmu.2022.955035
23. Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–1049. doi:10.1038/nm.3645
24. Dai Z, Sezin T, Chang Y, Lee EY, Wang EHC, Christiano AM. Induction of T cell exhaustion by JAK1/3 inhibition in the treatment of alopecia areata. Front Immunol. 2022;13:955038. doi:10.3389/fimmu.2022.955038
25. Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183(6):1083–1093. doi:10.1111/bjd.19040
26. Simakou T, Butcher JP, Reid S, Henriquez FL. Alopecia areata: a multifactorial autoimmune condition. J Autoimmun. 2019;98:74–85. doi:10.1016/j.jaut.2018.12.001
27. Jacobsen EW, Pedersen OB, Andorsdottir G, Jemec GBE, Bryld LE. Family recurrence risk of alopecia areata in the Faroe Islands. Clin Exp Dermatol. 2019;44(7):e224–e229. doi:10.1111/ced.13974
28. Martinez-Mir A, Zlotogorski A, Ott J, Gordon D, Christiano AM. Genetic linkage studies in alopecia areata. J Investig Dermatol Symp Proc. 2003;8(2):199–203. doi:10.1046/j.1087-0024.2003.00809.x
29. Kavak A, Baykal C, Ozarmagan G, Akar U. HLA in alopecia areata. Int J Dermatol. 2000;39(8):589–592. doi:10.1046/j.1365-4362.2000.00921.x
30. Kim HJ, Kazmi SZ, Kang T, et al. Familial risk and incidence of alopecia areata among first degree relatives-A nationwide population-based study in Korea. J Am Acad Dermatol. 2021;85(5):1360–1362. doi:10.1016/j.jaad.2020.10.063
31. Martinez-Mir A, Zlotogorski A, Gordon D, et al. Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet. 2007;80(2):316–328. doi:10.1086/511442
32. Hashimoto K, Yamada Y, Sekiguchi K, Matsuda S, Mori S, Matsumoto T. Induction of alopecia areata in C3H/HeJ mice using cryopreserved lymphocytes. J Dermatol Sci. 2021;102(3):177–183. doi:10.1016/j.jdermsci.2021.04.009
33. Liu TY, Lin CF, Wu HT, et al. Comparison of multiple imputation algorithms and verification using whole-genome sequencing in the CMUH genetic biobank. Biomedicine. 2021;11(4):57–65. doi:10.37796/2211-8039.1302
34. Dai YX, Tai YH, Chen CC, Chang YT, Chen TJ, Chen MH. Bidirectional association between alopecia areata and sleep disorders: a population-based cohort study in Taiwan. Sleep Med. 2020;75:112–116. doi:10.1016/j.sleep.2020.06.015
35. Ho CY, Wu CY, Chen JY, Wu CY. Clinical and genetic aspects of alopecia areata: a cutting edge review. Genes. 2023;14(7). doi:10.3390/genes14071362
36. Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466(7302):113–117. doi:10.1038/nature09114
37. Betz RC, Petukhova L, Ripke S, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun. 2015;6:5966. doi:10.1038/ncomms6966
38. Liao WL, Liu TY, Cheng CF, et al. Analysis of HLA variants and graves’ disease and its comorbidities using a high resolution imputation system to examine electronic medical health records. Front Endocrinol. 2022;13:842673. doi:10.3389/fendo.2022.842673
39. Lu HF, Liu TY, Chou YP, et al. Comprehensive characterization of pharmacogenes in a Taiwanese Han population. Front Genet. 2022;13:948616. doi:10.3389/fgene.2022.948616
40. Redler S, Albert F, Brockschmidt FF, et al. Investigation of selected cytokine genes suggests that IL2RA and the TNF/LTA locus are risk factors for severe alopecia areata. Br J Dermatol. 2012;167(6):1360–1365. doi:10.1111/bjd.12004
41. Forstbauer LM, Brockschmidt FF, Moskvina V, et al. Genome-wide pooling approach identifies SPATA5 as a new susceptibility locus for alopecia areata. Eur J Hum Genet. 2012;20(3):326–332. doi:10.1038/ejhg.2011.185
42. Megiorni F, Pizzuti A, Mora B, et al. Genetic association of HLA-DQB1 and HLA-DRB1 polymorphisms with alopecia areata in the Italian population. Br J Dermatol. 2011;165(4):823–827. doi:10.1111/j.1365-2133.2011.10466.x
43. AlFadhli S, Nanda A. Genetic evidence for the involvement of NOTCH4 in rheumatoid arthritis and alopecia areata. Immunol Lett. 2013;150(1–2):130–133. doi:10.1016/j.imlet.2013.01.002
44. Lee CH, Ko AM, Warnakulasuriya S, et al. Population burden of betel quid abuse and its relation to oral premalignant disorders in South, Southeast, and East Asia: an Asian Betel-quid Consortium study. Am J Public Health. 2012;102(3):e17–24. doi:10.2105/AJPH.2011.300521
45. Hennig EE, Kluska A, Piatkowska M, et al. GWAS links new variant in long non-coding RNA LINC02006 with colorectal cancer susceptibility. Biology. 2021;10(6). doi:10.3390/biology10060465
46. Xia L, Ou J, Li K, et al. Genome-wide association analysis of autism identified multiple loci that have been reported as strong signals for neuropsychiatric disorders. Autism Res. 2020;13(3):382–396. doi:10.1002/aur.2229
47. Yamada Y, Sakuma J, Takeuchi I, et al. Identification of EGFLAM, SPATC1L and RNASE13 as novel susceptibility loci for aortic aneurysm in Japanese individuals by exome-wide association studies. Int J Mol Med. 2017;39(5):1091–1100. doi:10.3892/ijmm.2017.2927
48. Chen J, Zhang J, Hong L, Zhou Y. EGFLAM correlates with cell proliferation, migration, invasion and poor prognosis in glioblastoma. Cancer Biomark. 2019;24(3):343–350. doi:10.3233/CBM-181740
49. Ranganathan S, Noyes NC, Migliorini M, et al. LRAD3, a novel low-density lipoprotein receptor family member that modulates amyloid precursor protein trafficking. J Neurosci. 2011;31(30):10836–10846. doi:10.1523/JNEUROSCI.5065-10.2011
50. Ma H, Kim AS, Kafai NM, et al. LDLRAD3 is a receptor for Venezuelan equine encephalitis virus. Nature. 2020;588(7837):308–314. doi:10.1038/s41586-020-2915-3
51. Liu PH, Chuang GT, Hsiung CN, et al. A genome-wide association study for melatonin secretion. Sci Rep. 2022;12(1):8025. doi:10.1038/s41598-022-12084-w
52. Petukhova L, Christiano AM. Functional interpretation of genome-wide association study evidence in alopecia areata. J Invest Dermatol. 2016;136(1):314–317. doi:10.1038/JID.2015.402
53. Duvefelt K, Anderson M, Fogdell-Hahn A, Hillert J. A NOTCH4 association with multiple sclerosis is secondary to HLA-DR*1501. Tissue Antigens. 2004;63(1):13–20. doi:10.1111/j.1399-0039.2004.00135.x
54. Ando A, Shigenari A, Naruse TK, et al. Triplet repeat polymorphism within the NOTCH4 gene located near the junction of the HLA class II and class III regions in narcolepsy. Tissue Antigens. 1997;50(6):646–649. doi:10.1111/j.1399-0039.1997.tb02924.x
55. Lopez-Lopez S, Romero de Avila MJ, Hernandez de Leon NC, et al. NOTCH4 exhibits anti-inflammatory activity in activated macrophages by interfering with interferon-gamma and TLR4 signaling. Front Immunol. 2021;12:734966. doi:10.3389/fimmu.2021.734966
56. Ryu S, Lee Y, Hyun MY, et al. Mycophenolate antagonizes IFN-gamma-induced catagen-like changes via beta-catenin activation in human dermal papilla cells and hair follicles. Int J Mol Sci. 2014;15(9):16800–16815. doi:10.3390/ijms150916800
57. Rajabi F, Amoli MM, Robati RM, Almasi-Nasrabadi M, Jabalameli N, Moravvej H. The association between genetic variation in wnt transcription factor TCF7L2 (TCF4) and alopecia areata. Immunol Invest. 2019;48(6):555–562. doi:10.1080/08820139.2019.1597109
58. Lee YJ, Park SH, Park HR, Lee Y, Kang H, Kim JE. Mesenchymal stem cells antagonize IFN-induced proinflammatory changes and growth inhibition effects via Wnt/beta-Catenin and JAK/STAT pathway in human outer root sheath cells and hair follicles. Int J Mol Sci. 2021;22(9). doi:10.3390/ijms22094581
59. Yue Z, Yang F, Zhang J, Li J, Chuong CM. Regulation and dysregulation of hair regeneration: aiming for clinical application. Cell Regen. 2022;11(1):22. doi:10.1186/s13619-022-00122-x
60. Coda AB, Sinha AA. Integration of genome-wide transcriptional and genetic profiles provides insights into disease development and clinical heterogeneity in alopecia areata. Genomics. 2011;98(6):431–439. doi:10.1016/j.ygeno.2011.08.009
61. Ann S, Ibo J, Megha M, et al. Treatment of in vitro generated Langerhans cells with JAK-STAT inhibitor reduces their inflammatory potential. Clin Exp Med. 2022. doi:10.1007/s10238-022-00899-w
62. Kim JE, Lee YJ, Park HR, Lee DG, Jeong KH, Kang H. The effect of JAK inhibitor on the survival, anagen re-entry, and hair follicle immune privilege restoration in human dermal papilla cells. Int J Mol Sci. 2020;21(14). doi:10.3390/ijms21145137
63. Aota K, Yamanoi T, Kani K, Ono S, Momota Y, Azuma M. Inhibition of JAK-STAT signaling by baricitinib reduces interferon-gamma-induced CXCL10 production in human salivary gland ductal cells. Inflammation. 2021;44(1):206–216. doi:10.1007/s10753-020-01322-w
64. Jia Y, Jing J, Bai Y, et al. Amelioration of experimental autoimmune encephalomyelitis by plumbagin through down-regulation of JAK-STAT and NF-kappaB signaling pathways. PLoS One. 2011;6(10):e27006. doi:10.1371/journal.pone.0027006
65. Kaneko F, Suzuki M, Takiguchi Y, Itoh N, Minagawa T. Immunohistopathologic studies in the development of psoriatic lesion influenced by gamma-interferon and the producing cells. J Dermatol Sci. 1990;1(6):425–434. doi:10.1016/0923-1811(90)90012-3
66. McDonagh AJ, Snowden JA, Stierle C, Elliott K, Messenger AG. HLA and ICAM-1 expression in alopecia areata in vivo and in vitro: the role of cytokines. Br J Dermatol. 1993;129(3):250–256. doi:10.1111/j.1365-2133.1993.tb11842.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



