Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Generalized Vitiligo After Stem Cell Transplantation: A Case Report
Authors Wang Y, Hu W , Lin F, Xu A
Received 26 May 2023
Accepted for publication 18 July 2023
Published 25 July 2023 Volume 2023:16 Pages 1945—1948
DOI https://doi.org/10.2147/CCID.S420342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yunxia Wang,1– 3 Wenting Hu,2 Fuquan Lin,2,3 Ai′e Xu2
1Department of Dermatology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui Province, People’s Republic of China; 2Department of Dermatology, Hangzhou Third People’s Hospital, Hangzhou, Zhejiang Province, People’s Republic of China; 3Department of Dermatology, Hangzhou Clinical College of Anhui Medical University, Hangzhou, Zhejiang Province, People’s Republic of China
Correspondence: Ai′e Xu, Hangzhou Third People’s Hospital, No. 38 Xihu Avenue, Shangcheng District, Hangzhou, Zhejiang Province, People’s Republic of China, Tel +86-131 5513 0536, Fax +86+ 571+87827534, Email [email protected]
Abstract: Graft versus host disease (GVHD) is a complex immune-mediated pathophysiological process, which is caused by allogenic immune reactions between donors and recipients. No matter ac-ute or chronic GVHD, skin involvement is the most common, severe skin damage can lead to permanent disfigurement, which seriously affects the long-term quality of life of patients. We herein report a patient with generalized vitiligo after allogeneic peripheral hematopoietic stem cell transplantation (allo-HSCT) for aplastic anemia.
Keywords: graft versus host disease, vitiligo, aplastic anemia, allogeneic peripheral hematopoietic stem cell transplantation
Introduction
In vivo, GVHD is a transitional reaction in which donor-derived immune-active cells mediated attack host cells and organs.1,2 Previous reports have documented the occurrence of localized or generalized pigment loss following transplantation, followed by the development of mossy-like skin rashes that merge into patches.3,4 The patient experienced two separate episodes of acute GVHD after receiving an allogenic peripheral hematopoietic stem cell transplant for severe aplastic anemia. These episodes were characterized by successive systemic light red and brown rashes. After shedding, it evolved into the vitiligo-like chronic GVHD, which is rarely reported in both domestic and international publications. This report aims to draw the attention of dermatological clinicians to this possibility and early intervention, and treatment.
Case Presentation
A 13-year-old female child came to our hospital with “white spots scattered all over the body for 2 years”. The patient was diagnosed with “severe aplastic anemia” at another hospital three years ago. During this period, the patient was treated with repeated red blood cells and platelets transfusion. In April 2019, an unrelated, blood group incompatible, HLA10/10 homozygous, and allo-HSCT was performed following the planned pretreatment regimen (FLU+CTX+A1G). On the 22 postoperative day, the child’s extremities gradually started to show signs of pale red rash on the extremities, accompanied by multiple scattered watery pustules up to 7–8 cm in diameter. Then, the patient developed extensive desquamation. In the second month after surgery, the patient developed intermittent high fever (38°C to 39.5°C) without obvious inducement, along with a broad brown florid rash, desquamation, which was later diagnosed as “acute graft-versus-host disease, EBV infection, and cytomegalovirus infection”. In the eighth month after surgery, white spots began to appear on the patient’s hands, which gradually increased and became larger. The white spots further enlarged in size and spread to the entire body. Due to the improvement in bone marrow biopsy results 15 months after surgery, medications such as cyclosporin, Mycophenolate Mofetil, and hormone were discontinued. At 20 months after surgery, phalangeal joint size showed systemic scattered color recovery, which connected into flake-like, with a gradually larger area. The patient came to our hospital for a diagnosis and was diagnosed with vitiligo-like GVHD. Auxiliary examinations: skin CT (neck): which was considered progressive vitiligo. Laboratory tests revealed Hematology parameters: Lymphocyte subsets: CD45+: 6200 (1200–3700), CD3+: 4210 (690–2540), CD19+: 1494 (90–560), CD8+: 1556 (190–1140), CD4+: 2306 (410–1440), B lymphocytes: 24.1%(5–20%), NK: 6.4%(7–40%), with no other obvious abnormalities observed. Physical examination at the Dermatology Department suggested that the entire body had well-defined depigmented plaques in various areas were observed in the whole body, and it took up about 64% of the body’s surface area. (Figure 1). In the treatment, Compound Betamethasone Injection is administered via intramuscular injection to control the progression of the disease. Additionally, Large topical application of Halometasone/Triclosan Cream once daily, 0.1% Tacrolimus Ointment twice daily, and systemic NB-UVB phototherapy once a week are implemented to manage the condition. The patient pays clinical follow-up visits currently.
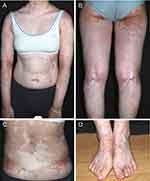 |
Figure 1 (A–D) Spot or patchy hyperpigmentation after generalized depigmentation. |
Discussion
The main mechanism of GVHD, an unfavorable type IV delayed-type hypersensitivity secondary to allogenic peripheral hematopoietic stem cell transplant (allo-HSCT). Excessive pretreatments and blood transfusion of the patients before allo-HSCT increased the risk of sensitization to minor histocompatibility antigen (MIHA) in the blood donor, thus leading to a higher risk of GVHD.5 According to laboratory analyses of lymphocyte subpopulations, the proportions of CD3+ and CD8+T Lymphocytes increased, while those of CD4+T lymphocytes, B lymphocytes, and CD4+/CD8+ ratio decreased, revealing the immune dysfunction of the patient. Meanwhile, A clear diagnosis of progressive vitiligo in combination with auxiliary examinations. High levels of CD8+ cytotoxic T cells were detected in the peripheral blood of progressive vitiligo patients, which have cytotoxic and skin-homing abilities and may form a local immune microenvironment and migrate towards the skin tissue mediated under the mediation of chemokines and were localized within melanocytes at the dermal-epidermal junction.6 Besides, they specifically killed melanocytes through perforin and granzyme-B.7,8 Due to the repeated attacks of GVHD, the repeated inflammatory reactions induced persistent immune dysfunction of B cells and T cells, leading to gradual destruction, aging, acute apoptosis, and necrosis of melanocytes. These pathological changes exceeded the compensatory capacity of the body, leading to the systemic progression of skin color evolution from light red to brown to white. With the subsequent withdrawal of drugs like immunosuppressants, the immune system was restored, and the existing melanin stem cells in the hair follicles proliferated and migrated, gradually resulting in the occurrence of spontaneous color recovery.9,10
Conclusion
Early recognition, intervention, and treatment are essential to avoid further development of skin reactions. Considering that the patient developed systemic skin decoloration, it is crucial to prevent color recovery when necessary, maintain permanent decoloration, and identify the disease’s progression mechanism, which requires further investigation.
Abbreviations
GVHD, graft versus host disease; allo-HSCT, allogeneic peripheral hematopoietic stem cell transplantation.
Consent Statement
The patient’s guardian provided informed consent to publish their case details and any accompanying images. Institutional approval is not required for this case study.
Acknowledgments
The authors thank all participants and their guardians. This study was supported by the National Natural Science Foundation of China Joint Fund Project (U22A20310), National Natural Science Foundation of China (82003322), Natural Science Foundation of Zhejiang Province (LY21H110002), Hangzhou medical key discipline construction project (No [2021]21-3).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Kröger N. Preventing graft-versus-host disease without losing graft-versus-leukemia effect after allogeneic stem-cell transplantation. J Clin Oncol. 2020;38(29):3357–3360. doi:10.1200/JCO.20.01756
2. Socié G, Kean LS, Zeiser R, Blazar BR. Insights from integrating clinical and preclinical studies advance understanding of graft-versus-host disease. J Clin Invest. 2021;131(12). doi:10.1172/JCI149296
3. Dai J, Hight RS, Grullon K, Xiao TL, Stein SL. Vitiligo-like manifestations of graft-versus-host disease in a pediatric population. Pediatr Dermatol. 2023;40(1):157–161. doi:10.1111/pde.15121
4. Sanli H, Akay BN, Arat M, et al. Vitiligo after hematopoietic cell transplantation: six cases and review of the literature. Dermatology. 2008;216(4):349–354. doi:10.1159/000117705
5. Koyama M, Mukhopadhyay P, Schuster IS, et al. MHC class II antigen presentation by the intestinal epithelium initiates graft-versus-host disease and is influenced by the microbiota. Immunity. 2019;51(5):885–898. doi:10.1016/j.immuni.2019.08.011
6. Richmond JM, Bangari DS, Essien KI, et al. Keratinocyte-derived chemokines orchestrate T-Cell positioning in the epidermis during vitiligo and may serve as biomarkers of disease. J Invest Dermatol. 2017;137(2):350. doi:10.1016/j.jid.2016.09.016
7. Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6(223):223ra23–ra23. doi:10.1126/scitranslmed.3007811
8. Sabat R, Wolk K, Loyal L, Döcke W-D, Ghoreschi K. T cell pathology in skin inflammation. Semin Immunopathol. 2019;41(3):359–377. doi:10.1007/s00281-019-00742-7
9. Yardman-Frank JM, Fisher DE. Skin pigmentation and its control: from ultraviolet radiation to stem cells. Exp Dermatol. 2021;30(4):560–571. doi:10.1111/exd.14260
10. Zeiser R, Blazar BR, Longo DL. Pathophysiology of chronic Graft-versus-host disease and therapeutic Targets. N Engl J Med. 2017;377(26):2565–2579. doi:10.1056/NEJMra1703472
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
