Back to Journals » Journal of Pain Research » Volume 11
Genetic and psychological factors interact to predict physical impairment phenotypes following exercise-induced shoulder injury
Authors Borsa PA , Parr JJ, Wallace MR , Wu SS, Dai Y , Fillingim RB, George SZ
Received 18 April 2018
Accepted for publication 26 July 2018
Published 23 October 2018 Volume 2018:11 Pages 2497—2508
DOI https://doi.org/10.2147/JPR.S171498
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Paul A Borsa,1 Jeffrey J Parr,2 Margaret R Wallace,3 Samuel S Wu,4 Yunfeng Dai,4 Roger B Fillingim,5 Steven Z George6
1Department of Applied Physiology and Kinesiology, University of Florida, Gainesville, FL, USA; 2School of Health Professions, University of Southern Mississippi, Hattiesburg, MS, USA; 3Department of Molecular Genetics and Microbiology, Center for Epigenetics, UF Genetics Institute, University of Florida, Gainesville, FL, USA; 4Department of Biostatistics, University of Florida, Gainesville, FL, USA; 5Department of Community Dentistry and Behavioral Science, University of Florida, Gainesville, FL, USA; 6Duke Clinical Research Institute and Department of Orthopaedic Surgery, Duke University, Durham, NC, USA
Background: We investigated interactions between genetic and psychological factors in predicting shoulder impairment phenotypes. We hypothesized that pro-inflammatory genes would display stronger relationships compared with pain-related genes when combined with psychological factors for predicting phenotypic changes.
Subjects and methods: Altogether, 190 participants completed a 5-day experimental protocol. An experimental shoulder injury model was used to induce physical impairment, and a priori selected genetic (pain-related, pro-inflammatory) and psychological (anxiety, depressive symptoms, pain catastrophizing, fear of pain, kinesiophobia) factors were included as predictors of interest. Impairment phenotypes were injury-induced deficits in range of motion (ROM) and strength. After controlling for age, sex, and race, genetic and psychological predictors were entered separately as main effects and interaction terms in regression models for each phenotype.
Results: Strong statistical evidence was provided for interactions between: 1) IL-1β (rs1143634) and fear of pain for predicting loss of shoulder flexion and abduction, 2) IL-1β (rs1143634) and anxiety for predicting loss of flexion, and 3) IL-1β (rs1143634) and depressive symptoms for predicting loss of internal rotation. In addition, the interaction between OPRM1 (rs1799971) and fear of pain as well as COMT (rs4818) and pain catastrophizing provided strong statistical evidence for predicting strength loss.
Conclusion: Pro-inflammatory gene variants contributed more to physical impairment with two single nucleotide polymorphisms (SNPs; IL-1β [rs1143634] and TNF/LTA [rs2229094]) interacting with psychological factors to predict six shoulder impairment phenotypes. In comparison, two pain-related gene SNPs (OPRM1 [rs1799971] and COMT [rs4818]) interacted with psychological factors to predict four shoulder impairment phenotypes (abduction: 5-day average loss; strength loss: 5-day average, peak, and relative loss).
Keywords: single nucleotide polymorphisms, inflammation, IL-1β, fear of pain, pain catastrophizing
Introduction
Shoulder injuries are a common cause of persistent musculoskeletal pain and dysfunction.1–5 Studies have shown that athletes involved in overhead throwing sports experience shoulder injuries at prevalence rates between 20% and 91%.2–4 Shoulder injury is characterized by prolonged strength loss and restricted range of motion (ROM) along with pain and disability.6,7 Consequently, only 50% of shoulder injuries in primary care settings resolve within the first 6 months after injury, with 40% of cases persisting for more than 12 months.8–10 Traditionally, sports medicine practitioners use self-report ratings of pain and disability along with objective measures of physical impairment as outcomes when reporting the severity of the injury as well as the extent of recovery during rehabilitation.7 It is well known that the symptomatic response following injury can be impacted by biological and psychological factors; however, very little is known about how biological and psychological factors interact to influence the magnitude of physical impairment after shoulder injury.
Diatchenko et al11 proposed a theoretical model that identified environmental, genetic, psychological, and pain amplification factors as important in the development of acute and chronic pain conditions. Psychological factors are predictive of prolonged recovery following musculoskeletal injuries and have been associated with increased pain or disability in studies of low back,12 knee,13 cervical,14 and shoulder pain.15–17 Recently, genetic factors have been implicated in the development of shoulder pain following exercise-induced shoulder injury.18–21 A select group of pain-related18–20 and pro-inflammatory21 genes when combined with pain-associated psychological factors has shown predictive value for identifying individuals who may be at risk for experiencing increased pain intensity and duration following shoulder injury. For example, George et al18,19 reported that an interaction between elevated pain catastrophizing and a catechol-O-methyltransferase (COMT) genotype for high pain sensitivity resulted in higher shoulder pain in exercise-induced injury and surgical cohorts. More recently, George et al20 were able to confirm that the COMT genotype interacts with pain-associated psychological factors and identified additional interactions with other pain modulatory genes such as AVPR1A, KCNS1, and ADRB2 that were thought to increase or prolong the pain experience. In addition, George et al21 investigated the combined influences of pro-inflammatory and psychological factors on several shoulder pain phenotypes. The findings indicate strong statistical evidence for the interactions between TNF/LTA single nucleotide polymorphism (SNP) rs2229094 and depressive symptoms for pain intensity and duration and IL1β 2-SNP diplotype and kinesiophobia for average shoulder pain intensity.
What is not currently known is whether these same pro-inflammatory and pain-related genes when combined with pain-associated psychological factors have predictive value for identifying individuals at risk for experiencing increased physical impairment following musculoskeletal injury. If pro-inflammatory genes have a role in the upregulation of pro-inflammatory mediators, a greater magnitude of physical impairment may be expected. Therefore, our primary objective was to identify interactions between selected pain-related and pro-inflammatory genes and pain-associated psychological factors that predict shoulder impairment phenotypes better than individual genetic or psychological factors alone. We hypothesized that pro-inflammatory genes will display stronger relationships compared with pain-related genes when combined with psychological factors for predicting these phenotypic changes.
Subjects and methods
Participants
Participants were otherwise healthy men and women of any racial/ethnic background. Participants were paid volunteers. To meet the inclusion criteria, participants had to be between the age of 18 and 85 years and not currently performing resistance exercise of the upper extremity during the previous 6 weeks. Participants were also excluded if they 1) were currently experiencing neck or shoulder pain, 2) had any neurological impairment of the upper extremity, such as loss of sensation, muscle weakness, or reflex changes, 3) were currently taking pain medication, or 4) had previous history of shoulder surgery. These eligibility criteria are the same as those used in our previous studies.15–21 All participants provided signed informed consent, and the study was approved by the institutional review board of the University of Florida. All experimental procedures followed in this study conformed to the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Procedures
All participants underwent five testing sessions on consecutive days. During the first session, subjects 1) read and signed the informed consent approved by the institutional review board; 2) completed a series of brief questionnaires asking for demographic data including age, height, and weight; 3) filled out previously validated questionnaires; 4) had pre-injury (baseline) impairment measures taken; 5) had DNA collected via buccal swabs; and 6) performed a concentric–eccentric isokinetic exercise protocol on their dominant shoulder. Our study used a fatigue protocol for the shoulder that induced controlled micro-trauma resulting in inflammation, muscular pain, and loss of physical function. We used the exercise-induced injury protocol, because it is a validated model of muscle-related shoulder pain and associated impairment that lasts several days up to a week.15–21 We included shoulder impairment phenotypes (isometric strength and ROM) as outcomes to represent different aspects of the musculoskeletal injury experience.
Subjects were asked to return to the laboratory post injury at 24-hour intervals for the next 4 days. If shoulder pain and disability continued after the 5th study day, subjects were sent an email prompting them to report symptom intensity via a web-based data collection tool.
Self-report measures
Negative mood
Depressive symptoms were assessed through the Patient Health Questionnaire (PHQ). The PHQ is a nine-item measure that assesses both symptoms and severity of depression.22 The PHQ examines how often you have particular thoughts or feeling and is rated on a 4-point scale, where 0 means “not at all” and 3 means “nearly every day.” Anxiety was assessed with the State-Trait Anxiety Inventory (STAI) which is a 40-item measure for symptoms of anxiety.23 Only the 20-item trait portion of the STAI was used in the data analysis to capture a dispositional construct.
Fear-avoidance model
Fear of pain, fear of reinjury/movement, and pain catastrophizing were the fear-avoidance model-specific constructs of interest for this study. We used a shortened version of the Fear of Pain Questionnaire (FPQ)-III. The FPQ-III is a well-validated instrument that is appropriate for use in nonclinical and clinical populations.24 The shortened version contains nine items that correlated highly with the original 30-item scale in previous studies.15,16 The items assess fear of specific situations that would normally produce pain on a 5-point rating scale, where a score of 5 represents “extremely painful” and a score of 1 represents “not at all painful.” The Tampa Scale of Kinesiophobia (TSK) consists of eleven items and is used to measure the fear of movement/reinjury. It is rated on a 4-point scale, where a score of 4 represents “strongly agree” with the statement and a score of 1 represents a “strongly disagree.” Subjects were asked to complete the TSK on each visit to the laboratory. The total score was used in the current study. The TSK has been deemed a valid and reliable method for determining fear of movement/reinjury in both clinical and nonclinical populations.25 The Pain Catastrophizing Scale (PCS) consists of 13 items and assesses different thoughts that may be associated with experiencing pain. It is rated on a 5-point scale, where a score of 4 means “you worry all the time about the pain” and a score of 0 means “not at all”.26 Subjects were instructed to rate the degree to which they have specified feelings when experiencing pain. Three dimensions of pain catastrophizing have been identified, but only the total score was used for the current study. The PCS has been validated for clinical and nonclinical populations.27
Genetic data generation
Gene and SNP selection
Genetic predictors were selected a priori based on allele frequencies, status as tagging SNPs, functional data, and promising findings in human association studies involving experimental or clinical pain phenotypes. All 19 SNPs (eight pro-inflammatory and eleven pain-related) chosen were bi-allelic. The specific SNPs selected for each gene had minor allele frequencies in white populations of European descent (the majority of our subjects) that ensured adequate power in statistical analyses. The pro-inflammatory SNPs included the following: IL-1β (rs1143627, rs16944, and rs1143634), IL-6 (rs1800797, rs2069840), TNF/LTA (rs229094, rs1800683), and TNF-308 (rs1800629). The pain-related SNPs included the following: COMT (rs6269, rs4633, rs4818, and rs4680), OPRM1 (rs1799971), ADRB2 (rs1042713 and rs1042714), AVPR1A (rs1042615 and rs1087796), GCH1 (rs2149482), and KCNS1 (rs734784).
Genotyping
The distribution of the genotypes and genotyping of the 19 SNPs was performed using standard methods as described in our previous publications.20,21 Briefly, DNA was extracted from saliva (buccal swabs) using the PureGene system (Qiagen NV, Venlo, the Netherlands). DNA quality and quantity were verified with spectrophotometry, and sample aliquots were diluted to 10 ng/µL. The DNA samples were genotyped in 96-well plate format using ABI/Life Technologies TaqMan SNP genotyping assays (ABI/Invitrogen Inc., Carlsbad, CA) at the UF Pharmacogenetics Core, with Applied Biosystems 7900 HT platform (Applied Biosystems, Foster City, CA). The plates included several blanks and duplicates for quality control. Distribution of genotypes is summarized in Tables 1 and 2.
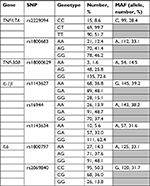  | Table 1 Descriptive statistics for inflammatory genes and SNPs Abbreviations: MAF, minor allele frequency; SNP, single nucleotide polymorphism. |
  | Table 2 Descriptive statistics for pain candidate genes and SNPs Abbreviations: MAF, minor allele frequency; SNP, single nucleotide polymorphism. |
Physical impairment measures
ROM
Shoulder ROM was assessed using a standard plastic goniometer in the following movement planes: forward flexion and abduction (active ROM) and internal rotation (passive ROM). Forward flexion and abduction of the glenohumeral joint was measured actively with the subject in the standing position. Subjects were instructed to either flex or abduct the arm until the end ROM was attained and hold the position until a measurement was obtained. The procedures for measuring active flexion and abduction followed guidelines established by Clarkson28 and have previously been reported in the literature to have good intrarater reliability with intraclass correlation coefficients of ≥0.85. Internal rotation of the glenohumeral joint was measured passively. Subjects were placed in a supine position on a padded table with their shoulder abducted to 90° and their elbow slightly off the table. The stationary arm of the goniometer was held perpendicular to the floor, and the moment arm was aligned with the medial styloid of the ulna. The fulcrum of the goniometer was aligned with the olecranon process of the ulna. The subject was instructed to relax as the examiner passively moved the limb into internal rotation. The end point for internal rotation was determined when the subject’s shoulder began to lift off the table. Each ROM measurement was performed three times, and an average was calculated for that session.
Maximal voluntary isometric contraction (MVIC)
MVIC was measured on a Kin-Com dynamometer (125 AP; Isokinetics International, Chattanooga, TN, USA). Subjects were secured in the Kin-Com with strappings as per recommended standards by the manufacturer. The dominant arm was positioned at 45° of abduction and 45° of external rotation. This position has been found to limit impingement of the rotator cuff under the acromion process.29 The arm remained stationary while subjects pushed against a pad that had a load cell embedded to measure the applied force. The subject performed three trials, and the highest strength measurement was recorded in pounds of force. This measurement was then converted to torque (force × distance). Distance was recorded as the length of the level arm, which remained constant throughout the week.
Shoulder fatigue protocol
Controlled muscle injury was induced using the Kin-Com isokinetic dynamometer. Detailed methods for the exercise-induced injury model have been described in our previous studies,15–21 and a brief description is provided in this paper. An MVIC was determined by having the participants perform three repetitions of maximal isometric shoulder external rotation. The highest torque value was recorded as their MVIC. After initial MVIC was determined, subjects completed maximal isokinetic concentric/eccentric external rotation repetitions to induce an experimental muscle injury. The speed was set at 60°/s for three sets of ten repetitions. Subjects were given 30 seconds of rest between sets. Following the isokinetic repetitions, MVIC was measured, and if subjects could still generate >50% of their initial MVIC, they performed an additional one to eight sets of ten repetitions at 60°/s. This was continued until their peak force was <50% of the initial MVIC. Previous research indicated that the inability to achieve 50% of initial peak MVIC is a consistent indicator of muscle fatigue.30
Statistical analyses
All statistical analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). Summary statistics were calculated for all demographic, psychological, genetic, and shoulder impairment outcome measures. Impairment measures for ROM were recorded as a peak and average value. Impairment measures for strength included a peak and an average value as well as a percentage value. The peak loss value was the greatest deficit measure from baseline (pre-exercise) over the postexercise recovery period, while the average loss value was recorded as the average deficit over the postexercise recovery period. The percentage value for strength was recorded as the ratio of the average of the postexercise period to the pre-exercise, baseline day.
For every pro-inflammatory and pain-related gene, a general linear model was fitted to assess its main effect (genotype level) and a series of expanded models were fitted to study its interaction with five psychological factors for each shoulder impairment outcome. Each model had the same structure with four increments, including 1) demographic data (age, sex, and race), 2) genotype, 3) psychological factor, and 4) the gene-by-psychological factor interaction. In this approach, the inflammatory/pain gene-by-psychological interaction effect was determined individually after accounting for the other predictor variables to identify its unique prediction of variability for the respective shoulder impairment phenotype.
In this secondary analysis, we took a structured approach to interpreting the statistical findings that followed our earlier papers.20,21 Interaction terms with P-values of <0.01 were considered as showing “strong” statistical evidence for predicting the impairment phenotype of interest, whereas those interaction terms with P-values of ≥0.01 but ≤0.05 were considered as showing “moderate” statistical evidence for predicting the impairment phenotype of interest. Interaction terms with P-values of ≥0.05 were not further considered for interpretation. Models meeting our criterion for strong or moderate statistical evidence of a genotype-by-psychological factor interaction are summarized in Tables 3 and 4.
Results
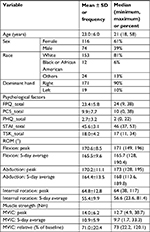
Altogether, 190 subjects completed the study, and the cohort had an average age of 23.0±6.0 years (mean ± SD) with 61% female participants. Sample size was determined a priori using data collected from preliminary studies.18,19 These data provided estimation of effect parameters for the genetic and psychological factors as well as their interactions. These parameters were specified in terms of R2 of the full model and R2 difference between full and the reduced models. The SAS POWER procedure (version 9.2, SAS Institute, Cary, NC, USA) was adopted to evaluate the required sample sizes to achieve a target power of 80% to test each effect at a type I error level of 0.005. Descriptive data (mean ± SD) for participant characteristics, psychological factors, and impairment measures are summarized in Table 5.
Active ROM: flexion
The interaction between IL-1β (rs1143634) and FPQ demonstrated strong statistical evidence for predicting 5-day average loss of active shoulder flexion associated with exercise-induced injury. The complete regression model was able to explain 14.6% of the total variance (P=0.002), while the gene and psychological interaction was able to independently account for 10.5% of the overall variance (P<0.0001; Figure 1). The interaction between IL-1β (rs1143634) and STAI demonstrated strong statistical evidence for predicting loss of peak flexion associated with exercise-induced injury. The complete regression model was able to explain 10.3% of the total variance (P=0.030), while the gene and psychological interaction was able to independently account for 5.7% of the overall variance (P=0.0058; Figure 1).
Active ROM: abduction
The interaction between IL-1β (rs1143634) and FPQ demonstrated strong statistical evidence for predicting loss of 5-day average abduction associated with exercise-induced injury. The complete regression model explained 14.5% of the total variance (P=0.003), while the gene and psychological interaction was able to independently account for 8.5% of the overall variance (P=0.0006; Figure 2). The interaction between IL-1β (rs1143634) and FPQ demonstrated strong statistical evidence for predicting loss of peak abduction associated with exercise-induced injury. The complete regression model was able to explain 9.9% of variance (P=0.026), while the gene and psychological interaction was able to independently account for 7.2% of the overall variance (P=0.0027; Figure 2).
Passive ROM: internal rotation
The interaction between IL-1β (rs1143634) and PHQ demonstrated strong statistical evidence for predicting peak internal rotation loss associated with exercise-induced injury. The complete regression model was able to explain 12.1% of variance (P=0.009), while the gene-by-psychological interaction was able to independently account for 6.2% of the overall variance (P=0.0035; Figure 3).
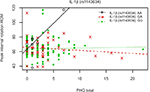  | Figure 3 Interaction of genetic (IL-1β rs1143634) and psychological (PHQ) factors for peak loss of internal rotation ROM. Abbreviations: PHQ, Patient Health Questionnaire; ROM, range of motion. |
Figures 1–3 show that the strength of the interactions for the IL-1β (rs1143634) variant is primarily driven by the AA homozygotes. However, this is a very small group (n=10; 5.6%) making the interaction vulnerable to chance findings.
Maximum voluntary isometric contraction
Two separate genetic and psychological interactions provided strong statistical evidence for predicting post-injury strength loss. The interaction between OPRM1 (rs1799971) and FPQ demonstrated strong statistical evidence for predicting 5-day average MVIC impairment phenotype. The complete regression model was able to explain 62.0% of the total variance (P<0.0001), while the gene and psychological interaction was able to independently account for 2.2% of the overall variance (P=0.0066; Figure 4). The interaction between OPRM1 (rs1799971) and FPQ also demonstrated strong statistical evidence for predicting peak MVIC impairment phenotype. The complete regression model was able to explain 50.4% of the total variance (P<0.0001), while the gene and psychological interaction was able to independently account for 2.9% of the overall variance (P=0.0067; Figure 4). Figure 4 shows that the strength of the interactions for the OPRM1 (rs1799971) variant is primarily driven by the GG homozygotes. This is a very small group (n=10; 5.3%) making the interaction vulnerable to chance findings.
Three separate genetic and psychological interactions had moderate to strong statistical evidence for predicting relative post-injury strength loss. First, in the full regression model for COMT (rs4818) and PCS, total variance explained was an estimated 20.1% (P<0.0001), with the interaction term independently accounting for 5.9% of the overall variance (P=0.0048). Second, in the complete regression model, the OPRM1 (rs1799971) and FPQ total variance explained was an estimated 18.6% (P<0.0001), with the interaction term independently accounting for 4.4% of the overall variance (P=0.0192). Third, in the full regression model for TNF/LTA (rs2229094) and PCS, total variance explained was an estimated 16.3% (P=0.001), with the interaction term independently accounting for 4.3% of the overall variance (P=0.0171).
Discussion
Our primary objective was to investigate how selected pain-related and pro-inflammatory genes interact with pain-associated psychological factors to predict phenotypic changes in shoulder impairment. This particular experimental injury and pain model was selected because it allows us to control and standardize the mechanism of injury (eccentric overload of muscle coupled with fatigue), and it produces a local inflammatory response with associated pain, disability, and functional deficits.16,17,20 This model also allows us to track symptomatic response and physical impairment in a more clinically relevant manner when compared with other experimental pain models that are controllable but of shorter duration (eg, thermal or pressure stimuli). Furthermore, previous findings of increased pain and disability from this validated preclinical exercise model have an established link to a postoperative clinical pain model.31,32
The genotype-by-psychological interactions investigated the combined effect of both factors rather than the predictive value of either factor separately. The results of this study expand upon our previous findings that identified interactions between psychological factors and gene SNPs associated with pain and inflammation involved in heightened pain and disability.20,21 The present study adds to our understanding of genotype-by-psychological interactions by determining how they may predict commonly measured shoulder impairments.
We hypothesized that pro-inflammatory genes will display stronger relationships compared with pain-related genes when combined with psychological factors for predicting physical impairment outcomes. Our findings confirmed this hypothesis by indicating that pro-inflammatory gene variants contribute more to physical impairment, with two pro-inflammatory gene SNPs (IL-1β [rs1143634] and TNF/LTA [rs2229094]) interacting with psychological factors to predict a total of six separate shoulder impairment phenotypes (flexion and abduction: peak and 5-day average loss; internal rotation: peak loss and relative strength loss). In direct comparison, two pain-related gene SNPs (OPRM1 [rs1799971] and COMT [rs4818]) interacted with psychological factors to predict four separate shoulder impairment phenotypes (abduction: 5-day average loss; strength loss: 5-day average, peak, and relative loss). Pro-inflammatory gene polymorphisms have been implicated in extending the length of the acute inflammatory response.33 Elevated levels of pro-inflammatory cytokines both locally at the site of injury and in the bloodstream could extend the peripheral sensitization of nociceptors as well as centrally in the spinal cord, thus leading to prolonged decrements in strength and joint mobility.
The IL-1β (rs1143634) SNP was the most consistent gene variant that interacted with several psychological factors for predicting functional phenotypic changes with induced injury. IL-1β (rs1143634) interacted most with fear of pain in predicting three impairment phenotypes related to loss of shoulder ROM (flexion: 5-day average loss; abduction: 5-day average and peak loss), and it also interacted with anxiety for predicting loss of peak shoulder flexion as well as depressive symptoms for predicting loss of peak internal rotation. All IL-1β (rs1143634) SNP-by-psychological factor interactions provided strong statistical evidence for predicting functional phenotypic changes, indicating that this gene variant may be a significant contributor to shoulder impairment following musculoskeletal injury.
IL-1β (rs1143634) had a significant impact on predicting ROM deficits post injury, interacting with several psychological factors (eg, fear of pain, anxiety, and negative mood) consistently across the different planes of motion, both active and passive. This is an encouraging finding because IL-1β is a cytokine protein produced by many cell types, including activated macrophages, following musculoskeletal injury.34 IL-1β acts locally as an important mediator of acute inflammation and is a known contributor to inflammatory pain hypersensitivity through induction of the cyclo-oxygenase-2 (Cox-2) cascade.35 IL-1β has also been found to induce hyperalgesia directly by lowering nociceptive membrane thresholds for activation or indirectly through upregulation of other pro-nociceptive mediators such as prostaglandin E2, substance P, bradykinin, nerve growth factor (NGF), and calcitonin gene-related peptide (CGRP).34–36 IL-1 polymorphisms have been implicated in the amplification of acute inflammation following resistance exercise. Dennis et al37 found that IL-1 polymorphisms (both haplotypes and individual SNPs) influenced the inflammatory response in skeletal muscle after a single bout of strenuous resistance exercise in young adult men. Individuals with the IL-1β genotype (+3,954 also known as rs1143634) were found to have an increased expression of inflammatory cytokines per macrophage compared with individuals who did not have the genotype. IL-1 region polymorphisms have also been linked to common chronic pain conditions such as knee33 and hand osteoarthritis.38
More recently, IL-1β has been examined as a candidate for susceptibility to depressive disorders.39 Peripheral IL-1β communicates with the brain via neural and humoral pathways to induce brain expression of IL-1β, which elicits mood changes,40 making it a potential candidate for interaction with pain-associated psychological factors for influencing pain perception and physical impairment phenotypes. Interestingly, our regression models indicated that IL-1β (rs1143634) interacted significantly with PHQ (depressive symptoms) and STAI (anxiety) to predict peak deficits in active shoulder flexion and passive internal rotation post injury. This is an indication that those with the variation in IL-1β will have worse deficits when linked with depressive symptoms and anxiety. IL-1β appears to be a good candidate for increased study due to its versatility as a modifier of biological (inflammation and pain) as well as psychological processes (mood changes and depressive symptoms).
IL-1β is an important mediator of acute inflammation and contributes to inflammatory pain hypersensitivity. Elevated IL-1β gene expression in and around injured tissue could act as a source of heightened inflammatory pain and could lead to impaired limb movement and function. The combination of heightened inflammatory pain and risk of fear-avoidance behavior could be a robust predictor of physical impairment. Continued research on IL-1 polymorphisms is necessary to confer the risk of heightened pain and physical impairments following musculoskeletal injury.
Post-injury strength loss was another functional phenotype that was predicted by three gene SNPs: two were pain-related (COMT rs4818 and OPRM1 rs1799971) and one was pro-inflammatory (TNF/LTA rs2229094). Pain catastrophizing and fear of pain were two psychological factors that interacted significantly with the gene SNPs to predict relative post-injury strength loss. The ability of a muscle to generate force can be negatively influenced by a number of factors, including heightened pain sensitivity, increased tissue inflammation, and maladaptive pain-coping strategies. Lingering symptoms from the induced injury in combination with heightened pain sensitivity and higher pain catastrophizing and fear of pain (indicative of maladaptive pain coping) could have likely contributed to deficits in force production and the delayed recovery of strength. Previous studies were able to show that interactions between COMT genotypes and pain catastrophizing were strong predictors of shoulder pain and disability more than either factor alone.18–20 In a preclinical cohort, we identified a subgroup composed of patients with a COMT genotype associated with low enzyme activity plus elevated pain catastrophizing that was at higher risk for increased pain intensity and delayed recovery from the induced shoulder injury. In a separate clinical pain cohort, the high-risk subgroup was validated by demonstrating that the subgroup from the clinical pain cohort experienced significantly poorer 12-month postsurgical outcomes.32 These findings provide further evidence that the COMT genotype coupled with elevated pain catastrophizing is a robust predictor, and these additional findings provide new insights into how this interaction predicts pain and prolonged physical impairment following shoulder injury.
Overall, fear of pain was the most consistent psychological factor interacting with six SNPs totally (three pro-inflammatory and three pain-related genes), followed by pain catastrophizing (two SNPs), anxiety (one SNP), and depressive symptoms (one SNP) in predicting deficits in six separate functional phenotypes post injury (5-day average loss of shoulder flexion and abduction; peak loss of abduction; and 5-day average, peak, and relative loss of shoulder strength post injury). Our data suggest that therapeutic approaches that focus solely on the inflammatory response may fail to meet the standards of full recovery if you ignore the potential for psychological factors to amplify the effects of musculoskeletal injury and inflammatory pain.
The results of this study along with our other studies18–21 have identified multiple genetic and psychological factors that when considered simultaneously are predictive of symptomatic responses that may be relevant for the transition from acute to chronic pain states. These analyses add to the existing literature in this area by demonstrating how genetic and psychological factors (in combination) also may predict prolonged physical impairment that could also play an important role in the development of impaired movement that co-occurs with chronic pain states. This is an important issue for clinical practice, because there is considerable debate about application of biopsychosocial approaches to understanding pain, but very few studies incorporate biological and psychological combinations as we have.
Future research in clinical populations should consider the combination of genetic and psychological factors when predicting physical impairment outcomes. Broadening this approach during the screening of clinical populations is important to determine the ecological validity of these predictors for physical impairment phenotypes and to identify potential treatment targets that could be used to develop tailored therapeutic intervention strategies that would improve physical impairment before the development of a chronic condition. In addition, future research should attempt to replicate our findings in other anatomical regions that commonly experience musculoskeletal injuries (eg, back, neck, and knee).
Conclusion
Pro-inflammatory gene variants contributed more to physical impairment with 2 SNPs [IL-1β (rs1143634) and TNF/LTA (rs2229094)] interacting with psychological factors to predict 6 shoulder-impairment phenotypes. In comparison, 2 pain-related gene SNPs [OPRM1 (rs1799971) and COMT (rs4818)] interacted with psychological factors to predict 4 shoulder-impairment phenotypes (abduction: 5-day average loss; strength loss: 5-day average, peak, and relative).
Acknowledgments
The authors would like to thank Alberto Bursian, Brianna Castillo, Lauren Hardin, Andy Hogan, Kelly Larkin Kaiser, Natalie Martinez, Pamela McCurdy, Rachel Montgomery, Hannah Spilker, and Nhi Thieu who assisted with exercise-induced injury protocol and data collection. They also thank Will Eaton and Michelle Burgh for assisting with genetic analyses. This study was completed with funding from the National Institutes of Health – National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR055899) and National Institute of Neurological Disorders and Stroke (NS045551).
Author contributions
PAB conceived and designed the research, interpreted the results of experiments, drafted the manuscript, edited and revised the manuscript, and approved the final version of the manuscript. JJP performed the experiments, edited and revised the manuscript, and approved the final version of the manuscript. MRW performed the experiments, interpreted the results of experiments, drafted the manuscript, edited and revised the manuscript, and approved the final version of the manuscript. SSW and YD analyzed the data, prepared the figures, edited and revised the manuscript, and approved the final version of the manuscript. RBF conceived and designed the research, interpreted the results of experiments, drafted the manuscript, edited and revised the manuscript, and approved the final version of the manuscript. SZG conceived and designed the research, interpreted the results of experiments, drafted the manuscript, edited and revised the manuscript, and approved the final version of the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The authors report no conflicts of interest in this work.
References
Roquelaure Y, Ha C, Leclerc A, et al. Epidemiologic surveillance of upper-extremity musculoskeletal disorders in the working population. Arthritis Rheum. 2006;55(5):765–778. | ||
Sein ML, Walton J, Linklater J, et al. Shoulder pain in elite swimmers: primarily due to swim-volume-induced supraspinatus tendinopathy. Br J Sports Med. 2010;44(2):105–113. | ||
Tate A, Turner GN, Knab SE, Jorgensen C, Strittmatter A, Michener LA. Risk factors associated with shoulder pain and disability across the lifespan of competitive swimmers. J Athl Train. 2012;47(2):149–158. | ||
Mcfarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10–13. | ||
Powell JW, Barber-Foss KD. Injury patterns in selected high school sports: a review of the 1995-1997 seasons. J Athl Train. 1999;34(3):277–284. | ||
Hakala P, Rimpelä A, Salminen JJ, Virtanen SM, Rimpelä M. Back, neck, and shoulder pain in Finnish adolescents: national cross sectional surveys. BMJ. 2002;325(7367):743. | ||
Roy JS, Macdermid JC, Boyd KU, Faber KJ, Drosdowech D, Athwal GS. Rotational strength, range of motion, and function in people with unaffected shoulders from various stages of life. Sports Med Arthrosc Rehabil Ther Technol. 2009;1:4. | ||
van der Heijden GJ . Shoulder disorders: a state-of-the-art review. Baillieres Clin Rheumatol. 1999;13(2):287–309. | ||
Croft P, Pope D, Silman A. The clinical course of shoulder pain: prospective cohort study in primary care. Primary Care Rheumatology Society Shoulder Study Group. BMJ. 1996;313(7057):601–602. | ||
van der Windt DA, Koes BW, Boeke AJ, Devillé W, de Jong BA, Bouter LM. Shoulder disorders in general practice: prognostic indicators of outcome. Br J Gen Pract. 1996;46(410):519–523. | ||
Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders – pathways of vulnerability. Pain. 2006;123(3):226–230. | ||
Scholich SL, Hallner D, Wittenberg RH, Hasenbring MI, Rusu AC. The relationship between pain, disability, quality of life and cognitive-behavioural factors in chronic back pain. Disabil Rehabil. 2012;34(23):1993–2000. | ||
Domenech J, Sanchis-Alfonso V, López L, Espejo B. Influence of kinesiophobia and catastrophizing on pain and disability in anterior knee pain patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1562–1568. | ||
Mclean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12(1):101–107. | ||
Parr J, Borsa P, Fillingim R, et al. Psychological influences predict recovery following exercise induced shoulder pain. Int J Sports Med. 2014;35(3):232–237. | ||
Parr JJ, Borsa PA, Fillingim RB, et al. Pain-related fear and catastrophizing predict pain intensity and disability independently using an induced muscle injury model. J Pain. 2012;13(4):370–378. | ||
George SZ, Dover GC, Fillingim RB. Fear of pain influences outcomes after exercise-induced delayed onset muscle soreness at the shoulder. Clin J Pain. 2007;23(1):76–84. | ||
George SZ, Dover GC, Wallace MR, et al. Biopsychosocial influence on exercise-induced delayed onset muscle soreness at the shoulder: pain catastrophizing and catechol-o-methyltransferase (COMT) diplotype predict pain ratings. Clin J Pain. 2008;24(9):793–801. | ||
George SZ, Wallace MR, Wright TW, et al. Evidence for a biopsychosocial influence on shoulder pain: pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict clinical pain ratings. Pain. 2008;136(1-2):53–61. | ||
George SZ, Parr JJ, Wallace MR, et al. Biopsychosocial influence on exercise-induced injury: genetic and psychological combinations are predictive of shoulder pain phenotypes. J Pain. 2014;15(1):68–80. | ||
George SZ, Parr JJ, Wallace MR, et al. Inflammatory genes and psychological factors predict induced shoulder pain phenotype. Med Sci Sports Exerc. 2014;46(10):1871–1881. | ||
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. | ||
Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State and Trait Anxiety Inventory (Form Y). Palo Alto, CA: Consulting Psychologists Press. 1983. | ||
Mcneil DW, Rainwater AJ. Rainwater AJ 3rd. Development of the Fear of Pain Questionnaire-III. J Behav Med. 1998;21(4):389–410. | ||
Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117(1–2):137–144. | ||
Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7(4):524–532. | ||
Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23(4):351–365. | ||
Clarkson HM. Musculoskeletal Assessment: Joint Range of Motion and Manual Muscle Strength. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2000:110–114. | ||
Plotnikoff NA, Macintyre DL. Test-retest reliability of glenohumeral internal and external rotator strength. Clin J Sport Med. 2002;12(6):367–372. | ||
Carpenter JE, Blasier RB, Pellizzon GG. The effects of muscle fatigue on shoulder joint position sense. Am J Sports Med. 1998;26(2):262–265. | ||
George SZ, Staud R, Borsa PA, et al. Biopsychosocial influence on shoulder pain: Rationale and protocol for a pre-clinical trial. Contemp Clin Trials. 2017;56:9–17. | ||
George SZ, Wallace MR, Wu SS, et al. Biopsychosocial influence on shoulder pain: risk subgroups translated across preclinical and clinical prospective cohorts. Pain. 2015;156(1):148–156. | ||
Jotanovic Z, Etokebe GE, Mihelic R, et al. IL1B -511(G>A) and IL1RN (VNTR) allelic polymorphisms and susceptibility to knee osteoarthritis in Croatian population. Rheumatol Int. 2012;32(7):2135–2141. | ||
Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60(1):57–64. | ||
Samad TA, Moore KA, Sapirstein A, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410(6827):471–475. | ||
Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361(1–3):184–187. | ||
Dennis RA, Trappe TA, Simpson P, et al. Interleukin-1 polymorphisms are associated with the inflammatory response in human muscle to acute resistance exercise. J Physiol. 2004;560(Pt 3):617–626. | ||
Moxley G, Han J, Stern AG, Riley BP. Potential influence of IL1B haplotype and IL1A-IL1B-IL1RN extended haplotype on hand osteoarthritis risk. Osteoarthritis Cartilage. 2007;15(10):1106–1112. | ||
Misener VL, Gomez L, Wigg KG, et al. Tagging SNP association study of the IL-1beta gene (IL1B) and childhood-onset mood disorders. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(5):653–659. | ||
Dantzer R, Cytokine DR. Cytokine, sickness behavior, and depression. Neurol Clin. 2006;24(3):441–460. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.