Back to Journals » Clinical Ophthalmology » Volume 16
Genetic Testing of Inherited Retinal Disease in Australian Private Tertiary Ophthalmology Practice
Authors Gocuk SA, Jiao Y, Britten-Jones AC , Kerr NM , Lim L, Skalicky S , Stawell R, Ayton LN , Mack HG
Received 13 December 2021
Accepted for publication 28 March 2022
Published 13 April 2022 Volume 2022:16 Pages 1127—1138
DOI https://doi.org/10.2147/OPTH.S353787
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sena A Gocuk,1 Yuanzhang Jiao,2 Alexis Ceecee Britten-Jones,1,3,4 Nathan M Kerr,5 Lyndell Lim,3,5 Simon Skalicky,5 Richard Stawell,5 Lauren N Ayton,1,3,4 Heather G Mack3– 5
1Department of Optometry and Vision Sciences, University of Melbourne, Melbourne, Victoria, Australia; 2University Hospital Geelong, Geelong, Victoria, Australia; 3Centre for Eye Research Australia, Royal Victorian Eye and Ear Hospital, Melbourne, Victoria, Australia; 4Ophthalmology, Department of Surgery, University of Melbourne, Melbourne, Victoria, Australia; 5Eye Surgery Associates, East Melbourne, Victoria, Australia
Correspondence: Lauren N Ayton, Email [email protected]
Background: To assess the prevalence of genetic testing for inherited retinal diseases (IRDs) in a tertiary practice setting.
Methods: Single-centre retrospective analysis of patients with diagnosed or suspected IRD.
Results: Four hundred and sixty-four patient records were analysed. Patients had received care for different IRDs grouped as follows: panretinal pigmentary retinopathies (283, 61%), macular dystrophies (136, 29.3%), stationary diseases (23, 5%), hereditary vitreoretinopathies (14, 3%), and other IRDs (8, 1.7%). The suspected pattern of inheritance of patients’ IRD was predominantly autosomal recessive (205, 44.2%). Genetic testing was performed with the corresponding results available for 44 patients (9.5%). Diagnostic yield was 65.9% for the results received. Genetic test results were available mostly for younger patients (13.1% for < 45 years vs 6.2% ≥ 45 years of age, p = 0.01) and those who received greater than 12 months of care (16% for ≥ 12 months vs 4% for < 12 months, p < 0.01). For patients without genetic testing results, reasons include awaiting a geneticist consultation (17.9%), awaiting test results (4.5%), or patient refusal (8.4%). Most clinical records (69.2%) did not document genetic testing status.
Conclusion: Genetic testing is increasingly being utilised in the work-up for patients with IRD worldwide. This large Australian private practice IRD cohort shows a low uptake of testing (around 10%), reflecting historical management patterns and accessibility of genetic counselling and testing. The results show that younger patients and those with a longer duration of care were more likely to have received genetic testing. As the importance of IRD genetic testing continues to increase, we expect to see a change in patient management within the Australian private ophthalmology system and testing rates to increase. Further research is required to identify and address clinician and patient barriers to improving genetic testing rates for IRD.
Keywords: inherited retinal disease, retinitis pigmentosa, macular dystrophy, genetic testing
Introduction
Inherited retinal diseases (IRDs) are a group of heterogeneous degenerative retinal conditions estimated to occur in up to 1 in 1000 individuals.1,2 IRDs are now the most common cause of legal blindness in adults of working age in Australia3 and the United Kingdom (UK).4 Previous experimental treatments for IRD have included Vitamin A supplementation, valproate,5 ciliary neurotrophic factor supplementation6 and electrical stimulation through the skin7 or cornea,8 but their efficacies are unclear, and none have reached regulatory approval.
Recently, gene augmentation therapy for RPE65-associated IRD (Leber Congenital Amaurosis) has been approved by the United States (US) Food and Drug Administration (FDA, 2017), European Medicines Agency (2018), and the Therapeutic Goods Administration in Australia (2020). This has accelerated the development of further gene therapies for other forms of IRD, including gene augmentation, gene editing (CRISPR/Cas9) and RNA-based therapies.9 Currently, there are over 30 active clinical trials for gene therapy for patients with IRD.10
Assessment of eligibility for ocular gene therapies requires identification of patients’ pathogenic genetic variant. Therefore, genetic testing is recommended as standard of care in Australia11 and internationally.12 In addition to exploring potential gene therapy opportunities, genetic testing is recommended to confirm the clinical diagnosis and inheritance of the condition, which may inform prognosis for patients and their family members, including family planning considerations.13–15
Genetic testing has evolved over the years, allowing case-by-case selection of appropriate molecular testing strategies.16 While Sanger sequencing is typically chosen for suspected monogenic disorders, more advanced methods such as next-generation sequencing (NGS) and whole-exome sequencing (WES) are available for patients with uncertain clinical diagnoses and/or inheritance patterns.16 These novel methods have increased the success rate of IRD genetic testing (defined as identification of at least one pathogenic variation) to between 56% and 76% in most developed countries.14,17–19 The success of genetic testing in identifying the disease-causing variant varies depending on patients’ specific diagnosis,17 age,20 and whether the responsible gene and/or pathogenic variant has been previously identified in IRD patients and/or family members.21 New developments in testing methodology and gene therapy have further highlighted the important role of genetic testing for IRDs.
A recent study by Strait et al (2020) explored self-reported genetic testing practices of optometrists and ophthalmologists managing patients with IRDs in the US.15 Respondents indicated that while there are discussions surrounding genetics (64.7% and 70.6% of the clinicians reported taking family history of IRD and explaining inheritance patterns to their IRD patients, respectively), 78.4% of the clinicians have not ordered genetic testing for their patients with IRD.15 Reported reasons for not completing genetic testing included the opinion that genetic test results do not alter IRD patients’ clinical management, lack of clinicians’ confidence in their ability to order the appropriate test, preference to refer to experienced clinicians, and/or patient refusal.15
To our knowledge, there are no studies exploring the rate and outcomes of IRD genetic testing ordered by Australian ophthalmologists in a clinical private tertiary care setting. This study sought to evaluate the current prevalence of genetic testing, distribution of IRDs and genetic diagnoses in a private tertiary retinal practice in Victoria, Australia. This should be taken as an indication of historical referral processes, when genetic testing was not key in the management of IRD. We aim to reassess in several years to observe the changes following the recent Royal Australian and New Zealand College of Ophthalmologists (RANZCO) IRD management guidelines,11 which have highlighted the need for more widespread genetic testing with the availability of gene-based therapies for these patients.
Methods
This retrospective analysis involved evaluation of electronic medical records of pre-existing patients of Eye Surgery Associates, a large private ophthalmic practice in Victoria, Australia, with 18 sub-specialty ophthalmologists. Patients are referred to this clinic for tertiary level medical retina care and/or diagnostic retinal electrophysiology services.
The senior author and ophthalmologist HM completed a search of the practice’s electronic database (VIP.net Version Ruby, Best Practice Software, Bundaberg, QLD) to identify all confirmed or suspected IRD patients seen between 1995 and 2021 using the following search terms: retinitis pigmentosa (or abbreviation, RP), retinal dystrophy, cone dystrophy, cone-rod dystrophy, macular dystrophy, Best, Stargardt, congenital stationary night blindness, monochromat, achromatopsia, Bietti, choroideremia, familial exudative vitreoretinopathy, Usher, Wagner, gyrate and Sorsby.
After removing duplicate records, clinical records were reviewed by HM for accuracy of diagnosis, and those with incorrect or uncertain diagnoses as documented by clinicians were excluded, including 20 cases of possible adult vitelliform macular dystrophy, which were not possible to distinguish from age-related macular degeneration from clinical records.
Data Collection
A two-stage clinical record review was undertaken by the senior author (HM), followed by two co-first authors experienced in IRD (YJ, SG). The analysis was completed between June and August 2021. The senior author (HM) is an experienced ophthalmologist in the management of medical retina disorders, particularly IRDs. Both co-first authors are optometry trained with further training in research (MPhil, SG) and medicine (MD, YJ). Data were captured as documented in the clinical records by the treating clinician. Unclear records (n=10) were discussed by the broader research team (YJ, SG, HM, LA, ACBJ) to obtain consensus.
The following de-identified information was collected, based only upon information available in the patient record: patient age, gender (female, male, non-binary), duration of care at the practice (months), clinical diagnosis of IRD, suspected mode of inheritance, history of consanguinity, and genetic testing results for the patient and/or family members. Suspected mode of inheritance was determined through family history (Supplementary Figure 1), and when present, genetic test results of the patient and their family members.
If a genetic test report was available, the following data were collected: testing methodology (NGS, WES, Sanger sequencing, microarray, unknown), clinical grade or research grade testing, and whether the pathogenic or likely pathogenic variant was identified.
If no genetic test results were available, the status of planned testing was captured (awaiting geneticist, awaiting test results, patient refused, or not further specified). Clinical records that did not capture whether genetic testing was ordered or the patient’s response to genetic testing, were considered “not further specified.”
Data Management and Privacy
De-identified data were collected using REDCap, a secure web application for building and managing online surveys and databases. REDCap includes a full analysis trail and specified user-based privileges. Access to study data in REDCap was restricted to the members of the study team. Only de-identified data was exported for the purposes of analysis and reporting.
Statistical Analysis
De-identified data were imported into R (R Core Team, Vienna, Austria) for descriptive statistical analyses. IRD clinical diagnosis was grouped into panretinal pigmentary retinopathies, macular dystrophies, stationary diseases, and hereditary vitreoretinopathies according to Coco-Martin et al.22
Age subgroups are presented as young patients (less than 45 years of age) versus older patients (45 years and older) as an appropriate cut-off age for family planning23 and childbearing.24 The distribution of the data was explored and comparison between subgroups was performed using Wilcoxon rank sum test for non-parametric continuous variables and Fisher exact test for categorical variables. An alpha value of 0.05 was used to define statistical significance. Binary logistic regression was performed using IBM SPSS Statistics for Windows, version 27 (IBM Corp., Armonk, NY, USA), to calculate the odds of patients having had genetic testing based on patients’ gender, age, and duration of care.
Ethics
All patients had provided written consent for their health information to be used for research, and audit purposes at the time of their initial visit at Eye Surgery Associates, therefore, were not re-contacted for consent specifically for this study. Ophthalmologists of all reviewed patients gave permission for record access. This study was approved by the Human Research Ethics committee of the RANZCO (#124.21) and abided by the Declaration of Helsinki.
Results
An initial search of the database containing 194,716 unique patient records at Eye Surgery Associates revealed 541 patients with an IRD. Exclusion of incomplete patient records and/or incorrect or uncertain clinical diagnoses resulted in 464 patient records in this retrospective study.
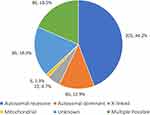
Demographic variables are presented in Table 1. Approximately half of the patients were male (239, 51.5%). Included patients had a median age of 46 years (interquartile range [IQR]: 28–60) and a median duration of care of 5 months (IQR: 0–63 months). Based on clinical diagnosis, patients were grouped as having panretinal pigmentary retinopathies (284, 61.2%), macular dystrophies (137, 29.5%), stationary diseases (23, 5%), hereditary vitreoretinopathies (14, 3%), and other IRDs (6, 1.3%). The suspected pattern of inheritance of patients’ IRD was predominantly autosomal recessive (205, 44.2%), followed by autosomal dominant (60, 12.9%), X-linked (22, 4.7%), and mitochondrial (6, 1.3%). There were patients with unknown (85, 18.3%) or multiple (86, 18.5%) possible modes of inheritance based on clinical records (Figure 1). Consanguinity was noted in a small percentage of patients (17, 3.6%).
 |
Table 1 Demographics of All Patients and as Categorised by Age (Less Than 45 Years of Age, 45 Years or Older) |
In the study cohort, there was a predominance of younger males (less than 45 years of age) and older females (45 years or older). Age-stratified analysis showed that the younger patients were less likely to have attended the practice for more than a year (younger vs older: 61.1% vs 48.1%, p<0.01) but more likely to have genetic testing performed (13.1% vs 6.2%, p=0.01) than older patients. Younger patients were also more likely to have received care for stationary disease (8.6% vs 1.6%, p<0.01). More patients in the older age group had macular dystrophies (34.6% vs 24%, p<0.01); however, the number of patients with panretinal pigmentary retinopathies (60.5% vs 62%, p=0.78) was similar in both groups.
Genetic Testing
Genetic testing results were available in patients’ clinical records for 44 patients (9.5%). Genetic testing was performed mostly for patients less than 45 years of age (13.1% for <45 years vs 6.2% ≥45 years of age, p=0.01) and those with duration of care of 12 months or longer (16% for ≥12 months of care vs 4% for <12 months of care, p<0.01). For three patients, immediate family members had genetic testing results available. While clinical information from a family member or research grade testing is useful in a clinical setting, only patients who have undergone clinical testing themselves were included in this analysis.
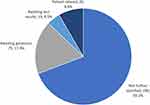
Reasons for not having genetic testing results available were documented as: awaiting an appointment with a geneticist (75, 17.9%), awaiting test results following sample collection (19, 4.5%), and patient refusal of genetic testing (35, 8.4%). However, in most cases, the reason was “not further specified” (290, 69.2%) (Figure 2).
Multivariate logistic regression revealed that younger patients (OR: 2.95, p<0.01) and those with duration of care of 12 months or longer (OR: 5.48, p<0.01) are more likely to have had genetic testing performed (Table 2). There was no association between gender and the likelihood of patients having genetic testing results available (univariate OR: 0.79, p=0.46).
 |
Table 2 Univariate and Multivariate Logistic Regression Assessing Predictors of Having Genetic Testing Results Among Patients |
Of the genetic testing results obtained, 43.2% were clinical grade and 6.8% were research grade; however, for 50% of the genetic tests, this information was not documented in the patient’s clinical record or genetic report. In this cohort, the diagnostic yield of genetic testing was 65.9%. Among the genes identified, the most common was ABCA4 (13.6%), followed by BEST1 and USH2A (6.8% each), MFRP, RHO, CRB1 (4.5% each) and BBS1, BBS9, CHM, CNGA3, CRX, CSPP1, EYS, HFE, IFT2, INPP5E, FSCN2, MT-ND5, MT-TL1, NMNAT1, PEX7, PRPF8, PRPS1, RGR, RP1, RP1L1, RPGR, SPATA7 (2.3% each). In all cases, the ABCA4 gene variant was determined to be pathogenic from laboratory reports, and there were two to three pathogenic variants identified per patient. No further familial testing data was reported within the clinical records for any of the patients with an ABCA4 gene mutation. Two patients had only one ABCA4 mutation identified; therefore, these patients were not included in the diagnostic yield of genetic testing reported. In 31.8% of the genetic reports, the disease-causing variant was not documented or undetected. The most common genes and their frequency in our cohort are summarised in Table 3.
 |
Table 3 Frequency of Genes Identified During Genetic Testing |
Discussion
This retrospective, single centre study presents data of the frequencies of IRD at a private subspecialty tertiary referral retinal practice, servicing predominantly Victoria, Australia. To our knowledge, this is the first Australian study reporting genetic test ordering in a large tertiary practice with a large database of patients with IRD. This information is valuable for ophthalmologists and other healthcare professionals to reflect on their current genetic test ordering and the benefits of identifying patient-specific variants. The rate of genetic testing results was 9.5%, which lags behind similar cohorts in developed countries such as the US (55%)25 and Spain (26.85%).26 This is likely due to several factors: the very recent approval of gene-based therapies that require this information (voretigene neparvovec-rzyl approved in Australia in 2020), improvements in genetic testing technologies, and slower introduction of genetic testing programs in Australia. Sponsored IRD genetic testing programs were introduced in Australia in 2021 but have been available overseas for several years. Access to free testing for patients undoubtedly has the potential to increase genetic testing uptake. In addition, the RANZCO guidelines for IRD management,11 which emphasise the importance of genetic testing for a broader group of patients than previously thought beneficial, will change future practice. Finally, this practice is a specialist tertiary care provider, where patients are often referred for specialised testing (such as electrophysiology or confirmation of diagnosis, etc). Hence, there is a high percentage of single-visit patients in this cohort, which means it is less likely that genetic testing would have been discussed. The results of this study are intended as a benchmark of historical practice (1995–2021), and we will reassess in the future to determine the changes due to the above factors.
The predominant phenotypic diagnosis in this patient cohort was retinitis pigmentosa/rod-cone dystrophy. Macular dystrophy with flecks was the second most common IRD category, suggesting ABCA4 retinopathy as the most common macular IRD diagnosis. The distribution of IRD phenotypes in our cohort is similar to those reported in Spain,26,27 the US,14,28 the UK,29 Iran,30 and Norway.31 The Australian Inherited Retinal Disease Registry and DNA Bank also reported that retinitis pigmentosa and Stargardt disease are the most common two diagnoses among over 9000 Australian patients.32
Among those who had genetic testing performed, the most common molecular diagnoses were ABCA4, followed by BEST1, USH2A, RHO, RP1, CRB1. This compares well to other study cohorts in Brazil,31 New Zealand33 and UK.29 Similarly, a study by Mansfield et al (2020) reported that ABCA4, USH2A, RHO, BEST1 and CRB1 are among the top 10 genes identified in the My Retina Tracker® Registry containing approximately 27,000 registered individuals with IRD.28
Obtaining a history of consanguinity in patients with an IRD may assist in selecting appropriate genes for screening and interpreting whole-genome sequencing results.29 In the current cohort, 3.5% of the patients reported consanguinity, which is mid-range between reported Chinese (<1%)34 and Norwegian (6%)31 IRD patient cohorts. However, our results are less than those reported in Brazil (>10%),35 Spain (11%),22 and Iran (76%).30 A study by Khan et al (2017) found that diagnostic yield increased from 45% to 60% when consanguinity was considered to select the most appropriate test.36 This result supports the importance of capturing patients’ ethnic background and pedigree structure to increase detection rates of the disease-causing variant.36
In the current study cohort, the predominant inheritance pattern was autosomal recessive (44.2%) followed by autosomal dominant (12.9%) and X-linked inheritance (4.7%). A study by Liu et al (2021) similarly reported that in a registry containing 800 Chinese families, the inheritance pattern was also predominantly autosomal recessive (43.88%), followed by X-linked (9.25%) and autosomal dominant (7%).34 Studies in the UK20,29,36 and the US14 also report similar frequencies of inheritance patterns. However, a study by Coco-Martin et al (2021) reported that the most common inheritance pattern based on family history in their cohort of IRD patients was autosomal dominant (52%) followed by autosomal recessive (23%) and X-linked (10%) inheritance.22 This may be attributed to a greater proportion of macular dystrophies in their study (n=161), mainly following an autosomal dominant inheritance, compared to panretinal pigmentary retinopathies (n=39) following an autosomal recessive inheritance pattern.22 This variation in IRD phenotype may further be explained by the extensive macular dystrophies reported in the Spanish cohort,22 potentially as a result of geographic disparities and greater frequencies of certain mutations in common racial classifications (Africa, Europe, Asia, Oceania, Americas).37
Diagnostic Yield of Genetic Testing
A proportion of our cohort had inconclusive results, which included both negative (31.8%) results from genetic test reports and unavailable or pending (22.4%) results from tests ordered. Our “solve rate” was 65.9% for those patients who had genetic testing, which is comparable to diagnostic yield reported by studies in the US (76%),14 China (60%),34 and New Zealand (83.6%).33 Motta et al (2017) reported results similar to the current study, with 71.6% of their cohort receiving a conclusive molecular diagnosis compared to 28% individuals receiving negative or inconclusive results.35 Our results were significantly greater than the solution rate reported in Norway (32%).31 Gene-panel testing for IRD was not available at the time of that publication (prior to 2016) in Norway; therefore, arrayed primer extension was the test of choice which involves testing each patient for a panel of known disease-causing genes.31 NGS testing increases diagnostic yield; however, it may also increase detection of variant of unknown significance (VUS). Therefore, further investigation is required in this area.11,38
The diagnostic yield for genetic testing also varies depending on the provisional IRD diagnosis, testing methodology and whether the IRD is genetically simple or exhibits complex disease phenotypes.38,39 Jiman et al (2020) reported a significant improvement in genetic diagnosis for people with a provisional clinical diagnosis compared to individuals without a clinical diagnosis at the time of genetic testing (71% compared to 25%).39 Furthermore, Li et al (2019) suggested that tailoring the panel of genes to the clinical presentation increases the diagnostic yield of genetic testing and reduces the false-positive rate of VUS.40 Incorporation of clinical diagnoses into genetic testing must be considered along with genetic testing methods and gene panel selection.
Barriers to Genetic Testing
Among the patients who did not have genetic testing results available, 8.4% of clinical records documented patient refusal; however, this figure may be higher since approximately 70% of clinical records did not have documented counselling regarding genetic testing. It is important to consider the clinical context of genetic testing. At the time of care, genetic testing was often clinically unjustified in many of our patients with an established IRD diagnosis, stable clinical phenotype, or beyond reproductive age. Patient visits with the sole intention of providing legal blindness certification to established IRD patients or performing single procedure services such as electroretinography were considered exempt from genetic testing counselling and ordering.
Patient-related barriers to uptake of genetic testing have been explored in several studies. Li et al (2019) found that patients were reluctant to agree to genetic testing due to cost involved, advanced age, mobility challenges due to poor vision and difficulty arranging transportation among the visually impaired.40 However, 73% of the eligible patients consent to genetic testing when at no cost to them.40 Recently announced industry sponsored testing programs (including Invitae and the Blueprint/Novartis collaboration, both commencing in 2021) offer IRD patients free access to panel testing in Australia, which may overcome this barrier. However, whether clinicians are aware of such programs remains unknown. Previous studies also recognise patients’ education, family status and age affect acceptance of genetic testing.23,41,42 The main reasons for negative attitudes were due to the assumption that abortion rates will increase, exposure to social discrimination, misuse of results by ordering clinician, and anxieties surrounding their own health and that of their child’s.23,42 Therefore, there is a role for clinicians to earn their patients’ trust and provide informative advice regarding the advantages of genetic testing.
In addition, Neiweem et al (2021) recognised that many clinicians in medicine and ophthalmology are unfamiliar with genetic testing due to the several complexities involved.43 Clinicians may be unaware which patients are suitable candidates, the appropriate test to order, how to interpret results, or the associated cost of genetic testing.21,43 Further education may be required to educate clinicians and patients regarding the benefits of genetic testing using informative resources such as the Retina International Campaign, “Know Your Code” (www.kyc.retinaint.org).44 Confoundingly, there is also variation in testing guidelines between international and Australian guidelines, with international patient advocacy groups such as Retina International detailing a need for global consensus in published guidelines.44 The RANZCO have recently published comprehensive IRD management guidelines, which emphasise the importance of genetic testing in accordance with clinical benefits.11 With emerging gene-dependent treatment options such as gene therapy, it is important to screen IRD patients to facilitate appropriate referral for clinical trials efficiently when it becomes available. Of note, in unsolved cases, the current literature recommends a retest interval of at least 18 months.45
Previously reported resource-related barriers to genetic testing include long turnaround times of genetic testing (up to 6 months in some cases),46 limitations of genetic testing methods,39 and limited integration of different medical specialities such as ophthalmology and genetic counsellors.21 The latter challenge is being addressed in Australia, and other countries, through multi-disciplinary clinics such as the Ocular Genetics Clinic at the Royal Victorian Eye and Ear Hospital. Another Australian-based resource for genetic data on IRD is the Australian Inherited Retinal Disease Register and DNA Biobank (https://www.scgh.health.wa.gov.au/Research/DNA-Bank), which holds the largest collection of DNA samples in Australia.
A key strength of our study is the relatively large patient cohort, consisting of 464 patients from a single large tertiary ophthalmic practice. Furthermore, the study constituted a rigorous process of selecting appropriate patients using a two-stage clinical record review by the senior author (HM), followed by an ophthalmology registrar (YJ) and an optometrist experienced in IRD (SG) to assess clinical diagnoses and genetic testing results.
Study limitations include the large heterogeneity in patient follow-up duration, ranging from single visits to regular patients attending for up to 27 years. The relatively high number of single visits at this clinic is due to high numbers of referrals solely for electrophysiological testing, diagnosing patients and/or certifying legal blindness. Once patients receive their clinical diagnosis, they return to their primary eyecare provider for ongoing management, who may have ordered genetic testing however forwarded these results with patient referrals. Furthermore, the relatively high “not further specified” reason for lack of genetic testing may be indicative of the variation of clinicians’ clinical record documentation patterns that did not capture discussions, referrals, and/or patient opinions. For pathogenicity determination, we relied on information provided by the laboratory and/or geneticist or genetic counsellor available in patients’ clinical records. In some cases, the letter provided to the ophthalmologist contained only information on the name of the affected gene and number of variants identified but no information on the specific variants.
In the future, we expect these figures to improve with availability of higher precision genetic testing methods, free sponsored programs, FDA-approved gene therapy, and potentially greater awareness of genetic testing benefits. We aim to repeat this study in 2 years, to assess the impact these policy and practice changes have on genetic test ordering for people with IRD. Future research should evaluate genetic testing in the public system, as well as additional barriers, policies, and patient perceptions of the genetic testing process in Australia.
Our study cohort shows low uptake of genetic testing of patients with IRD in a large private tertiary retinal practice in Australia, compared to international studies. Currently, our cohort demonstrates that younger patients with longer duration of care are more likely to have received genetic test results. This study provides a snapshot of ophthalmic practices in genetic test ordering for definitive clinical diagnoses, establishing inheritance patterns, family planning, and assessing patients’ suitability for gene-targeted therapies, which will be of interest to many general and specialised retinal ophthalmologists. We expect that the availability of sponsored testing programs and increased awareness relating to the importance of genetic testing will increase uptake of genetic testing in the future. To achieve this, we advocate further clinician and patient education based upon the established IRD guidelines (such as RANZCO11), streamlined access to public genetic clinics, detailed and standardised reporting of genetic test results, continued support of large IRD databases, and funding for reduced-cost testing to improve ongoing management and clinical outcomes for IRD patients.
Abbreviations
DNA, deoxyribonucleic acid; FDA, Food and Drug Administration; IRD, inherited retinal disease; NGS, next-generation sequencing; QLD, Queensland; RANZCO, Royal Australian and New Zealand College of Ophthalmologists; RNA, ribonucleic acid; RP, RETINITIS PIGmentosa; UK, United Kingdom; US, United States; VUS, variant of unknown significance; WES, whole-exome sequencing.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
The authorship team would like to thank the many IRD patients who have been seen at Eye Surgery Associates and the ophthalmologists caring for them who agreed to patient file review: Jacqueline Beltz, Ben Connell, Anthony JH Hall, Andrew Symons, Wilson Heriot and Grant Snibson. LA is supported by a National Health and Medical Research Council (NHMRC) MRFF Fellowship (MRF# 1151055) and EL2 Investigator Grant (GNT#1195713). CERA receives Operational Infrastructure Support from the Victorian Government. Sena A. Gocuk and Yuanzhang Jiao are co-first authors, and Lauren N. Ayton and Heather G. Mack are co-senior authors, on this paper.
Disclosure
Dr Lyndell Lim reports grants, personal fees from Bayer, personal fees from Novartis, personal fees from Allergan, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Hanany M, Rivolta C, Sharon D. Worldwide carrier frequency and genetic prevalence of autosomal recessive inherited retinal diseases. Proc Natl Acad Sci. 2020;117(5):2710–2716.
2. Rattner A, Sun H, Nathans J. Molecular genetics of human retinal disease. Annu Rev Genet. 1999;33(1):89–131.
3. Crewe JM, Morlet N, Morgan WH, et al. Mortality and hospital morbidity of working-age blind. Br J Ophthalmol. 2013;97(12):1579–1585.
4. Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open. 2014;4(2):e004015.
5. Clemson C, Tzekov R, Krebs M, Checchi J, Bigelow C, Kaushal S. Therapeutic potential of valproic acid for retinitis pigmentosa. Br J Ophthalmol. 2011;95(1):89–93.
6. Birch DG, Bennett LD, Duncan JL, Weleber RG, Pennesi ME. Long-term follow-up of patients with retinitis pigmentosa receiving intraocular ciliary neurotrophic factor implants. Am J Ophthalmol. 2016;170:10–14.
7. Miura G, Sugawara T, Kawasaki Y, et al. Clinical trial to evaluate safety and efficacy of transdermal electrical stimulation on visual functions of patients with retinitis pigmentosa. Sci Rep. 2019;9(1):1–8.
8. Schatz A, Röck T, Naycheva L, et al. Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled exploratory study. Invest Ophthalmol Vis Sci. 2011;52(7):4485–4496.
9. Simunovic MP, Mack HG, Ayton L, Hassall M. Gene Therapy, Diet, and Drug Approaches to Treating Inherited Retinal Disease. In: Kenakin T, Editor. Reference Module in Biomedical Sciences - Comprehensive Pharmacology. Elsevier; 2021.
10. Hu ML, Edwards TL, O’Hare F, et al. Gene therapy for inherited retinal diseases: progress and possibilities. Clin Exp Optom. 2021;104(4):444–454.
11. Grigg J, Jamieson R, Chen FK, et al. Guidelines for the assessment and management of patients with Inherited Retinal Degenerations. 2020. Available from: www.ranzco.edu.
12. Duncan J, Bernstein P, Birch D, Fishman G, Heon E, Jacobson S. Recommendations on Clinical Assessment of Patients with Inherited Retinal Degenerations-2016. Clin J Med. 2016;1:242.
13. Moore AT. Genetic testing for inherited retinal disease. Ophthalmology. 2017;124(9):1254–1255.
14. Stone EM, Andorf JL, Whitmore SS, et al. Clinically focused molecular investigation of 1000 consecutive families with inherited retinal disease. Ophthalmology. 2017;124(9):1314–1331.
15. Strait S, Loman R, Erickson L, DeBenedictis M. Inherited retinal degeneration current genetics practices - a needs assessment. Ophthalmic Genet. 2020;41(6):533–538.
16. Lee K, Garg S. Navigating the current landscape of clinical genetic testing for inherited retinal dystrophies. Genet Med. 2015;17(4):245–252.
17. Carss KJ, Arno G, Erwood M, et al. Comprehensive rare variant analysis via whole-genome sequencing to determine the molecular pathology of inherited retinal disease. Am J Hum Genet. 2017;100(1):75–90.
18. Sharon D, Ben‐Yosef T, Goldenberg‐Cohen N, et al. A nationwide genetic analysis of inherited retinal diseases in Israel as assessed by the Israeli inherited retinal disease consortium (IIRDC). Hum Mutat. 2020;41(1):140–149.
19. Whelan L, Dockery A, Wynne N, et al. Findings from a genotyping study of over 1000 people with inherited retinal disorders in Ireland. Genes. 2020;11(1):105.
20. Shah M, Shanks M, Packham E, et al. Next generation sequencing using phenotype-based panels for genetic testing in inherited retinal diseases. Ophthalmic Genet. 2020;41(4):331–337.
21. Branham K, Schlegel D, Fahim AT, Jayasundera KT. Genetic testing for inherited retinal degenerations: triumphs and tribulations. Am J Med Genet Part C. 2020;1:571–577.
22. Coco-Martin RM, Diego-Alonso M, Orduz-Montana WA, Sanabria MR, Descriptive S-TH. Study of a Cohort of 488 Patients with Inherited Retinal Dystrophies. Clin Ophthalmol. 2021;15:1075–1084.
23. Aro AR, Hakonen A, Hietala M, et al. Acceptance of genetic testing in a general population: age, education and gender differences. Patient Educ Couns. 1997;32(1–2):41–49.
24. Vahratian A. Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family Growth. Matern Child Health J. 2009;13(2):268–273.
25. Mansfield BC, Yerxa BR, Branham KH. Implementation of a registry and open access genetic testing program for inherited retinal diseases within a non-profit foundation. Am J Med Genet Part C. 2020;184(3):838–845.
26. Coco-Martin RM, Diego-Alonso M, Orduz-Montaña WA, Sanabria MR, Sanchez-Tocino H. Descriptive Study of a Cohort of 488 Patients with Inherited Retinal Dystrophies. Clin Ophthalmol. 2021;15:1075–1084.
27. Holtan JP, Selmer KK, Heimdal KR, Bragadóttir R. Inherited retinal disease in Norway - a characterization of current clinical and genetic knowledge. Acta Ophthalmol. 2020;98(3):286–295.
28. Mansfield BC, Yerxa BR, Branham KH. Implementation of a registry and open access genetic testing program for inherited retinal diseases within a non-profit foundation. Am J Med Genet C Semin Med Genet. 2020;184(3):838–845.
29. Pontikos N, Arno G, Jurkute N, et al. Genetic basis of inherited retinal disease in a molecularly characterized cohort of more than 3000 families from the United Kingdom. Ophthalmology. 2020;127(10):1384–1394.
30. Sabbaghi H, Daftarian N, Suri F, et al. The first inherited retinal disease registry in Iran: research protocol and results of a pilot study. Arch Iran Med. 2020;23(7):445–454.
31. Holtan JP, Selmer KK, Heimdal KR, Bragadóttir R. Inherited retinal disease in Norway–a characterization of current clinical and genetic knowledge. Acta Ophthalmol. 2020;98(3):286–295.
32. De Roach JN, McLaren TL, Thompson JA, et al. The Australian Inherited Retinal Disease Registry and DNA Bank. Tasman Med J. 2020;2(3):60–67.
33. Hull S, Kiray G, Chiang JP, Vincent AL. Molecular and phenotypic investigation of a New Zealand cohort of childhood-onset retinal dystrophy. Am J Med Genet C Semin Med Genet. 2020;184(3):708–717.
34. Liu X, Tao T, Zhao L, Li G, Yang L. Molecular diagnosis based on comprehensive genetic testing in 800 Chinese families with non-syndromic inherited retinal dystrophies. Clin Exp Ophthalmol. 2021;49(1):46–59.
35. Motta FL, Martin RP, Filippelli-Silva R, Salles MV, Sallum JMF. Relative frequency of inherited retinal dystrophies in Brazil. Sci Rep. 2018;8(1):15939.
36. Khan K, Chana R, Ali N, et al. Advanced diagnostic genetic testing in inherited retinal disease: experience from a single tertiary referral centre in the UK National Health Service. Clin Genet. 2017;91(1):38–45.
37. Tishkoff SA, Kidd KK. Implications of biogeography of human populations for’race’and medicine. Nat Genet. 2004;36(11):S21–S7.
38. Kohl S, Biskup S. [Genetic diagnostic testing in inherited retinal dystrophies]. Klin Monbl Augenheilkd. 2013;230(3):243–246.
39. Jiman OA, Taylor RL, Lenassi E, et al. Diagnostic yield of panel-based genetic testing in syndromic inherited retinal disease. Eur J Hum Genet. 2020;28(5):576–586.
40. Li AS, MacKay D, Chen H, Rajagopal R, Apte RS. Challenges to routine genetic testing for inherited retinal dystrophies. Ophthalmology. 2019;126(10):1466–1468.
41. Suther S, Goodson P. Barriers to the provision of genetic services by primary care physicians: a systematic review of the literature. Genet Med. 2003;5(2):70–76.
42. Suther S, Kiros G-E. Barriers to the use of genetic testing: a study of racial and ethnic disparities. Genet Med. 2009;11(9):655–662.
43. Neiweem AE, Hariprasad SM, Ciulla TA. Genetic testing prevalence, guidelines, and pitfalls in large, university-based medical systems. Ophthalmic Surg Lasers Imaging Retina. 2021;52(1):6–10.
44. Retinal International Campaign - Know Your Code. 2021. Available from: https://kyc.retinaint.org/.
45. Tan NB, Stapleton R, Stark Z, et al. Evaluating systematic reanalysis of clinical genomic data in rare disease from single center experience and literature review. Mol Genet Genomic Med. 2020;8(11):e1508.
46. Stone EM, Aldave AJ, Drack AV, et al. Recommendations for genetic testing of inherited eye diseases: report of the American Academy of Ophthalmology task force on genetic testing. Ophthalmology. 2012;119(11):2408–2410.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.