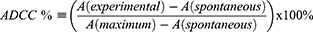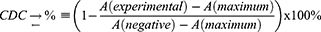Back to Journals » International Journal of Nanomedicine » Volume 18
Generation of a CD70-Specific Fusion Nanobody with IgG Recruiting Capacity for Tumor Killing
Authors Liu C, Li J, Hu Q, Xu X, Zhang X
Received 15 March 2023
Accepted for publication 2 June 2023
Published 19 June 2023 Volume 2023:18 Pages 3325—3338
DOI https://doi.org/10.2147/IJN.S410281
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Israel (Rudi) Rubinstein
Chang Liu, Jiangwei Li, Qianqian Hu, Xinlan Xu, Xin Zhang
Xinjiang Key Laboratory of Biological Resources and Genetic Engineering, College of Life Science and Technology, Xinjiang University, Urumqi, People’s Republic of China
Correspondence: Jiangwei Li, Xinjiang Key Laboratory of Biological Resources and Genetic Engineering, College of Life science and technology, Xinjiang University, 777 Huarui Street, Urumqi, 830046, People’s Republic of China, Email [email protected]
Purpose: Due to its competitive advantages such as small size, high stability, easy production, and good tissue penetration compared with monoclonal antibodies (mAb), nanobodies (Nbs) were considered the next generation of therapeutics. However, the absence of Fc fragments and Fc-triggered immune effectors limits their clinical applications. In order to overcome these limitations, we develop a novel approach by attaching an IgG binding domain (IgBD) to Nbs for recruiting endogenous IgG and recovering the immune effectors for tumor killing.
Material and Methods: We linked a Streptococcal Protein G-derived IgBD, termed C3Fab, at the C-terminus of a CD70-specific Nb 3B6 to construct an endogenous IgG recruitment antibody (termed EIR). The recombinant Nb3B6-C3Fab was expressed in E. coli BL21 (DE3) and purified by nickel affinity chromatography. We further evaluated the binding, recruitment of IgG, and the serum half-life of Nb3B6-C3Fab. The tumor-killing effects on CD70 positive cells mediated by antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity were also detected.
Results: We successfully constructed a IgBD fused Nb3B6-C3Fab with high affinity for CD70 and mouse IgG (mIgG). Nb3B6-C3Fab can specifically bind to CD70 positive tumor cells and recruit mIgG on the cell surface. Ligating of Nb3B6 with C3Fab increased its serum half-life in mice almost 39-fold from 0.96 h to 37.67 h. Moreover, we demonstrated remarkable cytotoxicity of Nb3B6-C3Fab to CD70 positive tumor cells via C3Fab by immune effector cells.
Conclusion: Our study demonstrates that IgBD fusion endows Nbs with the ability for endogenous IgG recruitment and half-life promotion. Linking IgBD to Nbs is an effective strategy to recovering immune effectors for tumor killing.
Keywords: CD70, nanobody, endogenous IgG recruitment, IgG binding domain, serum half-life, tumor killing
Introduction
In the past decade, immunotherapy has made great progress in the treatment of hematological and solid cancers by targeting immune checkpoint molecules such as CTLA-4, PD-1, and PD-L11. However, the clinical application of cancer immunotherapy is quite limited due to the existing primary, adaptive, and acquired resistance,2 and only a minority of patients benefit from the therapy. Therefore, there is a need to develop novel targets for immunotherapy.
CD70 is a type II transmembrane glycoprotein that belongs to the tumor necrosis factor (TNF) family. Its binding with receptor CD27 activates signal transduction and differentiates T and B cells.3–5 However, CD70 expression is usually restricted to activated T cells, B cells, and mature dendritic cells in normal tissues6–8 while being highly expressed in hematological and solid tumors, such as B-cell lymphoma (BCL, 71%), diffuse large B-cell lymphoma (DLBCL, 72%), chronic lymphocyte leukemia (CLL, 50%), renal cell carcinomas (RCC, 87%), glioblastoma (42%), pancreatic (25%), lung (16.3%), ovarian (15%), melanoma (15%), colon (9%) and breast cancer (2%).9–11 The overexpression of CD70 in tumors may contribute to immune escape and promote tumor survival and growth,11,12 making CD70 an attractive target for tumor immunotherapy.
Currently, only one CD70-targeted monoclonal antibody (mAb), cusatuzumab (ARGX-110)13 has entered clinical trials and is expected to be approved soon. In addition, several CD70-targeted ADC and CAR-T therapeutic agents, including SNG-70A, SEA-CD70, SNG-75,14,15 have been tested in clinical trials. However, all the tested CD70-targeted agents are mAbs, despite better performance and success in targeting, mAbs have several significant disadvantages such as large size, low permeability to solid tumors, complex manufacturing process, high production costs, relatively low stability, and the need for intravenous administration. Therefore, there is a need to develop the next generation therapeutics that ideally combine the advantages of small molecules with the benefits of mAbs.
Nbs are naturally the smallest antibody fragments derived from camel heavy-chain antibody (HCAb), consisting of only a single antigen-binding domain. In addition to their small size, Nbs have several advantages over conventional mAbs, including preferential binding with cryptic epitopes in the cleft of receptors, high stability, ready-to-be-engineered, and easy to be manufactured in microorganisms,16–18 which makes them ideal candidates for use as blockers or agonists in the development of next-generation cancer therapeutics.
However, there are significant challenges in developing Nb-based therapies for clinical use. The anti-tumor effectors of mAbs are typically produced through the Fc domain, which triggers antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cell-mediated phagocytosis (ADCP). Nbs lack Fc fragments, making them pharmacokinetically inferior, and they cannot elicit a potent immune response in cancer immunotherapy. Currently, Nbs are primarily used as imaging, detecting, and diagnostic agents, and Nb-based therapeutics are rarely developed for clinical use. Up to now, there are only three approved Nb drugs, including caplacizumab, the first approved Nb drug by the EMA and FDA for the treatment of rare blood clotting disorders in adults with acquired thrombotic thrombocytopenic purpura (TPP),19 envafolimab, a subcutaneously administered anti-PD-L1 Nb approved in China for the treatment of various solid tumors20 and ozoralizumab (Nanozora®), a trivalent anti-TNFα Nb compound approved in Japan for the treatment of rheumatoid arthritis.21 All of these Nb drugs had to be fused with the human IgG Fc region to restore immune functions in therapeutics, which compromises the advantages of Nbs, such as cost-effective production and multiple routes of administration. Furthermore, Fc-fused Nbs performed unsatisfactorily in clinical trials due to Fc-mediated undesirable immune responses and non-specific binding. Therefore, alternative strategies are required to improve Nb pharmacokinetics and achieve potent biological functions cost-effectively for clinical use.
IgG is one of the most abundant proteins in plasma and keeps a long half-life (~19 days) in circulation via the FcRn recycling pathway.22 Previous studies demonstrated that binding to IgG elongates the circulation time of recombinant proteins.23 A few immunoglobulin-binding domains (IgBDs) were identified from bacterial proteins, including protein A (SPA) from S. aureus and protein G (SPG) from Streptococcus. Recently, an IgBD from streptococcal mutant protein G (SpGC3) was generated and demonstrated with high affinity to selectively bind the Fab region of IgG and elongate the half-life of small therapeutic recombinant proteins. We proposed that ligation of IgBD with Nbs would either enhance its half-life and recruit the abundant endogenous IgGs in serum and thus generate immune effectors to kill CD70 expressed tumor cells.
To test the hypothesis, we selected SpGC3 (termed as C3Fab in our study) as IgBD and constructed a C3Fab-fused CD70 targeted Nbs as endogenous IgG recruited module. As shown in Figure 1, these Nbs were able to bind CD70 on cancer cells and recruit NK effector cells via Fc-FcR interaction and kill cancer cells by ADCC and CDC.
 |
Figure 1 The proposed mechanism of ADCC and CDC effects on cancer cell mediated by CD70 targeted EIR nanobody VHH-C3Fab. EIR, endogenous IgG recruiting. |
Material and Methods
Cell Lines and Bacterial Strains
Several human cancer cell lines were used in this study, including renal clear adenocarcinoma 786-O, pancreatic adenocarcinoma Panc-1, Burkitt’s lymphoma Raji, and ovarian cancer SKOV-3. These cells were purchased from PUMC National Cell Resource Corporation (Peking Union Medical College, Beijing, China) and cultured in RPMI-1640 medium (Gibco, Invitrogen) supplemented with 10% heat‐inactivated Fetal Bovine Serum (FBS; Gibco) and 1% penicillin-streptomycin at 37°C with 5% CO2. All cell lines were cultured for less than 6 passages before experiments. The host strains Escherichia coli (E. coli) DH5α and BL21 (DE3) were purchased from New England Biolabs (Neb, Beijing branch) and were used for cloning and expressing recombinant proteins, respectively.
Animals
Female Balb/c mice were purchased from Experimental Animal Center of Xinjiang Medical University. All animal experiments were approved by the Committee on the Ethics of Animal Experiments of Xinjiang Key Laboratory of Biological Resources and Genetic Engineering (No. XJUAE-2022-026) and performed under the guidelines of the Animal Care and Use Committee of College of Life Science and Technology, Xinjiang University.
Reagents and Antibodies
LDH cytotoxicity assay kit, CCK8 assay kit, and BCA protein quantification kit were purchased from Beyotime (Shanghai, China). The recombinant human CD70 (hrCD70) used in the study was obtained from Sinobiological (Beijing, China). Mouse anti-human CD70 mAb (Cat. No. 355,101) was purchased from Biolegend (San Diego, USA). HRP-conjugated mouse anti-His and anti-HA mAb and goat anti-mouse IgG pAb were purchased from GeneScript (Nanjing, China). FITC-conjugated Goat anti-mouse IgG was purchased from Sinobiological (Beijing, China), and all other materials and reagents were acquired commercially.
Recombinant Protein Expression and Purification
In order to better maintain Nb activity, a Fab-selective IgBD, called C3Fab from Streptococcal Protein G, was used and ligated to the C-terminus of Nb3B6 with a flexible linker (GGGGSGGGGS). The DNA sequences of the designed Nb3B6-C3Fab proteins were optimized based on the codon bias of E. coli and synthesized by Genewiz company (Suzhou, China). The synthesized gene was then cloned into the pET21a vector at the Bam HI and Xho I restriction sites to construct pET21a-Nb3B6-C3Fab recombinant plasmid, which was transformed into E. coli BL21 (DE3) for protein expression. The transformed BL21-pET21a-Nb3B6-C3Fab cells were grown with LB medium containing 0.1 µg/mL ampicillin and incubated with shaking until the OD600 reached 0.5–0.6. Isopropyl β-Dthiogalactoside (IPTG) was then added to a final concentration of 0.1mM in the medium, and the cells were incubated at 16 °C for 12 h with shaking at 150 rpm/min. The next day, the bacteria were pelleted by centrifuging at 8000 rpm for 15 min and resuspended in cold lysis buffer twice, and subjected to sonication for 45 min while keeping cold. The supernatant was collected, and His-tagged recombinant proteins were purified using Ni-NTA affinity chromatography (Qiagen, USA). The purified Nb3B6-C3Fab proteins were ultrafiltrated to remove ions, and the purity was examined by SDS-PAGE. Protein concentration was measured using a protein BCA assay kit.
Detecting the binding of EIR Nb to mouse IgG and serum with ELISA.
To detect the binding of EIR Nb to mIgG, a 96-well ELISA plate (BIOFIL, China) was coated with 100 ng/well of mIgG in carbonate buffer (pH 9.6) overnight at 4 °C. After washing with PBST and blocking with 5% skimmed milk powder for 2 h at 37 °C, a series of diluted Nbs (0.001–1000 nmol/L) were added and incubated for 1.5 h at 37 °C. Plates were washed 5 times with PBST. For detection of bound Nbs, HRP-conjugated anti-His tag antibody was diluted 1:3000 and incubated at 37 °C for 1 h. Peroxidase enzyme activity was determined after the addition of TMB (3,3’,5,5’-tetramethylbenzidine) substrate, and the optical density was measured at 450 nm in a plate reader.
To detect the binding of EIR Nb to mouse serum, a captive ELISA was conducted as the same protocol as above except coating with Nbs (100 ng/well) and incubating with series of diluted mouse serum (1:10,1:100 and 1:1000). For detection of bound mouse serum, HRP-conjugated rabbit anti-mouse IgG mAb (1:1000) was used.
Competitive Cell ELISA for EIR Nb Affinity on Cell Binding
To determine the cell-binding ability of Nb3B6-C3Fab, a total of 3 × 103 786-O cells were inoculated into 96-well plates to allow growth for 12 hours. Cells were fixed with 4% paraformaldehyde for 15 min on ice after washing with PBS and removing of culture medium. Then, the wells were blocked with 3% bovine serum albumin (BSA) at 37°C for 1.5 h. Subsequently, increasing concentrations (ranging from 0.0067 to 22.42 nmol/L) of hrCD70 proteins mixed with 3 μg/mL of the Nb3B6-C3Fab were added to incubate at 37°C for 1 h. After washing, a 1:3000 dilution of HRP-conjugated anti-His mAb as the secondary antibody was added to incubate at 37°C for 1 h for detecting the Nb binding. The color development and OD measurement were performed as described above.
Detecting the Cell Surface Expression of CD70 with Flow Cytometry
To detect the cell surface expression of CD70, several tumor cells were stained by anti-CD70 mouse mAb in flow cytometry (FCM). Firstly, cells were cultured for 3 to 4 days to allow 80% confluence. A total of 1 × 106 cells in FCM buffer (2% BSA in PBS) were collected. Then, 100 µL of anti-CD70 mouse mAb (1:500) were added to the cells on ice for 45 min. An isotypic mouse IgG was used as negative control. After washing with PBST, cells were treated with a FITC-conjugated rabbit anti-mouse IgG mAb (1:1000) and incubated on ice for another 45 min in the dark. At last, cells were washed and detected on FACS (BD FACSCalibur, USA). The results were analyzed on FlowJo software.
Immunofluorescence
To detect the binding of EIR Nb to CD70 positive cancer cells and recruiting endogenous mIgGs onto cell surfaces, the immunofluorescence experiment was conducted. Tumor cells were seeded in 12-well plates at 3000 cells per well and allowed to grow overnight until 70–80% confluence was reached. Then, medium was discarded and washed with PBST twice, and cells were fixed with 4% paraformaldehyde for 15 min. After washing, wells were blocked with 3% BSA at 37°C for 1.5 h. A 200 µL of a mixture containing 3 µg/mL of Nb3B6-C3Fab and 3 µg/mL of mIgG were added to the cells and incubated at 37°C for 30 min. Meanwhile, 3 µg/mL of Nb3B6 and Nb1D1-C3Fab mixed with 3 µg/mL of mIgG were also added as negative and irrelevant control, respectively. The cells were then washed 3 times and treated with FITC-conjugated rabbit anti-mouse IgG mAb (1:1000) and incubated for another 30 min at 37°C in the dark. After washing with PBST, the cell nucleus was stained with 100 µL of 4’,6-diamidino-2-phenylindole (DAPI) solution at room temperature for 15 min. Finally, the cells were imaged with a fluorescence microscope and processed using ImageJ.
Quantitative Evaluation of IgG-Recruiting Ability of EIR Nbs Using FCM
To evaluate the IgG-recruiting ability of EIR Nbs quantitatively, CD70 positive cells were cultured for 3 to 4 days to allow 80% confluence. A total of 1 × 106 cells in FCM buffer (2% BSA in PBS) were collected. Then, 100 µL of Nbs diluted in a concentration of 0.3–243 nmol/L were added to the cells and incubated on ice for 45 min, and then, mouse IgG was added. After incubation on ice for 30 min and washing, cells were treated with a FITC-conjugated anti-mouse IgG mAb (1:1000) and incubated on ice for 45 min in the dark. The FACS detection and analysis was conducted as above.
In vivo Pharmacokinetics (PK) Assay
In order to determine the half-life of Nb and its C3Fab fusion in serum, six Balb/c mice were divided into two groups, each containing three mice. Nb3B6 and Nb3B6-C3Fab proteins were intravenously (i.v.) injected into the tail vein of each Balb/c mouse at a dose of 2 mg/kg and a volume of 100 µL. Blood samples were collected from the orbit at 0.05, 0.16, 0.5, 1, 2, 6, 12, 24, 36, 48, 72, 96 h, and serum was isolated and stored at −20°C. A sandwich ELISA was developed using T7- and His-tag, which simultaneously occurred in Nbs, to create standard curves. A T7-Tag specific mouse mAb was used as captive Ab and was coated on the 96-well plate at 1:1000 dilution overnight at 4°C. After washing with PBST, wells were blocked with 5% skimmed milk powder at 37 °C for 2 h. Nbs at different concentrations ranging from 0 to 16.384 µg/mL were added and incubated at 37°C for another 2 h. After incubation with HRP conjugated anti-His mAb and colorimetric substrate TMB, the OD450 measurement was performed as described above. To detect Nbs in mouse serum, the same procedure was performed except the mouse serum collected at different times was diluted 1:100 and added to the T7-Tag mAb coated plate. The concentration of Nbs in blood was calculated from the standard curve and plasma half-lives and clearance rates were obtained using PK parameters.
ADCC Assay
To evaluate the ADCC effects of Nb3B6-C3Fab on CD70 overexpressed tumor cells, peripheral blood mononuclear cells (PBMC) were isolated from mouse blood by a Ficoll-Hypaque isolation kit and used as effector cells. CD70 overexpressed tumor cells were used as target cells. Tumor cells were seeded in 96-well plates at 5 × 103/well and incubated overnight. The next day, 50 µL of different concentrations of Nb3B6-C3Fab (20,40,80,160 and 320 nmol/L) and 50 µL of 2% mouse serum were added and incubated at 37°C for 2 hours. After washing, freshly isolated PBMC at 8 × 104/well were added and incubated at 37°C for 4 h. The ADCC effects of Nbs on target cells were assayed using an LDH cytotoxicity kit, and the cell lysis rate was calculated using the following equation:
where A (experimental) is the OD490 value of LDH released from tumor cells treated with Nb samples in the presence of mouse serum and PBMC; A (spontaneous) is the OD490 value of LDH released from spontaneous death of tumor cells; A (maximum) is the OD490 value at the maximum enzyme activity. A (spontaneous) is the OD490 value of LDH released from spontaneous death of tumor cells.
CDC Assay
To evaluate the ADCC effects, CD70 overexpressed tumor cells were seeded in 96-well plates at 5 × 103 cells/well and incubated overnight. The next day, 100 µL of Nb3B6-C3Fab at different concentrations (0.08, 0.8, 8 and 80 nmol/L) were mixed with 9% mouse serum and added to the wells of plate and incubated at 37°C for 2 h. The heat-inactivated mouse serum was also added as a negative control. After washing, the cell viability was assayed using an Cell Counting Kit-8 kit incubated at 37°C for 4 h in dark. The maximum cell lysis was obtained at cells treated with 1% Triton X-100. The cell lysis was calculated using the following equation:
where A (experimental) is the OD450 value of cells treated with Nb samples in the presence of mouse serum; A (maximum) is the OD450 value of cells completely lysed with 1% Triton X-100. A (negative) is the OD450 value of cells treated with PBS.
Statistical Analysis
GraphPad Prism 8.0 software was used for statistical analyses. The statistical significance level was set at P < 0.05. The results are expressed as mean ± standard error of the mean (SEM). The level of statistical significance was indicated in the figures with asterisks. Where * indicated P < 0.05, ** indicated P < 0.01, *** indicated P < 0.001.
Results
Design and Expression of Endogenous IgG Recruiting CD70 Nbs
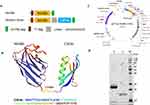
Human CD70 Nanobody Nb3B6 was identified by our group from a camel-derived VHH phage library reported in a previous study.24 Owing to the lack of an Fc region, it cannot trigger immune effectors in cancer immunotherapy. For recovery of its Fc-mediated function, we designed a bifunctional Nb by linking the CD70 target Nb with an IgG binding domain (IgBD). This Nb was termed endogenous IgG recruiting Nb (abbreviated to EIR Nb) in this work. A Fab-selective IgBD named C3Fab from Streptococcal Protein G25 was ligated to the C-terminus of the nanobody via the GGGGSGGGGS linker and served as an EIR module (Figure 2A). The 3D structure of Nb3B6-C3Fab was predicted with SWISS-MODEL. C3Fab contains four β-strands and oneα-helix as shown in Figure 2B. It linked with Nb3B6 by a flexible (G4S)2 linker at the C-terminus. The 3D model indicated that C3Fab might cause less steric hindrance effects on Nb. Previous study25 suggested C3Fab binds only the Fab region in IgG and does not interfere with Fc-FcR interaction. For expression, Nb3B6-C3Fab was cloned, and the recombinant plasmid pET21a-Nb3B6-C3Fab was constructed (as shown in Figure 2C). The fusions were endowed with a His-tag at the C-terminus and a T7-tag at the N-terminus and expressed in E. coli BL21 (DE3) cells by isopropyl-β-d-thiogalactopyranoside (IPTG) induction and purified by immobilized metal affinity chromatography. The purified proteins were analyzed by SDS-PAGE. Nb3B6 and Nb3B6-C3Fab appeared around 16 kDa and 23 kDa, respectively, which was consistent with the calculated molecular weights (Figure 2D).
Analysis of the Binding of EIR Nb with CD70 and mIgG
For evaluating the CD70 binding affinity of Nb3B6 after ligated with C3Fab, a competitive cell – ELISA was used to measure the CD70 positive cell binding rate of Nbs in the presence of increasing concentrations of rCD70 proteins. The bindings were detected by HRP conjugated anti-His secondary mAb. As shown in Figure 3A, Nb3B6-C3Fab and Nb3B6 bind with CD70 in a concentration-dependent manner, with an IC50 of 1.56 and 7.90 nmol/L, respectively, displayed high affinity to CD70. It was noted that ligation of C3Fab slightly increased the affinity of EIR Nb to CD70.
Subsequently, the binding of EIR Nb with mIgG was detected with an indirect ELISA. Various concentrations of Nb3B6-C3Fab were incubated with immobilized mIgG in ELISA plate, and HRP-modified anti-His mAb was used as secondary antibodies. Nb3B6 was used as a negative control. As shown in Figure 3B, Nb3B6-C3Fab bound mIgG in a concentration-dependent manner, while no binding signal was observed in Nb3B6 treated group. Furthermore, to test the binding of EIR Nb to mIgG in serum, a sandwich ELISA was performed, in which Nb3B6-C3Fab were coated as captive antibodies and incubated with various dilutions of mouse serum, and HRP-conjugated anti-mIgG was used as detecting antibodies. As shown in Figure 3C, EIR Nb positively binds serum IgG above the 1:1000 dilutions. Taken together, these results suggested that EIR Nb could specifically bind with CD70 and mIgG with a high affinity.
CD70 Overexpression on Multiple Cancer Cells
It was reported CD70 is overexpressed in several tumors. To comprehensively investigate CD70 expression across different tumor types, we used The Cancer Genome Atlas (TGGA) dataset to compare the CD70 gene differential expression between tumor and adjacent normal tissues. As shown in Figure 4A that CD70 was highly expressed in a number of tumor types, tumors including BRCA (Breast invasive carcinoma), CHOL (Cholangiocarcinoma), COAD (colon adenocarcinoma), ESCA (esophageal carcinoma), HNSC (Head and Neck squamous cell carcinoma), KICH (Kidney Chromophobe), KIRC (Kidney renal clear cell carcinoma), KIRP (Kidney renal papillary cell carcinoma), LIHC (Liver hepatocellular carcinoma), LUAD (lung adenocarcinoma), LUSC (lung squamous cell carcinoma), STAD (Stomach adenocarcinoma), THCA (thyroid carcinoma) and UCEC (Uterine Corpus Endometrial Carcinoma). These results suggested that the expression of CD70 was up-regulated on multiple tumors, compared with the corresponding normal tissues. To validate these findings experimentally, we used flow cytometry with commercially available anti-CD70 monoclonal to detect CD70 expression on four available human tumor cell lines, including 786-O (renal clear cell adenocarcinoma), SKOV-3 (Ovarian adenocarcinoma), Panc-1 (pancreatic carcinoma), and Raji (Burkitt’s lymphoma). As shown in Figure 4B, CD70 was highly expressed in these cell lines compared with the negative control. These experimental results support the in silico findings.
Recruitment of Mouse IgG on the Surface of CD70 Expressing Cancer Cells by EIR Nb
Firstly, we assessed the binding ability of EIR Nb with CO70 positive cells using FCM. We incubated 786-O and Panc-1 cells with different concentrations of Nb3B6 and Nb3B6-C3Fab, respectively, and detected the bindings using a fluorescent anti-His mAb in FACS. An irrelevant Nb 1D1-C3Fab was used as a negative control. As shown in Figure 5A, the median fluorescence intensity (MFI) of Nb3B6-C3Fab and Nb3B6 staining groups was significantly enhanced and displayed specifically and dose-dependent binding with the two cancer cells, while 1D1-C3Fab did not give any binding signals. Nb3B6-C3Fab bound 786-O cells stronger than Panc-1 (p<0.05) based on their EC50 values (19.5 and 24.85 nM, respectively).
Next, we provided evidence that endogenous IgG in mouse serum could be recruited on the CD70 positive tumor cells where Nb3B6-C3Fab binds. In immunofluorescence experiment, 786-O and Panc-1 were seeded in 12-well plates and treated with Nb3B6, Nb3B6-C3Fab and Nb1D1-C3Fab, respectively, followed by incubating with mouse serum and FITC-coupled anti-mouse IgG mAbs. The cells were imaged with a fluorescent microscope. As shown in Figure 5B, only Nb3B6-C3Fab treated cells displayed strong green fluorescent signals, whereas Nb without IgBD (3B6) and irrelvent Nb (1D1) treated cells did not show any signals. These results suggested that EIR Nb not only binds with CD70 overexpressed cells but also recruits mouse IgG on the surface of CD70 positive cells.
Half-Life Extension of Nbs by Linking IgBD
It is critical to keep a relatively long half-life in therapies. However, due to their small size, Nbs have a short half-life in vivo. The results presented earlier in this study showed Nb3B6 fused with C3Fab can recruit mouse IgG, and the Fc-FcRn interaction is a key contributor to the prolonged serum half-life of antibodies and their derivatives. Based on this finding, we hypothesized that ligating with C3Fab would enhance the plasma half-life of Nbs. To test this hypothesis, Nb3B6-C3Fab and unmodified Nb3B6 were injected into Babl/c mice through the tail vein, and blood samples were collected at different time points to measure the concentrations of Nbs in plasma in vivo. The serum concentrations of Nb3B6-C3Fab and Nb3B6 were determined according to the standard curves, which were assayed using a sandwich ELISA (Figure 6A). Nbs expressed in pET21a transformed BL21 (DE3) cells bear either 6 × His or T7 tags which facilitate detection. In sandwich ELISA, anti-T7 tag mAb was immobilized to capture Nbs in serum and followed by incubation with HRP conjugated anti-His tag for detecting their binding. As shown in Figure 6B, the unmodified Nb3B6 showed rapid clearance from the bloodstream with a terminal half-life of 0.96 h. In contrast, Nbs linked with IgBD, the Nb3B6-C3Fab fusion protein showed an enhanced terminal half-life of 37.67 h and a strongly increased AUC from 920.39 to 3256 (Table 1). Compared to the unmodified Nb3B6, the half-life of the Nb3B6-C3Fab fusion protein increased almost 39-fold.
 |
Table 1 Pharmacokinetic Parameters of EIR-CD70Nbs in Babl/c Mice (n = 3, mean±S.D.) |
ADCC and CDC Immune Effectors Can Be Triggered by EIR-Nbs via IgG and FcR
The main immune effect of antibodies for treating cancers is ADCC, which is dependent on targeted antibodies and mediated through FcgRIIIa receptors on immune cells, such as NK cells present in peripheral blood mononuclear cells (PBMC). To verify whether Nb3B6-C3Fab causes ADCC cytotoxicity by recruiting endogenous IgG and linking tumor cells to the immune system, CD70 overexpressed 786-O cells and Panc-1 cells were selected to evaluate ADCC effects of EIR Nb. Different concentrations of CD70 Nbs and its C3Fab fusions were incubated with mouse serum as a source of endogenous IgG, and an irrelevant Nb1D1-C3Fab was used as a negative control. The cell lysis was measured with a lactate dehydrogenase (LDH) cytotoxicity kit after co-culturing the cells with freshly isolated PBMC. As shown in Figure 7A, Nb3B6-C3Fab exhibited dose-dependent ADCC activities on 786-O and Panc-1 cells, while unmodified Nb3B6 and irrelevant Nb1D1-C3Fab had no effects on either cell even at high concentrations. The degree of cell lysis is enhanced with increasing concentrations of Nb. About 25% of 786-O cells and 22% of Panc-1 cells were killed by Nb3B6-C3Fab at a concentration of 320 nmol/L, whereas the cell viability of 786-O and Panc-1 was not influenced by Nb3B6 and Nb1D1-C3Fab. These results indicated that Nbs can combine with effector cells (NK) to kill targeted tumor cells by linking IgBD.
In addition to ADCC, CDC is another anti-tumor effect of antibodies. The tumor cells bound with targeted antibodies would be killed by membrane attack complex which was formed once component 1q (C1q) combined with antibody Fc region. To assess the effect of EIR Nb mediated CDC on tumor cells, CD70 Nb and its C3Fab fusions were co-incubated with tumor cells and mouse serum diluted at different concentrations. The cell lysis was tested by CCK8 kit. As shown in Figure 7B, significant cytotoxicity was observed in all Nb3B6-C3Fab treatments, while unmodified Nb3B6 and irrelevant Nb1D1-C3Fab had no effects on either cell even at high concentrations. Approximately 50% lysis of 786-O cells and Panc-1 cells were observed when treated with Nb3B6-C3Fab in 80 nmol/L, and even in low concentrations (20 ng/mL), Nb3B6-C3Fab lysis at least 20% of two cancer cells. Taken together, these results suggested that linking C3Fab to Nbs can obtain effective immune effectors and generate potent tumor killing.
Discussion
Since its discovery in 1993, Nb has received much attention in research. However, the clinical application of Nbs is often limited by their short serum half-life and lack of Fc-dependent immune effectors. Therefore, it is critical to develop approaches that can maintain the advantages of Nbs and achieve therapeutic efficacy at the same time. In this study, we provide a proof-of-concept that linking a C3Fab peptide derived from Streptococcal Protein G can enhance serum half-life of Nbs and also provide potent tumor cytotoxicity. This strategy could offer a novel and general solution to promote the effective application of Nbs in vivo.
The optimal IgBD to link Nbs should have several characteristics. Firstly, it should not interfere with the functions of Nbs. Secondly, the molecular size of IgBD should be small and not influence the conformational structure of Nbs. Thirdly, the IgBD should have no or minimal immunogenicity. Lastly, IgBD should not interfere the binding of IgG with their FcR. In our study, we selected Streptococcal Protein G derived C3Fab peptide as IgBD of Nbs to construct an endogenous IgG recruiting platform. C3Fab contains 56 amino acids (AA) and has a molecular weight of 5978.53 Da. Compared to the IgBD domain in protein G, there are three AA mutations (E27A, K28A, K31A) in C3Fab. Unverdorben confirmed26 that the modified C3Fab lost their IgG Fc binding activities and preserved the binding towards the Fab fragment and slightly improved the affinity to human and mouse IgG, and importantly, C3Fab eliminates the interference with the FcRn-mediated recycling process. Considering the characteristics of C3Fab, it is superior to wild IgBD in protein G or protein A and is suitable IgBD for constructing EIR Nbs. The secondary structure of C3Fab contains 4 β-strands and one α-helix to form a compact 3D structure. The small and compact structure makes C3Fab not interfere with its fusions. In our experiments, Nb3B6 fusing with C3Fab acquired endogenous IgG recruitment without interference with its target binding and prolonged their half-life in vivo. The recruited IgG can mediate Fc-FcRn interaction and contributed to the prolonged serum half-life of antibodies and their derivatives. However, there are several defects in the application of C3Fab as an IgBD One is its immunogenicity. Because it is derived from bacteria, C3Fab might elicit the anti-drug response in humans and mice, lowering the effect of EIR Nbs and generating side effects. Recently, a series of Nb-based IgBD were identified,27–29 and these Nbs have high affinity to human and mice IgG. Valuably, these Nbs can selectively bind with different IgG isotypes. Several studies confirmed that Nbs have minimal immunogenicity during therapeutic use in humans due to high homology (86–94%) with the human VH3 family.30 Furthermore, the immunogenicity of Nbs can be further reduced by humanization through genetic engineering approaches.31 Another defect of C3Fab as IgBD may be that it cannot selectively bind with specific IgG isotypes. There are four IgG subclasses, IgG1, IgG2, IgG3, and IgG4 in human serum. Different subclasses have different effector functions. Typically, IgG1 and IgG3 are potent triggers of effector mechanisms, whereas IgG2 and IgG4 have weak functions.32 C3Fab binds all IgG isotypes, this makes its effector functions dubiously. On the other hand, IgG-binding Nbs can selectively bind with specific IgG isotypes. Therefore, linking IgG-binding Nbs to targeted Nbs should be an optimal approach for constructing EIR Nbs. We are currently in the process of doing this work.
Small protein-based drugs, including Nbs and single-chain variable fragments (scFv), are characterized by rapid renal clearance, resulting in short serum half-lives. To address this limitation, various approaches have been developed to enhance their pharmacokinetics33 such as PEGylation, Fc-fusion, and human serum albumin (HSA) conjugation. However, these methods may compromise the advantages of Nbs in production, rendering them unsuitable for constructing Nb-based therapeutics. In this study, we demonstrated that linking with IgBD could improve both the effector functions and pharmacokinetics of Nbs. By attaching the Streptococcal Protein G-derived C3Fab peptide to Nb3B6, we were able to increase its serum half-life by 39-fold from 0.96 h to 37.67 h, without compromising its production in E. coli cells. Our findings suggest that IgBD-fusion is a promising approach for improving the pharmacokinetics of small protein-based drugs, including Nbs. Monoclonal antibodies as therapeutics have acquired great success in treating cancer and other diseases. However, their large size, complex structure, and high cost limit their applications. Nanobodies, which are considered the next-generation therapeutics,34 offer a versatile platform in drug development and therapeutic applications for their compact size and high flexibility in creating sophisticated structures. In a previous study, we identified Nb 3B6 as a high-affinity binder for human CD70, but its lack of effector functions has hindered its application in therapy.25 In this study, we demonstrated that EIR Nb can recruit abundant endogenous IgG to the surface of CD70 overexpressed tumor cells, which can thus be killed through Fc-mediated anti-tumor mechanisms. Currently, cusatuzumab is the only CD70 targeting mAb being tested in the clinical trial for cancer treatment through its immune effector functions. Therefore, there is a need to develop more such drugs to meet clinical needs and to better understand the mechanisms of CD70 in tumor immune evasion. The CD70 Nbs identified in our study recognized different epitopes on CD70 with cusatuzumab and strongly blocked CD70-CD27 binding.25 In this regard, the EIR Nb is a promising new therapeutic approach that may provide an alternative way to treat cancer.
Conclusions
In conclusion, our study demonstrated that EIR-Nbs, which recruit endogenous IgG and exhibit significant serum half-life improvement and tumor cytotoxicity, can be achieved through C3Fab IgBD-mediated ADCC and CDC immune functions. This approach offers a broad solution to enable Nbs and other small proteins to act effectively in disease therapy. Due to their high stability, small size, and cost-effective production in E. coli, EIR-Nbs may serve as a promising alternative to mAbs for targeted cancer and other disease treatments.
Ethics and Consent Statement
All animal experiments were approved by the Committee on the Ethics of Animal Experiments of Xinjiang Key Laboratory of Biological Resources and Genetic Engineering (No. XJUAE-2022-026) and performed under the guidelines of the Animal Care and Use Committee of College of Life Science and Technology, Xinjiang University.
Acknowledgments
This work was supported by grant of National Natural Science Foundation of China to Jiangwei Li (31570935).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dammeijer F, Lau SP, van Eijck CHJ, van der Burg SH, Aerts JGJV. Rationally combining immunotherapies to improve efficacy of immune checkpoint blockade in solid tumors. Cytokine Growth Factor Rev. 2017;36:5–15. doi:10.1016/j.cytogfr.2017.06.011
2. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707–723.
3. Flieswasser T, Van den Eynde A, Van Audenaerde J, et al. The CD70-CD27 axis in oncology: the new kids on the block. J Exp Clin Cancer Res. 2022;41(1):12.
4. Jacobs J, Deschoolmeester V, Zwaenepoel K, et al. CD70: an emerging target in cancer immunotherapy. Pharmacol Ther. 2015;155:1–10.
5. Seyfrid M, Maich WT, Shaikh VM, et al. CD70 as an actionable immunotherapeutic target in recurrent glioblastoma and its microenvironment. J Immunother Cancer. 2022;10(1):e003289.
6. Tesselaar K, Xiao Y, Arens R, et al. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J Immunol. 2003;170(1):33–40.
7. Bowman MR, Crimmins MA, Yetz-Aldape J, et al. The cloning of CD70 and its identification as the ligand for CD27. J Immunol. 1994;152(4):1756–1761.
8. Hintzen RQ, Lens SM, Koopman G, et al. CD70 represents the human ligand for CD27. Int Immunol. 1994;6(3):477–480.
9. Wajant H. Therapeutic targeting of CD70 and CD27. Expert Opin Ther Targets. 2016;20(8):959–973.
10. Pahl JH, Santos SJ, Kuijjer ML, et al. Expression of the immune regulation antigen CD70 in osteosarcoma. Cancer Cell Int. 2015;15(1):31. doi:10.1186/s12935-015-0181-5
11. Jacobs J, Zwaenepoel K, Rolfo C, et al. Unlocking the potential of CD70 as a novel immunotherapeutic target for non-small cell lung cancer. Oncotarget. 2015;6(15):13462–13475.
12. Lens SM, Drillenburg P, den Drijver BF, et al. Aberrant expression and reverse signalling of CD70 on malignant B cells. Br J Haematol. 1999;106(2):491–503.
13. Riether C, Schürch CM, Bührer ED, et al. CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J Exp Med. 2017;214(2):359–380.
14. Ryan MC, Kostner H, Gordon KA, et al. Targeting pancreatic and ovarian carcinomas using the auristatin-based anti-CD70 antibody-drug conjugate SGN-75. Br J Cancer. 2010;103(5):676–684.
15. McEarchern JA, Smith LM, McDonagh CF, et al. Preclinical characterization of SGN-70, a humanized antibody directed against CD70. Clin Cancer Res. 2008;14(23):7763–7772.
16. Muyldermans S, Baral TN, Retamozzo VC, et al. Camelid immunoglobulins and nanobody technology. Vet Immunol Immunopathol. 2009;128(1–3):178–183.
17. Steeland S, Vandenbroucke RE, Libert C. Nanobodies as therapeutics: big opportunities for small antibodies. Drug Discov Today. 2016;21(7):1076–1113.
18. Salvador JP, Vilaplana L, Marco MP. Nanobody: outstanding features for diagnostic and therapeutic applications. Anal Bioanal Chem. 2019;411(9):1703–1713.
19. Scully M, Cataland SR, Peyvandi F, et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N Engl J Med. 2019;380(4):335–346.
20. Markham A. Envafolimab: first Approval Drugs. Drugs. 2022;82(2):235–240.
21. Susan J, Keam O. Zoralizumab: first Approval Drugs. Drugs. 2023;83(1):87–92.
22. Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–725.
23. Hutt M, Färber-Schwarz A, Unverdorben F, Richter F, Kontermann RE. Plasma half-life extension of small recombinant antibodies by fusion to immunoglobulin-binding domains. J Biol Chem. 2012;287(7):4462–4469.
24. Sockolosky JT, Kivimäe S, Szoka FC. Fusion of a short peptide that binds immunoglobulin G to a recombinant protein substantially increases its plasma half-life in mice. PLoS One. 2014;9(7):e102566.
25. Zhang X, Liu C, Xie Y, Hu Q, Chen Y, Li J. Identification and characterization of blocking nanobodies against human CD70. Acta Biochim Biophys Sin. 2022;54(10):1518–1527.
26. Unverdorben F, Hutt M, Seifert O, Kontermann RE. A Fab-Selective Immunoglobulin-Binding Domain from Streptococcal Protein G with Improved Half-Life Extension Properties. PLoS One. 2015;10(10):e0139838.
27. Pleiner T, Bates M, Görlich D. A toolbox of anti-mouse and anti-rabbit IgG secondary nanobodies. J Cell Biol. 2018;217(3):1143–1154.
28. Hu B, Liu T, Li L, et al. IgG-Binding Nanobody Capable of Prolonging Nanobody-Based Radiotracer Plasma Half-Life and Enhancing the Efficacy of Tumor-Targeted Radionuclide Therapy. Bioconjug Chem. 2022;33(7):1328–1339.
29. Klarenbeek A, El Mazouari K, Desmyter A, et al. Camelid Ig V genes reveal significant human homology not seen in therapeutic target genes, providing for a powerful therapeutic antibody platform. MAbs. 2015;7(4):693–706.
30. Arbabi-Ghahroudi M. Camelid Single-Domain Antibodies: promises and Challenges as Lifesaving Treatments. Int J Mol Sci. 2022;23(9):5009.
31. Rossotti MA, Bélanger K, Henry KA, Tanha J. Immunogenicity and humanization of single-domain antibodies. FEBS J. 2022;289(14):4304–4327.
32. Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520.
33. Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol. 2011;22(6):868–876.
34. Yang EY, Shah K. Nanobodies: next Generation of Cancer Diagnostics and Therapeutics. Front Oncol. 2020;10:1182.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.