Back to Journals » Infection and Drug Resistance » Volume 11
Genetic characterization of Mycobacterium tuberculosis complex isolates circulating in Abuja, Nigeria
Authors Molina-Moya B , Abdurrahman ST, Madukaji LI, Gomgnimbou MK, Spinasse L, Gomes-Fernandes M, Gomes HM, Kacimi S, Dacombe R , Bimba JS , Lawson L , Sola C , Cuevas LE , Dominguez J
Received 1 March 2018
Accepted for publication 20 April 2018
Published 1 October 2018 Volume 2018:11 Pages 1617—1625
DOI https://doi.org/10.2147/IDR.S166986
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Barbara Molina-Moya,1,2 Saddiq T Abdurrahman,3 Laura I Madukaji,4 Michel Kiréopori Gomgnimbou,5,6 Lizania Spinasse,5 Meissiner Gomes-Fernandes,1,2,7 Harrison Magdinier Gomes,5 Sarah Kacimi,5 Russell Dacombe,8 John S Bimba,4 Lovett Lawson,4 Christophe Sola,5 Luis E Cuevas,8,* Jose Dominguez1,2,*
1Hospital Universitari Germans Trias i Pujol, Institut d’Investigació Germans Trias i Pujol, Universitat Autònoma de Barcelona, Badalona, Barcelona, Spain; 2CIBER Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Madrid, Spain; 3National TB, Buruli Ulcer and Leprosy Control Programme, Abuja, Nigeria; 4Bingham University, Nasarawa State, Nigeria; 5Institute for Integrative Biology of the Cell (I2BC), CEA, CNRS, Univ. Paris-Sud, Université Paris-Saclay, Gif-sur-Yvette cedex, France; 6Centre Muraz, Bobo-Dioulasso, Burkina Faso; 7CAPES Foundation, Ministry of Education of Brazil, Brasília, Brazil; 8Liverpool School of Tropical Medicine, Liverpool, UK
*These authors contributed equally to this work
Objective: Nigeria ranks fourth among the high tuberculosis (TB) burden countries. This study describes the prevalence of drug resistance and the genetic diversity of Mycobacterium tuberculosis in Abuja’s Federal Capital Territory.
Materials and methods: Two hundred and seventy-eight consecutive sputum samples were collected from adults with presumptive TB during 2013–2014. DNA was extracted from Löwenstein–Jensen cultures and analyzed for the identification of nontuberculous mycobacteria species, detection of drug resistance with line probe assays, and high-throughput spacer oligonucleotide typing (spoligotyping) using microbead-based hybridization.
Results: Two hundred and two cultures were positive for M. tuberculosis complex, 24 negative, 38 contaminated, and 15 positive for nontuberculous mycobacteria. Five (2.5%) M. tuberculosis complex isolates were resistant to rifampicin (RIF) and isoniazid (multidrug resistant), nine (4.5%) to RIF alone, and 15 (7.4%) to isoniazid alone; two RIF-resistant isolates were also resistant to fluoroquinolones and ethambutol, and one multidrug resistant isolate was also resistant to ethambutol. Among the 180 isolates with spoligotyping results, 164 (91.1%) were classified as lineage 4 (Euro-American), 13 (7.2%) as lineage 5 (West African 1), two (1.1%) as lineage 2 (East Asia), and one (0.6%) as lineage 6 (West African 2). One hundred and fifty-six (86.7%) isolates were grouped in 17 clusters (2–108 isolates/cluster), of which 108 (60.0%) were grouped as L4.6.2/Cameroon (spoligotype international type 61).
Conclusion: The description of drug resistance prevalence and genetic diversity of M. tuberculosis in this study may be useful for improving TB control in Nigeria.
Keywords: tuberculosis, isoniazid, rifampicin, line probe assay, microbeads, spoligotyping
Introduction
Despite advances in its diagnosis and treatment, tuberculosis (TB) remains a global public health problem. In 2015, there were an estimated 10.4 million new TB cases and 1.4 million TB deaths worldwide, 480,000 new cases of multidrug-resistant TB (MDR-TB), and 100,000 cases of rifampicin (RIF)-resistant TB (RR-TB).1 Multidrug resistance is defined as resistance to both RIF and isoniazid (INH), and WHO recommends that all patients with RR-TB should be treated with a second-line MDR-TB regimen. Nigeria has the fourth highest burden of TB in the world, with an estimated incidence of 322 per 100,000 people (2015). MDR-TB/RR-TB accounts for 4.3% of new cases and 25% of previously treated cases.1 However, these rates are likely to underestimate the problem, as a recent systematic review and meta-analysis reported that MDR-TB accounted for 6% of new and 32% of previously treated cases.2,3
WHO recommends the use of line probe assays (LPA) to rapidly detect drug resistance to the most important first-line (RIF and INH), second-line (fluoroquinolones [FLQ]), and injectable drugs (kanamycin [KAN], amikacin [AMK], and capreomycin [CAP]).4 LPA are based on PCR amplification of rpoB (RIF), katG and the promoter of inhA (INH), gyrA and gyrB (FLQ), and rrs and the promoter of eis (KAN, AMK, CAP), and subsequent detection of mutations through hybridization to probes immobilized to a nitrocellulose membrane. Several molecular methods are available for the detection of first- and second-line drug resistance,5–11 and a commercially available LPA for this purpose is Autoimmun Diagnostika (AID) TB Resistance (AID Diagnostika, Strassberg, Germany).12,13
Information on the Mycobacterium tuberculosis complex (MTBC) genotypes is useful for understanding the spread and phylogeographic specificity of predominant clones, as MTBC lineages have differences in virulence, transmissibility, and capacity of acquiring drug-resistance conferring mutations.14,15 One of the most used genotyping methods is spacer oligonucleotide typing (spoligotyping), which is based on the detection of 43 spacers between the repeats in the clustered regularly interspaced short palindromic repeats (CRISPR) locus.16 Spoligotyping can be performed with a high-throughput microbead-based assay, which can also be used to detect molecular drug resistance to RIF and INH status with the Tuberculosis Rifampicin Isoniazid Typing (TB-RINT) technique.17–20
The aim of the present study was to describe the molecular drug resistance and genetic diversity of MTBC in Abuja, Nigeria. For this, cultured isolates were tested with three molecular methods to detect drug resistance and with microbead-based spoligotyping to obtain a snapshot of the MTBC genetic diversity.
Materials and methods
Study design
Adults with presumptive TB attending TB diagnostic clinics at district hospitals of Abuja Federal Capital Territory during 2013 and 2014 were enrolled using consecutive sampling. Participants were asked to provide sputum samples on the spot, and samples were kept in a local refrigerator at 4°C until they were transported using a cold chain (4°C) to Zankli research laboratory in Abuja within 8 hours of collection. Specimens were cultured on duplicates using Löwenstein–Jensen medium. Duplicate cultures were then sent by courier at ambient temperature to the Hospital Universitari Germans Trias i Pujol (Badalona, Spain) for DNA extraction, identification of nontuberculous mycobacteria (NTM), and detection of drug resistance with LPA. DNA samples were then sent in dry ice to the Institut de Biologie Intégrative de la Cellule (Orsay, France) for spoligotyping and detection of drug resistance with microbead-based hybridization.
DNA extraction
For DNA extraction, few colonies were resuspended in 250 µL of molecular biology-grade water and were incubated at 95°C for 30 minutes to inactivate the mycobacteria. DNA was extracted using Maxwell® 16 Viral Total Nucleic Acid Purification Kit (Promega Corporation, Fitchburg, WI, USA) following the manufacturer’s instructions.
Identification of NTM species
Identification of NTM species was performed with the LPA Inno-Lipa Mycobacteria v2 assay (Fujirebio, Tokyo, Japan).21
Molecular detection of drug resistance
Molecular detection of drug resistance was performed with AID TB Resistance INH/RIF (AID Diagnostika), an LPA that targets wild-type regions and mutations in rpoB codons 516 (GAC [D] to GTC [V] or TAC [Y]), 526 (CAC [H] to TAC [Y], GAC [D], or CGC [R]), and 531 (TCG [S] to TTG [L] or TGG [W]), associated with RIF resistance; and in katG codon 315 (S to T) and inhA positions −16 (A to G), −15 (C to T), and −8 (T to A or C), associated with INH resistance.12 Four control probes (conjugate, amplification, Mycobacterium genus, and MTBC controls) are present in each strip to verify the test procedures. All four control bands should be present to consider a result valid. AID TB Resistance assay was performed following the manufacturer’s instructions and hybridization and detection were performed with Auto-Lipa (Fujirebio). The presence of all wild-type hybridization bands and absence of mutation bands indicate susceptibility to the drug considered. The absence of at least one wild-type hybridization band and/or the presence of any mutation band indicate resistance to the drug considered. The presence of all wild-type hybridization bands in combination with a mutation band in a target gene indicates heteroresistance, a combination of both susceptible and resistant M. tuberculosis.
In addition, molecular detection of drug resistance was performed with TB-RINT (Beamedex SAS, Orsay, France; www.beamedex.com), a 16-plex microbead-based hybridization assay, as described previously.18,19 Briefly, rpoB, katG, and the promoter region of inhA were simultaneously amplified by PCR using dual-priming oligonucleotide primers, and the PCR product was hybridized to oligonucleotide-precoupled microbeads. Subsequently, detection was performed either with the flow cytometry-based Luminex 200 system (Luminex Corporation, Austin, TX, USA), XPONENT software for LX100/LX200 (version 3.1.871.0), or BioPlex200 (Bio-Rad Laboratories Inc, Hercules, CA, USA) running under Bio-Plex Manager 5.0. This assay targets wild-type regions and mutations in rpoB codons 516 (GAC [D] and GTC [V]), 526 (CAC [H], TAC [Y], and GAC [D]), and 531 (TCG [S], TTG [L], and TGG [W]); katG codon 315 (ACG [S], ACC [T], and ACA [T]); and inhA positions −15 (C and T) and −8 (A). This assay may be used as a stand-alone assay or in association with spoligotyping, TB-SPOL, in one read only (TB-SPRINT, 59-Plex) on a Luminex 200 or on a FlexMap 3D, or in two reads (TB-SPOL and TB-RINT or TB-SPRINT-like) on a MagPix.
Isolates identified by LPA as resistant to RIF and/or INH underwent further molecular detection for FLQ, ethambutol (EMB), and injectable drugs (KAN, AMK, CAP) resistance using the LPA AID TB Resistance FQ/EMB, AID TB Resistance AG, or pyrosequencing.10,12 AID TB Resistance FQ/EMB targets wild-type regions and mutations in gyrA codons associated with FLQ resistance (codon 90 GCG [A] to GTG [V], codon 91 TCG [S] to CCG [P], and codon 94 GAC [D] to GCC [A], AAC [N], TAC [Y], and GGC [G]), and in embB codon 306 (ATG [M] to GTT [V], ATA [I], ATC [I], and ATT [I]) associated with EMB resistance. AID TB Resistance AG targets wild-type regions and mutations in rrs positions 1401 (A to G), 1402 (C to T), and 1484 (G to C or T), which are associated with KAN/AMK/CAP resistance. Finally, pyrosequencing was performed as previously described.7,10 Briefly, pyrosequencing targets gyrA codons 80 and 88–97, rrs positions 1401, 1402, and 1484, and embB codon 306. Pyrosequencing reaction and data analysis were performed as recommended by the PSQ96MA and SQA software manufacturer (Biotage AB, Uppsala, Sweden).
Spoligotyping
Spoligotyping was performed with the TB-SPOL kit (Beamedex SAS), a 43-plex microbead-based hybridization assay, as described previously.17 Briefly, the CRISPR region was amplified by PCR, and PCR products were hybridized to oligonucleotide-precoupled microbeads. Detection was performed as described for microbead-based detection of drug resistance. Numerical data were combined in a Microsoft Excel spreadsheet file and uploaded to BioNumerics version 6.1 software (Applied Maths, Kortrijk, Belgium). Spoligotyping patterns were compared with those in the International Spoligotyping Database (SITVITWEB) of the Pasteur Institute of Guadalupe (http://www.pasteur-guadeloupe.fr:8081/SITVIT_ONLINE/), and spoligotype international types (SIT) and major phylogenetic clades were assigned.22 SIT numbers designate spoligotyping patterns shared by two or more patient isolates, respectively, whereas “orphan” designates patterns reported for a single isolate. When no SIT numbers were available in SITVITWEB, the designation “new-x” (lower cases) was given for patterns detected in single isolates and the designation “NEW-X” for new intra-study clusters. TB lineages were assigned using TBminer (https://info-demo.lirmm.fr/tbminer/).23 Lineages and sublineages were reported using the recent whole-genome-based taxonomical nomenclature as suggested by Stucki et al24 and Coll et al.25
Ethics approval
Ethical approval was obtained from the Research Ethics Committees of the Liverpool School of Tropical Medicine, the Nigerian National Ethics Committee, and Zankli Medical Centre. For this study, written informed consent to participate was obtained from all participants. In addition, patient data were anonymized, most researchers were blind to the patient data, and all data reported in the manuscript were obtained from cultured isolates.
Results
A total of 278 cultures were performed. Of these, 221 (79.5%) were positive, 24 (8.6%) negative, and 33 (11.9%) contaminated. One culture had two different colonies, and therefore, 222 isolates were available for DNA extraction. Twenty of 222 isolates were suspected to be NTM. NTM identification with Inno-Lipa Mycobacteria v2 identified Mycobacterium intracellulare (sequevars Min-A, -B, -C and -D) in four isolates; Mycobacterium fortuitum–Mycobacterium peregrinum complex in three; Mycobacterium avium, Mycobacterium paratuberculosis, and Mycobacterium silvaticum in two; M. avium complex in one; Mycobacterium chelonae complex (group III, Mycobacterium abscessus) in one; Mycobacterium genus in four; and five were negative for Mycobacterium. Therefore, of 278 cultures, 202 (72.7%) had MTBC, 24 (8.6%) were negative, 38 (13.7%) were contaminated, and 15 (5.6%) had NTM (one was positive for both MTBC and NTM) (Table S1).
Molecular detection of drug resistance
AID TB Resistance INH/RIF results were obtained for the 202 MTBC isolates, and 173 (85.6%) were sensitive to both INH and RIF, five (2.5%) were resistant to both INH and RIF, nine (4.5%) resistant to RIF and sensitive to INH, and 15 (7.4%) sensitive to RIF and resistant to INH (Table S1).
The microbead-based TB-RINT assay could be performed for 179 (89%) of the 202 MTBC isolates but no results were obtained for the 20 isolates suspected to be NTM (Table S1). Interpretable results for rpoB, katG, and inhA were obtained for 109 (60.9%), 112 (62.6%), and 112 (62.6%) isolates respectively. Regarding rpoB, 104 isolates were wild type, two harbored the H526D mutation, two the S531L mutation, and one the D516V mutation. Regarding katG, 108 isolates were wild-type and four harbored the S315T mutation. As for inhA, 105 isolates were wild type and seven harbored the C-15T mutation. The results obtained with AID TB Resistance INH/RIF and TB-RINT had 100% concordance (Table S1).
The 29 INH and/or RR isolates detected with AID TB Resistance were subjected to molecular analysis of resistance to FLQ, EMB, and KAN/AMK/CAP. Twenty-four were susceptible to FLQ, EMB, and KAN/AMK/CAP, two were resistant to FLQ and EMB, and one was resistant to EMB only. Results for EMB and KAN/AMK/CAP were missing for two isolates that were susceptible to the remaining drugs (Table 1).
Spoligotyping
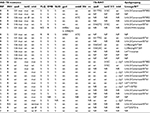
The microbead-based spoligotyping TB-SPOL assay was performed for 198 isolates, and an interpretable result was obtained for 180 (90.9%) (Table S1). Of these, 156 (86.7%) were grouped in 17 clusters, comprising between 2 and 108 isolates. One hundred and eight (60.0%) were grouped as L4.6.2/Cameroon (SIT 61). Forty eight (26.7%) were grouped into 16 clusters, each comprising between two and six isolates. Twenty four isolates had unique patterns, which resulted in 41 distinct spoligotyping patterns (Table 2). Of these, 26 were described and 15 were not described in the SITVITWEB. Four of the latter had been reported in an earlier study in Abuja in 2012.26 The distribution of sublineages among the 180 isolates is shown in Table 2 and included L4.6.2/Cameroon (n=120, 66.7%), L4.6.1/T2/Uganda (n=12, 6.7%), L4.1.1/X (n=4, 2.2%), L4.1.2/Haarlem (n=2, 1.1%), L4.3.2/LAM3 (n=1, 0.6%), L4/T1-not assigned (n=25, 10.6%), L5/Mycobacterium africanum WA1 (n=13, 7.2%), L2/Beijing (n=2, 1.1%), and L6/M. africanum WA2 (n=1, 0.6%). More broadly, of the 180 isolates, 164 (91.1%) were classified as L4 (Euro-American), 13 (7.2%) L5/M. africanum WA1, two (1.1%) L2 (East Asia), and one (0.6%) L6/M. africanum WA2 (Table 2).
Twenty three isolates with spoligotyping results were resistant to RIF and/or INH (Table 1). Four isolates with multidrug resistance were classified as L4.6.2/Cameroon: two as SIT852, one as SIT61, and one as SIT838. Six isolates were resistant to RIF and susceptible to INH. Of these, two were L2/Beijing/SIT269, two L4.6.2/Cameroon/SIT61, one L4/T1/orphan, and one L5/M. africanum WA1/orphan. Of 13 isolates susceptible to RIF and resistant to INH, eight were classified as L4.6.2/Cameroon/SIT61, two as L4/T1/SIT53, one as L4.6.2/Cameroon/SIT852, one as L4/T2/UgandaI/SIT316, and one as L4/new (pattern not described in SITVITWEB). Resistance to RIF and/or INH was detected in 11/108 (10.2%) isolates classified as L4.6.2/Cameroon/SIT61, 3/6 (50.0%) L4.6.2/Cameroon/SIT852, 1/5 (20.0%) L4/T2/UgandaI/SIT316, 1/4 (25.0%) L5/M. africanum WA1/orphan, 2/2 (100%) L4/T1/SIT53, 2/2 (100%) L2/Beijing/SIT269, 1/2 (50.0%) L4/T1/orphan (T1), 1/1 (100%) L4.6.2/Cameroon/SIT838, and 1/1 (100%) L4/new (pattern not described in SITVITWEB) (Table 3).
Discussion
We report a high proportion of isolates resistant to RIF, as detected with LPA and TB-RINT, and a large cluster of M. tuberculosis isolates classified as L4.6.2/Cameroon/SIT61, among patients with TB in Abuja, Nigeria.
In our study, according to genotypic drug susceptibility testing (DST), the rate of multidrug resistance was 2.5%, which is lower than the 11% reported in a previous study from our group, according to phenotypic DST.27 However, the limited sensitivity of genotypic DST for INH is noteworthy. In comparison, the rates of resistance in other states of Nigeria varied widely: 1.3% MDR in Kaduna (north Nigeria) according to genotypic DST,28 and 4.0% and 5.2% in two studies in Cross River, respectively,29,30 0% in Ibadan (south Nigeria),2 and 32% in Lagos (south Nigeria)2 according to phenotypic DST. The differences in the prevalence of drug resistance between north and south Nigeria were highlighted in a recent systematic review, with the prevalence of MDR-TB among new patients being 3.0% in the north and 12.0% in the south (P<0.001).3 The prevalence of MDR-TB among new patients in all of Nigeria was 6.0%, which is higher than the WHO estimate.1,3 Interestingly, according to the DST method used – genotypic or phenotypic – this rate varied among new patients from 4.0% to 7.0%, respectively, although the difference was not statistically significant.3
Genotypic and phenotypic DST present differences. The sensitivity of genotypic DST for detecting RIF resistance ranges from 95% to 98%, since the majority of mutations are located in the 81-bp core region of rpoB, which is targeted by most methods.31 However, a mutation located outside the 81-bp core region, the Ile572Phe, accounted for up to one-third of MDR-TB cases in Swaziland, resulting in many false negative results.32 Importantly, isolates harboring this mutation are not consistently identified as resistant by phenotypic methods.33 It is important that the molecular method identifies the specific mutations detected, since different mutations confer different levels of resistance to one or more drugs in a family. For example, mutations H526D and H526Y in rpoB confer high-level resistance to all rifamycins, and their detection should exclude all rifamycins from treatment.31 In this study, codon 526 was mutated in seven isolates according to LPA, but it was not possible to identify the specific mutation. However, mutation H526D could be identified with the TB-RINT microbead-based hybridization in two isolates.
Regarding INH resistance, the sensitivity of genotypic DST is lower than phenotypic methods. The most common mutations targeted by the molecular methods, katG315 and inhA-15, detected 64% and 19% of the phenotypically resistant cases globally.34 Thus, phenotypic resistance cannot be excluded for the isolates in our study that were classified as INH susceptible by LPA. It is especially important to detect INH resistance before starting the continuation phase of treatment, since only RIF would be effective. In addition, the level of resistance is important, since low-level INH resistance (inhA promoter mutations) can be overcome with higher doses. In the present study, among the INH monoresistant isolates, three harbored the katG mutation and 12 harbored a mutation in the inhA promoter. It is of note that high-dose INH, even in cases with high-level resistance (katG mutation), may still be effective.35
Mutations in gyrA were detected only in two isolates that were RR but INH-susceptible according to the LPA, and no mutations in rrs were detected; thus, no pre-extensively drug-resistant isolates were identified in this study. The specificity of molecular methods for detecting FLQ and second-line injectable drug resistance is high; thus, after detection of a mutation in gyrA or rrs, these drugs should not be used for treatment.31 It is important to test for first- and second-line drug resistance to prescribe an effective treatment. This is especially critical after detecting RIF resistance with Xpert MTB/RIF – which is increasingly being adopted as the initial diagnostic test for all individuals with presumptive TB in the Nigerian National Tuberculosis and Leprosy Control Programme.1 Despite these limitations, the rapid detection of drug resistance using molecular methods can be useful to exclude noneffective drugs and prevent the development of additional drug resistance.
The genetic diversity of M. tuberculosis isolates in Abuja described here updates that described by Lawson et al in 2009–2010.26 The proportion of isolates classified was similar to those reported earlier: L4.6.2/Cameroon (66.7% and 63.0%), L4.6.1/T2/UgandaI (6.7% and 11.7%), L4.1.1/X (2.2% and 0%), L4.1.2/Haarlem (1.1% and 5.2%), L4.3.2/LAM3 (0.6% and 0.6%), L4/T1-not assigned (10.6% and 7.1%), L5/M. africanum WA1 (7.2% and 8.4%), L2/Beijing (1.1% and 0.6%), L6/M. africanum WA2 (0.6% and 0.6%), and Mycobacterium bovis (0% and 2.6%). The clustering rate reached 87%, which is statistically similar to the 92% detected by spoligotyping by Lawson et al.26 However, 60% of the total isolates in the present study were classified as L4.6.2/Cameroon/SIT61. Due to this high clustering rate, further genotyping would be required to determine the real transmission rate of this clonal complex. In the previous study, isolates clustered with spoligotyping as L4.6.2/Cameroon/SIT61 were partially resolved in six clusters by 24 variable number tandem repeat.26 L4.6.2/Cameroon/SIT61, and the L4.6.2/Cameroon sublineage in general (spoligotyping signature lacking spacers 23–25 and 33–36) have been reported to be the main genotypic lineage in other Nigerian states (39.5% in Cross River36 and 66% in Ibadan)37 and in neighboring countries (66% in Cameroon,38 33% in Chad,39 33% in Benin,40 and 30% in Burkina Faso).41 The spread of this family could be associated with recent transmission of TB. In addition, L4.6.2/Cameroon/SIT61 has been associated with young patients (25–34 years), suggesting recent transmission.36 Also, the prevalence of M. africanum is similar between the present study and the previous one by Lawson et al (7.8% and 9.1%).26 In Cross River, M. africanum represented 12% of TB cases and was classified as West African 1.36 Recent results from our team based on spoligotyping performed directly on sputum smears extracts show that M. africanum West African 1 is geographically constrained to a limited number of regions in South-East Nigeria and likely associated with specific populations.42 On the contrary, the prevalence of M. africanum is highly variable in different regions of Africa (3% in Cameroon38 and 20% in Ghana)43 with a decreasing trend in transmission over the last three decades.44 Research is needed to determine the mechanism(s) underlying the putative decreasing trend of M. africanum and the increasing selection and dissemination of the Cameroon sublineage in certain parts of Africa.
Conclusion
We report a high proportion of isolates resistant to RIF, as detected with molecular methods, and a large cluster of L4.6.2/Cameroon/SIT61 isolates, indicating the spread of this sublineage in Nigeria. Future studies will be needed to describe changes on drug resistance patterns and genetic diversity of MTBC and to determine the evolution of TB transmission and drug resistance in this setting. These results may be useful to TB control programs to assess their impact on the control of drug resistance. The methods used here may be useful in other settings where phenotypic DST and more complex genotyping methods are not available.
Data availability
All data generated or analyzed during this study are included in the published article.
Acknowledgments
This study was funded by a Strategic Award grant from the European and Developing Countries Clinical Trials Partnership (grant SP.2011.41304.021) and its cofounders, the Medical Research Council UK, and Instituto de Salud Carlos III (ISCIII), Spain (PI13/01546 and PI16/01912). MKG was a Postdoctorate fellow from Centre Muraz, Bobo-Dioulasso, Burkina Faso. LS was a Postdoctorate fellow from UFRJ, Rio de Janeiro, Brazil, working in Sola’s Orsay team. MG-F was a PhD student funded by CAPES Foundation, Ministry of Education of Brazil, Brasília, Brazil. JD was funded by the “Miguel Servet” program of ISCIII (Spain).
The funders were not involved in any of the stages from study design to submission of the manuscript for publication. We are grateful to all TB program coordinators for the collection of samples in Nigeria.
Author contributions
Conception and design of the study: STA, LIM, RD, JSB, LL, LEC, JD, and CS. Acquisition of data: BM-M, STA, LIM, MKG, LS, MG-F, HMG, SK, JSB, and LL. Analysis and interpretation of data: BM-M, MKG, HMG, SK, and CS. Drafting the article: BM-M, LEC, JD, and CS. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
MKG and CS are among the founders of the Beamedex SAS company that produces and commercializes the spoligotyping (TB-SPOL) kit as a “Research Use Only” assay, to be run on Luminex 200 and on MagPix devices (Luminex Corporation, Austin, TX, USA). These authors held Beamedex shares during the study. However, CS does not hold any share in Beamedex anymore. MKG and CS also declare that they do not hold any share in Luminex Corporation nor were they financed by Luminex. The authors report no other conflicts of interest in this work.
References
World Health Organization. Global Tuberculosis Report. (WHO/HTM/TB/2016.13). Geneva, Switzerland: WHO; 2016. | ||
Gehre F, Otu J, Kendall L, et al. The emerging threat of pre-extensively drug-resistant tuberculosis in West Africa: preparing for large-scale tuberculosis research and drug resistance surveillance. BMC Med. 2016;14(1):160. | ||
Onyedum CC, Alobu I, Ukwaja KN. Prevalence of drug-resistant tuberculosis in Nigeria: a systematic review and meta-analysis. PLoS One. 2017;12(7):e0180996. | ||
World Health Organization. WHO Treatment Guidelines for Drug-Resistant Tuberculosis. (WHO/HTM/TB/2016.04). Geneva, Switzerland: WHO; 2016. | ||
Lacoma A, Garcia-Sierra N, Prat C, et al. GenoType MTBDRplus assay for molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical samples. J Clin Microbiol. 2008;46(11):3660–3667. | ||
Garcia-Sierra N, Lacoma A, Prat C, et al. Pyrosequencing for rapid molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol. 2011;49(10):3683–3686. | ||
Lacoma A, Garcia-Sierra N, Prat C, et al. GenoType MTBDRsl for molecular detection of second-line and ethambutol resistance in Mycobacterium tuberculosis strains and clinical samples. J Clin Microbiol. 2012;50(1):30–36. | ||
Molina-Moya B, Latorre I, Lacoma A, Prat C, Domínguez J. Recent advances in tuberculosis diagnosis: IGRAs and molecular biology. Curr Treat Options Infect Dis. 2014;6(4):377e91. | ||
Molina-Moya B, Lacoma A, Prat C, et al. Diagnostic accuracy study of multiplex PCR for detecting tuberculosis drug resistance. J Infect. 2015;71(2):220–230. | ||
Lacoma A, Molina-Moya B, Prat C, et al. Pyrosequencing for rapid detection of Mycobacterium tuberculosis second-line drugs and ethambutol resistance. Diagn Microbiol Infect Dis. 2015;83(3):263–269. | ||
Molina-Moya B, Kazdaglis G, Lacoma A, et al. Evaluation of Genoflow DR-MTB array test for detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis. J Clin Microbiol. 2016;54(4):1160–1163. | ||
Molina-Moya B, Lacoma A, Prat C, et al. AID TB resistance line probe assay for rapid detection of resistant Mycobacterium tuberculosis in clinical samples. J Infect. 2015;70(4):400–408. | ||
Ritter C, Lucke K, Sirgel FA, et al. Evaluation of the AID TB resistance line probe assay for rapid detection of genetic alterations associated with drug resistance in Mycobacterium tuberculosis strains. J Clin Microbiol. 2014;52(3):940–946. | ||
Coscolla M, Gagneux S. Does M. tuberculosis genomic diversity explain disease diversity? Drug Discov Today Dis Mech. 2010;7(1):e43–e59. | ||
Wlodarska M, Johnston JC, Gardy JL, Tang P. A microbiological revolution meets an ancient disease: improving the management of tuberculosis with genomics. Clin Microbiol Rev. 2015;28(2):523–539. | ||
Kamerbeek J, Schouls L, Kolk A, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35(4):907–914. | ||
Zhang J, Abadia E, Refregier G, et al. Mycobacterium tuberculosis complex CRISPR genotyping: improving efficiency, throughput and discriminative power of ‘spoligotyping’ with new spacers and a microbead-based hybridization assay. J Med Microbiol. 2010;59(Pt 3):285–294. | ||
Gomgnimbou MK, Abadia E, Zhang J, et al. “Spoligoriftyping,” a dual-priming-oligonucleotide-based direct-hybridization assay for tuberculosis control with a multianalyte microbead-based hybridization system. J Clin Microbiol. 2012;50(10):3172–3179. | ||
Gomgnimbou MK, Hernandez-Neuta I, Panaiotov S, et al. Tuberculosis-spoligo-rifampin-isoniazid typing: an all-in-one assay technique for surveillance and control of multidrug-resistant tuberculosis on Luminex devices. J Clin Microbiol. 2013;51(11):3527–3534. | ||
Molina-Moya B, Gomgnimbou MK, Lafoz C, et al. Molecular characterization of Mycobacterium tuberculosis strains with TB-SPRINT. Am J Trop Med Hyg. 2017;97(3):806–809. | ||
Tortoli E, Mariottini A, Mazzarelli G. Evaluation of INNO-LiPA MYCOBACTERIA v2: improved reverse hybridization multiple DNA probe assay for mycobacterial identification. J Clin Microbiol. 2003;41(9):4418–4420. | ||
Demay C, Liens B, Burguiere T, et al. SITVITWEB – a publicly available international multimarker database for studying Mycobacterium tuberculosis genetic diversity and molecular epidemiology. Infect Genet Evol. 2012;12(4):755–766. | ||
Aze J, Sola C, Zhang J, et al. Genomics and machine learning for taxonomy consensus: the Mycobacterium tuberculosis complex paradigm. PLoS One. 2015;10(7):e0130912. | ||
Stucki D, Brites D, Jeljeli L, et al. Mycobacterium tuberculosis lineage 4 comprises globally distributed and geographically restricted sublineages. Nat Genet. 2016;48(12):1535–1543. | ||
Coll F, McNerney R, Guerra-Assuncao JA, et al. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat Commun. 2014;5:4812. | ||
Lawson L, Zhang J, Gomgnimbou MK, et al. A molecular epidemiological and genetic diversity study of tuberculosis in Ibadan, Nnewi and Abuja, Nigeria. PLoS One. 2012;7(6):e38409. | ||
Lawson L, Yassin MA, Abdurrahman ST, et al. Resistance to first-line tuberculosis drugs in three cities of Nigeria. Trop Med Int Health. 2011;16(8):974–980. | ||
Aliyu G, El-Kamary SS, Abimiku A, et al. Mycobacterial etiology of pulmonary tuberculosis and association with HIV infection and multidrug resistance in northern Nigeria. Tuberc Res Treat. 2013;2013:650561. | ||
Otu A, Umoh V, Habib A, Ameh S, Lawson L, Ansa V. Drug resistance among pulmonary tuberculosis patients in Calabar, Nigeria. Pulm Med. 2013;2013:235190. | ||
Pokam BT, Asuquo AE, Abia-Bassey LN, et al. Multidrug resistance and demography of newly diagnosed tuberculosis patients in Cross River State, Nigeria. Int J Mycobacteriol. 2013;2(2):89–93. | ||
Dominguez J, Boettger EC, Cirillo D, et al. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: a TBNET/RESIST-TB consensus statement. Int J Tuberc Lung Dis. 2016;20(1):24–42. | ||
Sanchez-Padilla E, Merker M, Beckert P, et al. Detection of drug-resistant tuberculosis by Xpert MTB/RIF in Swaziland. N Engl J Med. 2015;372(12):1181–1182. | ||
Rigouts L, Gumusboga M, de Rijk WB, et al. Rifampin resistance missed in automated liquid culture system for Mycobacterium tuberculosis isolates with specific rpoB mutations. J Clin Microbiol. 2013;51(8):2641–2645. | ||
Seifert M, Catanzaro D, Catanzaro A, Rodwell TC. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: a systematic review. PLoS One. 2015;10(3):e0119628. | ||
Van Deun A, Chiang CY. Shortened multidrug-resistant tuberculosis regimens overcome low-level fluoroquinolone resistance. Eur Respir J. 2017;49(6):1700223. | ||
Thumamo BP, Asuquo AE, Abia-Bassey LN, et al. Molecular epidemiology and genetic diversity of Mycobacterium tuberculosis complex in the Cross River State, Nigeria. Infect Genet Evol. 2012;12(4):671–677. | ||
Cadmus S, Palmer S, Okker M, et al. Molecular analysis of human and bovine tubercle bacilli from a local setting in Nigeria. J Clin Microbiol. 2006;44(1):29–34. | ||
Koro Koro F, Um Boock A, Kaiyven AL, et al. Genetic structure and drug susceptibility patterns of Mycobacterium tuberculosis complex strains responsible of human pulmonary tuberculosis in the major rearing region in Cameroon. Biomed Res Int. 2016;2016:2904832. | ||
Diguimbaye C, Hilty M, Ngandolo R, et al. Molecular characterization and drug resistance testing of Mycobacterium tuberculosis isolates from Chad. J Clin Microbiol. 2006;44(4):1575–1577. | ||
Affolabi D, Sanoussi N, Codo S, et al. First insight into a nationwide genotypic diversity of Mycobacterium tuberculosis among previously treated pulmonary tuberculosis cases in Benin, West Africa. Can J Infect Dis Med Microbiol. 2017;2017:3276240. | ||
Godreuil S, Torrea G, Terru D, et al. First molecular epidemiology study of Mycobacterium tuberculosis in Burkina Faso. J Clin Microbiol. 2007;45(3):921–927. | ||
Molina-Moya B, Gomgnimbou MK, Spinasse L, et al. Mycobacterium tuberculosis complex genotypes circulating in Nigeria based on spoligotyping obtained from Ziehl-Neelsen stained slides extracted DNA. PLoS Negl Trop Dis. 2018;12(2):e0006242. | ||
Yeboah-Manu D, Asare P, Asante-Poku A, et al. Spatio-temporal distribution of Mycobacterium tuberculosis complex strains in Ghana. PLoS One. 2016;11(8):e0161892. | ||
Niobe-Eyangoh SN, Kuaban C, Sorlin P, et al. Genetic biodiversity of Mycobacterium tuberculosis complex strains from patients with pulmonary tuberculosis in Cameroon. J Clin Microbiol. 2003;41(6):2547–2553. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.