Back to Journals » Clinical Interventions in Aging » Volume 17
Genetic Effects of NDUFAF6 rs6982393 and APOE on Alzheimer’s Disease in Chinese Rural Elderly: A Cross-Sectional Population-Based Study
Authors Cheng Y, Li Y, Liang X, Wang P, Fa W, Liu C, Wang Y, Liu K, Wang N, Du Y
Received 29 October 2021
Accepted for publication 31 January 2022
Published 24 February 2022 Volume 2022:17 Pages 185—194
DOI https://doi.org/10.2147/CIA.S345784
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Yingzhe Cheng,1 Yuanjing Li,2 Xiaoyan Liang,3,4 Pin Wang,3 Wenxin Fa,1 Cuicui Liu,1,3,4 Yongxiang Wang,1,3,4 Keke Liu,5 Nan Wang,3,4 Yifeng Du1,3,4
1Department of Neurology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China; 2Aging Research Center and Center for Alzheimer Research, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet-Stockholm University, Stockholm, Sweden; 3Department of Neurology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China; 4Shandong Provincial Clinical Research Center for Neurological Diseases, Jinan, Shandong, People’s Republic of China; 5Shandong Academy of Clinical Medicine, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China
Correspondence: Yifeng Du, Department of Neurology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, No. 324 Jingwuweiqi Road, Jinan, Shandong, 250021, People’s Republic of China, Tel/Fax +86-531-68776354, Email [email protected]
Purpose: To investigate the associations of genotypes of NDUFAF6 rs6982393 and APOE and their combined genotypes with the risk of Alzheimer’s disease (AD) and mild cognitive impairment (MCI) in Chinese rural elderly.
Methods: This cross-sectional population-based study included 5096 older adults (age ≥ 60 years, 57.1% female). Genotypes of NDUFAF6 rs6982393 and APOE were detected using the multiple-polymerase chain reaction amplification. We diagnosed AD following the criteria of Diagnostic and Statistical Manual of Mental Disorders, the fourth edition and diagnosed MCI following the Petersen’s criteria MCI. Data were analyzed using the logistic regression model.
Results: The overall prevalence of AD and MCI was 3.57% (95% confidence interval [CI]: 0.040, 0.053) and 22.65% (95% CI: 0.223, 0.247), separately. The TT versus CC/CT genotype of NDUFAF6 rs6982393 was related to a higher risk of AD with the multi-adjusted odds ratio (95% CI) being 1.61 (1.02, 2.54) in the total sample, 3.36 (1.48, 7.60) in those aged 60– 69, and 1.24 (0.71, 2.17) in those aged 70 years and above. The interaction between genotype of NDUFAF6 rs6982393 with age groups (60– 69 versus ≥ 70 years) was significant on the risk of AD. The presence of APOE ϵ4 was not significantly associated with the risk of AD. Carrying both NDUFAF6 TT and APOE ϵ4 was related to a higher risk of AD with the multi-adjusted odds ratio (95% CI) being 2.69 (1.10, 2.56). In addition, there was no significant association between the above genotypes and MCI.
Conclusion: In Chinese rural elderly, the TT versus CT/CC genotype of NDUFAF6 rs6982393 was associated with an increased likelihood of AD; such an association only existed among young-old adults. Carrying both NDUFAF6 rs6982393-TT and APOE ϵ4 was related to a higher risk of AD. This finding highlights the importance of considering age and combined genotype in studying the genetic profiles of AD.
Keywords: NDUFAF6 rs6982393, APOE, dementia, population-based study
Introduction
By 2019, people aged 60 years and above have accounted for 17.9% in China.1 More than 15% of these older adults lived with cognitive impairment and among them approximately 27.9% had dementia,1 which had brought about heavy economic and labor burdens to the healthcare system.2,3 Among the dementia profiles, Alzheimer’s disease (AD) is the most common type, characterized by insidious onset, inevitable memory loss, cognitive decline in other domains, functional decline, and even increased mortality in the late stage.4 Besides, mild cognitive impairment (MCI) is an intermediate stage between normal cognition and dementia, characterized by subjective complaints of cognitive decline, or objectively measured cognitive deterioration in specific domains, while intact daily function.5 It has been well established that the presence of APOE ε4 could confer an increased risk of AD-type dementia and MCI in the European population.6–8 Whereas the influence of APOE ε4 on AD or MCI has not achieved consistency in the Chinese elderly.9–13
Recently, data from the genome-wide association studies suggested that a single nucleotide polymorphism of NDUFAF6 rs6982393 could bring about an increased risk of AD in the European population.14 In brief, NDUFAF6 rs6982393 encodes a protein involved in the metabolism of amyloid-β and tau protein in the etiopathology of AD.15,16 Rare study has examined the possible influence of NDUFAF6 rs6982393 on AD or MCI in the Chinese population. Most of the previous studies on genetic impacts on dementia have been conducted among highly educated elderly in general settings, where the findings may not be generalizable to the rural dwellers with no to little formal education. Considering that lower educational attainment serves as an independent risk factor of dementia, it is worth to explore possible genetic effect in Chinese rural elderly with limited education. Besides, it is also meaningful to investigate the possible joint effect of genotypes of NDUFAF6 rs6982393 and APOE and identified those with a higher inherited risk of MCI or AD. Furthermore, given that susceptibility genes of AD may exert differential genetic effects by age groups (such as APOE, PICALM and TOMM40 gene),17–19 it is plausible to hypothesize that the possible above genetic influence on AD or MCI may vary by age groups.
Therefore, in this population-based study, we aimed to investigate if genotypes of NDUFAF6 rs6982393, APOE, and their combined genotypes were related to the risks of AD and MCI in Chinese rural elderly with limited education attainment, and if such associations varied by age groups.
Methods
Study Population
This population-based study included participants from the ongoing Multimodal Interventions to Delay Dementia and Disability in Rural China (MIND-China).20–22 The MIND-China study was launched by Shandong Provincial Hospital, Shandong University Cheeloo College of Medicine, in collaboration with Yanlou Town Hospital. During baseline investigation (March–September 2018), the MIND-China study targeted older residents aged 60 years and in the 52 villages of Yanlou Town, Yanggu County, western Shandong Province. A total of 5765 participants were enrolled in this study. Of these participants, 669 were excluded due to missing genotyping information (n = 250) or missing diagnosis of dementia (n = 320) or other types of dementia than AD (n = 99). Thus, the analytical sample included 5096 participants. Figure 1 shows the flowchart of study participants.
The MIND-China study was conducted in accordance with the Declaration of Helsinki and has been approved by the Ethics Committee of Shandong Provincial Hospital in Jinan, Shandong. Written informed consents were acquired from all participants, or for people with severe cognitive or functional impairment, obtained from their proxies. The MIND-China study was registered in the Chinese Clinical Trial Registry (registration no.: ChiCTR1800017758).
Data Collection and Assessment
The procedure of baseline investigation was described previously.20,21 In brief, data were collected by trained staff through face-to-face interviews, clinical examinations, neuropsychological questionnaires, and laboratory tests. We collected data on demographics age, sex, and education), lifestyle factors (such as smoking and alcohol drinking), health status (such as hypertension, diabetes, dyslipidemia, coronary heart disease, and stroke), medical history (such as antihypertensive and hypoglycemic drugs), use of medications, and cognitive performance. All medications were classified according to Anatomical Therapeutic Chemical classification (ATC) system. Weight and height were measured in light clothes without shoes. After a rest of at least five minutes, the sitting arterial blood pressure of the right arm was measured using an electronic sphygmomanometer (hem-7127j, OMRON company, Kyoto, Japan). The 12-lead resting electrocardiogram was recorded by electrocardiograph (CM300, Shenzhen Branch, China) and then analyzed by local doctors. After an overnight fast, peripheral blood samples were taken and blood glucose, total cholesterol, triglyceride, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were measured by automatic biochemical analyzer (cs-600b, Changchun Dirui company, China) in the laboratory of Yanlou town hospital. Alcohol drinking and smoking were divided into never, former, and current, respectively. Body mass index was calculated as weight in kilograms divided by the square of height in meters. Hypertension was defined as the systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current use of antihypertensive drugs. Diabetes was defined as the fasting blood glucose ≥7.0 mmol/L, using hypoglycemic drugs, or a previous record of diabetes. Dyslipidemia was defined as the total cholesterol ≥6.2 mmol/L, triglyceride ≥2.3 mmol/L, low-density lipoprotein cholesterol ≥4.1 mmol/L, high-density lipoprotein cholesterol <1.0 mmol/L, or using lipid-lowering drugs.23 Coronary heart disease was defined by a previous record or evidence from the electrocardiograph, including angina pectoris, myocardial infarction, coronary angioplasty, and coronary artery bypass grafting. Stroke was defined according to a previous record or typical neurological signs.
Genomic DNA Extraction and Genotyping
The trained staff collected venous blood from participants and extracted genomic deoxyribonucleic acid (DNA) from venous blood leukocytes using the TIANamp blood DNA kit (Tiangen, Beijing, China). Then, DNA was quantified using Nanodrop 3300 spectrometry and a total amount of 100 ng genomic DNA per sample was used for the DNA sample preparation. Subsequently, sequencing libraries were generated using MultipSeqCustom Panel (iGeneTech, Beijing, China) following standard procedures and index codes were added to each sample. Finally, qualified libraries were subjected to next-generation sequencing on a Novaseq system (Illumina), and raw data were filtered to remove low-quality reads using FastQC. Genotyping was conducted by an operator who was blinded to all clinical data.
The genotypes of APOE and NDUFAF6 rs6982393 were detected using multiple-polymerase chain reaction amplification (iGeneTech Bioscience Co., Ltd, Beijing, China). The amplification of NDUFAF6 gene used the following primers: forward, 5′- CTGGGCGCGGACATGG −3′; reverse, 5′- CACCTTGGTTGTGTGATTTGTGAA −3′. The amplification of APOE gene used the following primers: forward, 5′- CTGGGCGCGGACATGG −3′; reverse, 5′- ttctGCAGGTCATCGGCATC −3′. The distribution of the NDUFAF6 rs6982393 and APOE ε4 conformed to the Hardy-Weinberg equilibrium.
Diagnosis on Dementia and Mild Cognitive Impairment
The diagnosis of dementia was based on the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) and followed a three-step diagnostic procedure.24 First, trained clinicians and interviewers performed the routine clinical examinations and comprehensive neuropsychological evaluations on each participant. Then, the experienced neurologists reviewed all neuropsychological information and made a preliminary diagnosis of dementia. Finally, these neurologists conducted additional face-to-face interviews with participants suspected of having dementia, and re-evaluated their medical history, cognitive and functional records, and any possible neuroimaging data. If the participants were unable to be interviewed (approximately 13%) due to severe cognitive impairment, neurologists would visit their family members, neighbors, or village doctors for further information. After all interviews and evaluations, neurologists ascertained the final diagnosis of dementia according to DSM-IV criteria.24 In the case of uncertainty, consultation would be provided with senior neuroscientist (L.C.) to discuss and reach a consensus diagnosis of dementia. The diagnosis of Alzheimer’s disease is based on the criteria of the American Alzheimer’s Association.25
We defined MCI following the Petersen’s criteria that were operationalized in the Mayo Clinic Study of Aging:26 (1) cognitive concern by subjects (according to memory complaints, Ascertain Dementia 8, or Clinical Dementia Rating Scale) or informants; (2) objective cognitive impairment evidenced in at least one of the four cognitive domains of memory, attention, execution, and language (according to cognitive test battery); (3) essentially preserved daily function (according to Activities of Daily Living); and (4) absence of dementia (according to DSM-IV).24
Statistical Analyses
First, we compared the characteristics of study participants using t-test for continuous variables and chi-square test for categorical variables. Next, we examined the associations of genotypes of NDUFAF6 rs6982393 (TT versus CC/CT) and APOE genotype (with or without ε4) with AD and MCI using the multinomial logistic regression models. We report results from three models: Model 1 was adjusted for age, sex, and education; Model 2 was additionally adjusted for vascular risk factors significantly related to the risks of MCI or AD (body mass index, alcohol consumption, smoking status, and coronary heart disease) based on Model 1; model 3 was additionally adjusted for diabetes, hypertension, dyslipidemia, and stroke and APOE or NDUFAF6 rs6982393 genotypes, if applicable, based on Model 2. In addition, we examined the statistical interactions of above genotypes with age groups (60–69 versus ≥70 years) on the prevalence of AD and MCI. Age-stratified analyses were performed once statistically significant interactions were detected (p for interaction <0.05). Finally, we examined the combined effect of NDUFAF6 rs6982393-TT and APOE ε4 on the risks of AD and MCI using multinomial logistic regression models. IBM SPSS Statistics version 22.0 (IBM SPSS Inc., Chicago, Illinois) was used for all analyses.
Results
Characteristics of Study Participants
Of the 5096 participants, the mean age was 70.4 (SD, 5.5) years, 57.1% were women, and the average years of formal schooling was 3.3 (SD, 3.5) years. Among all participants, 1154 (prevalence: 22.65%, 95% CI: 0.223,0.247) had MCI and 182 (prevalence: 3.57%, 95% CI, 0.040,0.053) had AD. Compared to cognitively normal participants, those with AD were older, less educated, had higher female proportion and lower BMI, and were less likely to smoke or to drink, while more likely to have coronary heart disease (≤0.005). Participants with different cognitive phenotypes did not differ in the proportions of diabetes, dyslipidemia, hypertension, or genotypes (Table 1).
 |
Table 1 Characteristics of Study Participants |
Associations of Polymorphisms of NDUFAF6 rs6982393 and APOE with Alzheimer’s Disease and Mild Cognitive Impairment
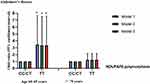
NDUFAF6 rs6982393-TT versus CC/CT genotype was significantly associated with a ~61% increased likelihood of AD after multiple adjusting, while was not significantly related to MCI (Table 2). There were significant interactions of age group (60–69 versus ≥70 years) with genotypes of NDUFAF6 rs6982393 on the likelihood of AD (P for interaction = 0.038). Age-stratified analyses revealed that compared to NDUFAF6 rs6982393-CC/CT, the TT genotype was significantly associated with a ~236% increased likelihood of AD among participants aged 60–69 years, but not among those aged ≥70 years after multiple adjusting (Figure 2). There was no significant interaction of age group with genotype of NDUFAF6 rs6982393 on the risk of MCI (Table 2). In addition, the presence of APOE ε4 was not significantly associated with the risk of AD or MCI (Supplementary Table 1).
 |
Table 2 Associations of Polymorphism of NDUFAF6 rs6982393 with Mild Cognitive Impairment and Alzheimer’s Disease |
Associations of Combined Effect Between Genotypes of NDUFAF6 Rs6982393 and APOE on Alzheimer’s Disease and Mild Cognitive Impairment
Carrying both NDUFAF6 rs6982393-TT and APOE ε4 was related to a ~69% increased risk of AD after multiple adjusting (Table 3). Carrying NDUFAF6 rs6982393-TT while not APOE ε4 was related to a higher risk of MCI after adjusting for age, sex, and education. Whereas, such association was not significant after adjusted for vascular risk factors (Table 3).
 |
Table 3 Associations of Combined Effect of Genotypes of NDUFAF6 rs6982393 with APOE on Mild Cognitive Impairment and Alzheimer’s Disease |
Discussion
In this population-based study of rural-dwelling Chinese older adults, we found that NDUFAF6 TT (versus CC/CT) genotype was associated with an increased likelihood of AD, especially among young-old people. The presence of APOE ε4 was not related to the risks of AD or MCI. Moreover, the combined genotype of NDUFAF6 TT and APOE ε4 was associated with a higher risk of AD.
To the best of our knowledge, this is the first population-based study to investigate the association of NDUFAF6 rs6982393 with AD and MCI. Our results were in line with the previous genome-wide association study in European countries.14 NDUFAF6-TT, encodes a protein involved in the assembly of mitochondrial respiratory chain complex I, which might contribute to oxidative stress, and further contribute to amyloid-β aggregation and tau phosphorylation.27 A slight inhibition of the activity of complex I would improve the cognitive function by inhibiting amyloid-β aggregation and tau phosphorylation in animal models.28 In addition, previous studies summarized the genome-wide association studies and found that NDUFAF6 rs6982393 might be the susceptibility gene of both type II diabetes and AD,14 which suggested that NDUFAF6 rs6982393 might confer risk of AD by pathophysiological mechanisms relevant to diabetes. Taken above, these findings supported the association between genotypes of NDUFAF6 rs6982393 and AD. On the other hand, we found that the association of NDUFAF6 rs6982393 TT genotype with AD was only evident in young-old, which was in accordance with the view that the potential genetic influence on the risk of AD might be weakened by advancing age.19,29 Moreover, the selective survival bias should be taken into consideration when interpreting the genetic effect in people aged 70 years and above.
We did not find a strong association between APOE ε4 and the risk of AD or MCI, which was consistent with other Chinese cohort studies.30,31 The frequency of the APOE ε4 allele differ between Asian and Caucasian population,10,32,33 which might lead to different statistical powers. In addition, the various molecular signatures of APOE region across ethics may also account for the different association between the APOE ε4 allele and AD among race/ethnic groups.34
Our study revealed a combined effect between genotypes of NDUFAF6 rs6982393 and APOE variants on AD. Previous studies via biochemical assays and proteomic profiling of mice neurons indicated Apolipoprotein E4 would lead to neurotoxicity by interfering with mitochondrial normal functions.35 The post-mortem studies in patients with AD found the isoform of Apolipoprotein E would affect the natural structure and functions of mitochondrion, which would further lead to increased level of oxidative stress, synaptic dysfunction, and cognitive decline.36 Taking into account the fact that NDUFAF6 rs6982393 might also lead to cognitive decline via interfering with mitochondrial function, the combined effect of NDUFAF6 and APOE risk variants on AD may be partly attributed to the common pathological mechanisms such as mitochondrial dysfunction.
The strength of our study was the population-based design and the rural-dwelling sample of Chinese older adults, integrating epidemiological, clinical, and genetic data. Our study also has limitations. First, in the cross-sectional design, the selective survival bias might be inevitable, which usually leads to an underestimated association. Second, the sample size of the study was not large enough for genetic research which might limit the statistical power in analyses. Thirdly, the study participants were recruited from a single rural area in north China, and characterized by lower educational attainment, which should be kept in mind when extending our findings to the other population.
Conclusion
In conclusion, among rural-dwelling Chinese older adults, the NDUFAF6 rs6982393-TT (versus CC/CT) genotype was related to a higher risk of AD especially in young-old people. The combined genotype of NDUFAF6 rs6982393-TT and APOE ε4 might be associated with a higher risk of AD. It is worth to explore the pathophysiological role of NDUFAF6 rs6982393 in the general population in the future studies. Besides, this finding highlights the importance of considering age and combined genotypes in studying the genetic profiles of AD.
Abbreviations
AD, Alzheimer’s disease; MCI, Mild cognitive impairment; NDUFAF6, NADH dehydrogenase (ubiquinone) complex I assembly factor 6; APOE, Apolipoprotein E; ATC, Anatomical Therapeutic Chemical; BMI, body mass index; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; MIND-China, Multimodal Intervention to Delay Dementia and Disability in Rural China; NIA-AA, National Institute on Aging-Alzheimer’s Association.
Data Sharing Statement
Data supporting the findings from this study will be available from the corresponding authors upon approval by the data management committee of MIND-China.
Ethics Approval and Informed Consent
MIND-China was approved by the Ethics Committee of Shandong Provincial Hospital in Jinan, Shandong. Written informed consent was obtained from all participants, or in the case of severely cognitively impaired participants, from informants. MIND-China was registered in the Chinese Clinical Trial Registry (registration no.: ChiCTR1800017758).
Consent for Publication
All authors confirm that the work described has not been published before, that it is not under consideration for publication elsewhere, and that its publication has been approved by all co-authors.
Acknowledgments
We would like to thank all the study participants, their caregivers, and the MIND-China Research Group. We also greatly appreciate the staff of the Yanlou Town Hospital of Yanggu County for their collaborations in data collection.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agreed to be accountable for all aspects of the work.
Funding
This study was supported by grants from the National Key R&D Program of China Ministry of Sciences and Technology (grant no.: 2017YFC1310100), the National Natural Science Foundation of China (grants no.: 81861138008 and 82011530139), the Academic Promotion Program of Shandong First Medical University, China (grant no.: 2019QL020), and the Taishan Scholar Program of Shandong Province, China. The funding body did not participate in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Disclosure
The authors declared no conflicts of interest in connection with this study.
References
1. Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020;5(12):e661–e671. doi:10.1016/S2468-2667(20)30185-7
2. Xu J, Wang J, Wimo A, Fratiglioni L, Qiu C. The economic burden of dementia in China, 1990–2030: implications for health policy. Bull World Health Organ. 2017;95(1):18–26. doi:10.2471/BLT.15.167726
3. Jia J, Wei C, Chen S, et al. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement. 2018;14(4):483–491. doi:10.1016/j.jalz.2017.12.006
4. Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327–406. doi:10.1002/alz.12328
5. Lai X, Wen H, Li Y, Lu L, Tang C. The comparative efficacy of multiple interventions for mild cognitive impairment in Alzheimer’s disease: a Bayesian network meta-analysis. Front Aging Neurosci. 2020;12:121. doi:10.3389/fnagi.2020.00121
6. Nicoll JA, Savva GM, Stewart J, Matthews FE, Brayne C, Ince P. Association between APOE genotype, neuropathology and dementia in the older population of England and Wales. Neuropathol Appl Neurobiol. 2011;37(3):285–294. doi:10.1111/j.1365-2990.2010.01130.x
7. Norberg J, Graff C, Almkvist O, et al. Regional differences in effects of APOE ε4 on cognitive impairment in non-demented subjects. Dement Geriatr Cogn Disord. 2011;32(2):135–142. doi:10.1159/000330492
8. Jefferson AL, Beiser AS, Seshadri S, Wolf PA, Au R. APOE and mild cognitive impairment: the Framingham Heart Study. Age Ageing. 2015;44(2):307–311. doi:10.1093/ageing/afu183
9. Jia L, Xu H, Chen S, et al. The APOE ε4 exerts differential effects on familial and other subtypes of Alzheimer’s disease. Alzheimers Dement. 2020;16(12):1613–1623. doi:10.1002/alz.12153
10. Katzman R, Zhang MY, Chen PJ, et al. Effects of apolipoprotein E on dementia and aging in the Shanghai survey of dementia. Neurology. 1997;49(3):779–785. doi:10.1212/WNL.49.3.779
11. Zheng L, Duan J, Duan X, et al. Association of apolipoprotein E (ApoE) polymorphism with Alzheimer’s disease in Chinese population. Curr Alzheimer Res. 2016;13(8):912–917. doi:10.2174/1567205013666160401115307
12. Hsieh TJ, Lee WJ, Liao YC, et al. Association between Alzheimer’s disease genes and trajectories of cognitive function decline in Han Chinese in Taiwan. Aging. 2021;13(13):17237–17252. doi:10.18632/aging.203204
13. Jiang Y, He T, Deng W, Sun P. Association between apolipoprotein E gene polymorphism and mild cognitive impairment: a meta-analysis. Clin Interv Aging. 2017;12:1941–1949. doi:10.2147/CIA.S143632
14. Wang XF, Lin X, Li DY, et al. Linking Alzheimer’s disease and type 2 diabetes: novel shared susceptibility genes detected by cFDR approach. J Neurol Sci. 2017;380:262–272. doi:10.1016/j.jns.2017.07.044
15. Joh Y, Choi WS. Mitochondrial complex I inhibition accelerates amyloid toxicity. Dev Reprod. 2017;21(4):417–424. doi:10.12717/DR.2017.21.4.417
16. Stojakovic A, Chang SY, Nesbitt J, et al. Partial inhibition of mitochondrial complex I reduces tau pathology and improves energy homeostasis and synaptic function in 3xTg-AD mice. J Alzheimers Dis. 2021;79(1):335–353. doi:10.3233/JAD-201015
17. Davidson Y, Gibbons L, Pritchard A, et al. Apolipoprotein E epsilon4 allele frequency and age at onset of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;23(1):60–66. doi:10.1159/000097038
18. Liu Z, Dai X, Zhang J, et al. The interactive effects of age and PICALM rs541458 polymorphism on cognitive performance, brain structure, and function in non-demented elderly. Mol Neurobiol. 2018;55(2):1271–1283. doi:10.1007/s12035-016-0358-5
19. Li G, Bekris LM, Leong L, et al. TOMM40 intron 6 poly-T length, age at onset, and neuropathology of AD in individuals with APOE ε3/ε3. Alzheimers Dement. 2013;9(5):554–561. doi:10.1016/j.jalz.2012.06.009
20. Han X, Jiang Z, Li Y, et al. Sex disparities in cardiovascular health metrics among rural-dwelling older adults in China: a population-based study. BMC Geriatr. 2021;21(1):158. doi:10.1186/s12877-021-02116-x
21. Dong Y, Wang Y, Liu K, et al. Olfactory impairment among rural-dwelling Chinese older adults: prevalence and associations with demographic, lifestyle, and clinical factors. Front Aging Neurosci. 2021;13:621619. doi:10.3389/fnagi.2021.621619
22. Cong L, Ren Y, Hou T, et al. Use of cardiovascular drugs for primary and secondary prevention of cardiovascular disease among rural-dwelling older Chinese adults. Front Pharmacol. 2020;11:608136. doi:10.3389/fphar.2020.608136
23. Zhu JR, Gao RL, Zhao SP, et al. 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15(1):1–29.
24. Rabe-Jabłońska J, Bieńkiewicz W. [Anxiety disorders in the fourth edition of the classification of mental disorders prepared by the American Psychiatric Association: diagnostic and statistical manual of mental disorders (DMS-IV – options book]. Psychiatr Pol. 1994;28(2):255–268. Polish.
25. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi:10.1016/j.jalz.2011.03.005
26. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The mayo clinic study of aging. Neurology. 2010;75(10):889–897. doi:10.1212/WNL.0b013e3181f11d85
27. Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67(4):360–368. doi:10.1001/archgenpsychiatry.2010.22
28. Zhang L, Zhang S, Maezawa I, et al. Modulation of mitochondrial complex I activity averts cognitive decline in multiple animal models of familial Alzheimer’s disease. EBioMedicine. 2015;2(4):294–305. doi:10.1016/j.ebiom.2015.03.009
29. Bellou E, Baker E, Leonenko G, et al. Age-dependent effect of APOE and polygenic component on Alzheimer’s disease. Neurobiol Aging. 2020;93:69–77. doi:10.1016/j.neurobiolaging.2020.04.024
30. Ding D, Zhao Q, Guo Q, et al. The Shanghai aging study: study design, baseline characteristics, and prevalence of dementia. Neuroepidemiology. 2014;43(2):114–122. doi:10.1159/000366163
31. Ding D, Zhao Q, Guo Q, et al. Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the Shanghai aging study. Alzheimers Dement. 2015;11(3):300–309.e302. doi:10.1016/j.jalz.2013.11.002
32. Hu P, Qin YH, Jing CX, Lu L, Hu B, Du PF. Does the geographical gradient of ApoE4 allele exist in China? A systemic comparison among multiple Chinese populations. Mol Biol Rep. 2011;38(1):489–494. doi:10.1007/s11033-010-0132-0
33. Liang S, Pan M, Geng HH, et al. Apolipoprotein E polymorphism in normal Han Chinese population: frequency and effect on lipid parameters. Mol Biol Rep. 2009;36(6):1251–1256. doi:10.1007/s11033-008-9305-5
34. Kulminski AM, Shu L, Loika Y, et al. APOE region molecular signatures of Alzheimer’s disease across races/ethnicities. Neurobiol Aging. 2020;87:
35. Orr AL, Kim C, Jimenez-Morales D, et al. Neuronal apolipoprotein E4 expression results in proteome-wide alterations and compromises bioenergetic capacity by disrupting mitochondrial function. J Alzheimers Dis. 2019;68(3):991–1011. doi:10.3233/JAD-181184
36. Yin J, Reiman EM, Beach TG, et al. Effect of ApoE isoforms on mitochondria in Alzheimer disease. Neurology. 2020;94(23):e2404–e2411. doi:10.1212/WNL.0000000000009582
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.