Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Generalized Asymptomatic Nodulo-Ulcerative Lesions Without Systemic Symptoms in a Secondary Syphilis Patient Co-Infected with HIV
Authors Maharani RH , Nugraha T , Sutedja E , Ruchiatan K , Usman HA , Achdiat PA
Received 1 November 2023
Accepted for publication 17 December 2023
Published 20 December 2023 Volume 2023:16 Pages 3645—3650
DOI https://doi.org/10.2147/CCID.S445155
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Rungsima Wanitphakdeedecha
Retno Hesty Maharani,1 Tomi Nugraha,1 Endang Sutedja,1 Kartika Ruchiatan,1 Hermin Aminah Usman,2 Pati Aji Achdiat1
1Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran – Dr. Hasan Sadikin Hospital, Bandung, Indonesia; 2Department of Anatomical Pathology, Faculty of Medicine, Universitas Padjadjaran – Dr. Hasan Sadikin Hospital, Bandung, Indonesia
Correspondence: Pati Aji Achdiat, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran – Dr. Hasan Sadikin Hospital, Jl. Pasteur 38, Bandung, West Java, 40161, Indonesia, Tel +62 81322750101, Email [email protected]
Abstract: Syphilis is a sexually-transmitted disease with various clinical stages. Secondary syphilis manifestations may mimic other skin lesions. Patient co-infected with Human Immunodeficiency Virus (HIV), with CD4 cell counts of 200– 499 cells/mm3, often manifests an atypical cutaneous lesion, which may also occur as nodular or ulcerative lesions. Generalized nodulo-ulcerative lesions without systemic symptoms in secondary syphilis patients with HIV co-infection are rarely reported. A 22-year-old man presented with generalized asymptomatic multiple erythematous papules and plaques with scales, as well as nodular and nodulo-ulcerative lesions on the trunk, both arms, and both legs. His lesions spread progressively without the presence of any prodromal symptoms or adenopathy. He was previously diagnosed with HIV and is currently on antiretroviral medications, with a CD4 cell count of 388 cells/μL. His venereal disease research laboratories (VDRL) result was reactive (titer of 1:256). His Treponema pallidum hemagglutination assay (TPHA) result was also reactive (titer of 1:10,240). A skin biopsy was performed from the nodulo-ulcerative lesion on his back. Hematoxylin-eosin staining revealed a hyperplastic epidermis, a massive influx of plasma cells, and lymphocyte infiltration into the deep dermis, especially in the peri-adnexal, peri-vascular, and peri-muscular regions. The patient was diagnosed with secondary syphilis with HIV co-infection. He had no previous history of drug allergy. A single dose of 2.4 million units of benzathine penicillin G was administered. Almost all the lesions became hyperpigmented macules after two weeks and resolved completely after one month. His VDRL titer declined to 1:32 after three months. The various atypical lesions of secondary syphilis may lead to misdiagnosis and delayed treatment. The presence of multiple asymptomatic nodulo-ulcerative lesion without prodromal symptoms may indicate the presence of secondary syphilis, notably in patients co-infected with HIV. Therefore, knowledge of atypical cutaneous manifestations of secondary syphilis is warranted in order to treat patients accordingly.
Keywords: secondary syphilis, human immunodeficiency virus, nodulo-ulcerative lesions
Introduction
In recent years, there is a high proportion of human immunodeficiency virus (HIV) co-infections among patients with syphilis. This can be related to the increased risk of HIV transmission, especially among men who have sex with men (MSM).1–3 This higher risk among MSM is thought to be caused by an increased frequency of sexual network from internet contact.2,3 Secondary syphilis skin lesions may be similar to other skin diseases. Hence, it is also known as “the great mimic” or “the great imitator”.1,2,4 Secondary syphilis lesions are typically asymptomatic and start to appear as scaly copper-colored macular rash (roseola syphilitica), papulosquamous lesion with white scaly ring (Biett collarette), or papular eruption. These lesions are sometimes overlooked and can be difficult to distinguish.1,2 The presence of HIV co-infection can lead to rapidly progressing lesions, and atypical lesions may sometimes appear.4,5 Atypical lesions in secondary syphilis include nodular, nodulo-ulcerative, annular, pustular, lues maligna, framboesiform, corymbose, photosensitive systemic lupus erythematosus (SLE)-like, leukoderma, as well as chancriform lesions.6 Nodulo-ulcerative lesions with prodromal symptoms are clinical manifestations of syphilis commonly seen in HIV-positive patients.4 This report presents a case of generalized asymptomatic erythematous papules and plaques with scales, nodular, and nodulo-ulcerative lesions without any prodromal symptoms in a secondary syphilis patient with HIV co-infection.
Case Report
A 22-year-old man presents with generalized asymptomatic erythematous papules and plaques with scales, as well as nodular and nodulo-ulcerative lesions on the trunk, both arms, and both legs. The lesions initially appeared as asymptomatic guttate erythematous macules and papules with scales on both arms and legs, whereas both palms and soles were spared. The lesions then spread progressively within three months, some of which evolved to nummular erythematous macules, papules, and nodular lesions. Lesions evolve slowly without abrupt changes. No prodromal symptoms or adenopathy were present prior to the eruption of skin lesions. There was also no previous history of solitary asymptomatic genital ulcers. The patient underwent voluntary counseling and testing for HIV in a primary care facility following his previous history of high-risk sexual behavior as an MSM with multiple sexual partners, and previous history of sexual contact as a receptive partner without the use of condoms. His anti-HIV test was reactive, with a CD4 cell count of 388 cells/μL, but other sexually transmitted infection was not ruled out. The patient had already started his antiretroviral therapy for two months during the evolution of the skin lesions. One month prior to his referral to our clinic, some of the lesions remained in the form of erythematous papules and plaques with scales, nodular lesions, and ulcerated nodular lesions.
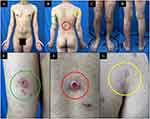
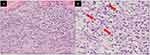
Physical examination revealed multiple erythematous papules and plaques with scales, as well as nodular and nodulo-ulcerative lesions on the trunk, both arms, and both legs (Figures 1A–D). Erythematous plaques and nodules with scales were mostly found on his extremities (Figure 1E). Nodulo-ulcerative lesions (Figure 1F) were found on his extremities and back. Other lesions, such as erythematous macules and plaques with scales (Figure 1G), erythematous papules, and nodular lesions are generalized. No genital ulcers were seen on physical examination. Vital signs and neurological examination were within normal limits. Venereal disease research laboratories (VDRL) and Treponema pallidum hemagglutination assay (TPHA) examinations were conducted to screen for syphilis, revealing reactive results with a titer of 1:256 for VDRL and 1:10,240 for TPHA. Cerebrospinal fluid examination for screening of neurosyphilis revealed clear fluid, non-reactive to VDRL, glucose level of 44 mg/dL, and total protein level of 45 mg/dL. Excisional biopsy was performed from the nodulo-ulcerative lesions on the back. Histopathological examination with hematoxylin-eosin staining shows hyperplastic epidermis, a massive influx of plasma cells around fibrous connective tissue in the dermis, as well as peri-adnexal, peri-vascular, and peri-muscular lymphocytic infiltration into the deep dermis (Figure 2A and B).
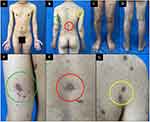
A diagnosis of secondary syphilis was established based on clinical manifestations, serological test results, and histopathological examination. According to CDC guidelines for secondary syphilis, we administered a single-dose intramuscular injection of 2.4 million units of benzathine penicillin G. Most of the lesions evolved to form hyperpigmented macules without scar formation at two weeks following treatment (Figure 3A–G). A large nodulo-ulcerative lesion on the left wrist (Figure 4A) regressed after two weeks (Figure 4B), and evolved to form a hyperpigmented macule without scar formation at one month (Figure 4C). His VDRL titer also declined to 1:32 at the third month of follow-up. After a 1-year of observation, his VDRL titer remained at 1:32. There was no suspected promiscuity, and no new skin lesions were seen during the observation period of one year.
Discussion
An estimated 12 million new cases of syphilis are diagnosed every year in developing countries.5 The seroprevalence of syphilis remains high worldwide, mainly in high-risk populations such as MSM and transgenders.2,7 Factors that may contribute to the high incidence of syphilis among MSM include high-risk sexual behaviors, internet-use for sexual contact, recreational drug use, having multiple sexual partners, as well as receptive anogenital sex as without the use of condoms.8–10 It is also known in the European population that the prevalence of MSM with HIV co-infection is 34%.3 The risk of HIV infection among patients with syphilis is higher in those who practice anogenital sex as the receptive partner.11 Our patient is a 22-year-old MSM, with previous history of promiscuity without condom use as a receptive partner, who was previously diagnosed with HIV.
Secondary syphilis is an infectious vasculitis due to the hematogenous spread of the bacteria characterized by diffuse lesions, generalized lymphadenopathy, and reactive serological test results.1 Symptoms of secondary syphilis can commonly be found three to six weeks after the appearance of primary genital ulcers.2 The skin lesions of secondary syphilis may mimic other skin diseases.6 The lesions usually start to appear as copper-colored macular rash, which can evolve a few days later to become asymptomatic symmetric papular eruptions.3 Various atypical skin lesions may appear, such as macular, nodular, nodulo-ulcerative, annular, pustular, framboesiform, corymbiform, or chancriform lesions.4,6 These atypical skin lesions can commonly be found in patients with HIV co-infections. Immune complexes will circulate in the body and result in immunologic abnormalities, marked by a high VDRL titer.2,12–14 Immunologic abnormalities will lead to over-reactivity of the immune system and increased number of peripheral T-helper cells.14 This co-infection also causes the skin lesion of secondary syphilis to become more severe, persistent, and rapidly progressive.4,5 Tambe et al15 reported two female patients with secondary syphilis and HIV co-infections, with nodulo-ulcerative lesions with ecthymatous crusts on the face and “kissing” ulcers, along with VDRL titers of 1:32 and 1:256, respectively. Burchak et al16 also reported nodular syphilitic lesions in an HIV-positive man with a VDRL titer of 1:256. The lesions present as small nodular lesions without ulcerations. Our patient also shows atypical lesions, including nodular and nodulo-ulcerative lesions, and had a VDRL titer of 1:256.
Treponema pallidum cannot be cultured. Hence, the diagnosis of syphilis is confirmed with serological testing, including TPHA and VDRL.1,2,4 The TPHA test will confirm the diagnosis of treponemal infection, whereas the VDRL test is performed to determine therapeutic response.2,17 The patient in this case was reactive to both TPHA and VDRL, with a history of skin lesions, suggestive of secondary syphilis. The VDRL titer was also used to evaluate the therapeutic response in this patient. Observations were performed for one year and his VDRL titer had declined at three months following antibiotic initiation.
Histopathological examination is also necessary if atypical lesions are found on a patient with secondary syphilis.4,17 The structure of the epidermis tend to be normal or hyperplastic with parakeratosis or acanthosis.1,4 Infiltration of plasma cells, lymphocytes, or histiocytes may be found on the dermis.1,18,19 Nodulo-ulcerative lesions of secondary syphilis may show acanthosis, spongiosis, and dermal edema, with lymphocytic and plasma cells infiltration on the peri-vascular and peri-adnexal regions.15 In the current patient, hyperplastic epidermis with massive lymphocytic and plasma cells infiltration on the dermis was found in the biopsy sample taken from the nodulo-ulcerative lesion.
Penicillin is used as a systemic treatment for syphilis.1 The treatment of secondary syphilis is the intramuscular administration of a single-dose (2.4 million units) of benzathine penicillin G.1,2,8 Therapeutic response to the antibiotic can be evaluated from the VDRL titer. A four-fold decline of VDRL titer can be considered as a successful response to antibiotics.2 In this patient, the treatment of syphilis was administered after the diagnosis of secondary syphilis was established following serological test results and histopathological examination. Clinical response was seen within two weeks and improvement was observed at the one-month follow-up. Serological response was seen at the three-month follow-up, with a four-fold decline in VDRL titer to 1:32 from the initial 1:256.
Conclusion
Secondary syphilis is a great imitator and may lead to misdiagnosis, including atypical lesions in patients with HIV co-infection, due to the presence of immunological abnormalities. Atypical skin manifestations of secondary syphilis may appear as multiple asymptomatic nodulo-ulcerative lesions without prodromal symptoms. After confirming the diagnosis with serological tests, it is also necessary to perform skin biopsy of atypical lesions to support the diagnosis of secondary syphilis and identify plasma cells as well as lymphocytic infiltrates. These atypical lesions may also lead to delayed treatment. Therefore, knowledge of atypical cutaneous manifestations of secondary syphilis is warranted in order to treat patients accordingly.
Ethics Statement
The publication of images were included in the patient’s consent for publication of the case. Institutional approval has been obtained to publish the case details.
Consent Statement
The authors certify that they have obtained all appropriate patient consent forms. The patient signed a consent form for the publication of the case details and images.
Acknowledgments
The authors would like to thank the staff of the Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia.
Funding
The authors declare that this study has received no financial support.
Disclosure
No conflicts of interest are declared in this case report.
References
1. Tuddenham SA, Zenilman JM. Syphilis. In: Kang S, Amagai M, Bruckner AL, et al. editors. Fitzpatrick’s Dermatology.
2. Sparling PF, Swartz MN, Musher DM, Healy BP. Clinical manifestations of syphilis. In: Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, editors. Sexually Transmitted Diseases.
3. Kojima N, Klausner JD. An update on the global epidemiology of syphilis. Curr Epidemiol Rep. 2018;5(1):24–38. doi:10.1007/s40471-018-0138-z
4. Lleó MI, Escribano PC, Prieto BM. Atypical cutaneous manifestations in syphilis. Actas Dermosifiliogr. 2016;107(4):275–283. doi:10.1016/j.ad.2015.11.002
5. Karp G, Schlaeffer F, Jotkowitz A, Riesenberg K. Syphilis and HIV co-infection. Eur J Intern Med. 2009;20(1):9–13. doi:10.1016/j.ejim.2008.04.002
6. Balagula Y, Mattei PL, Wisco OJ, Erdag G, Chien AL. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dematol. 2014;53(12):1434–1441. doi:10.1111/ijd.12518
7. World Health Organization. WHO Guidelines for the Treatment of Treponema Pallidum (Syphilis). Geneva: World Health Organization; 2016.
8. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. doi:10.15585/mmwr.rr7004a1
9. Arando M, Fernandez-Naval C, Mota-Foix M, et al. Early syphilis: risk factors and clinical manifestations focusing on HIV-positive patients. BMC Infect Dis. 2019;19(1):727. doi:10.1186/s12879-019-4269-8
10. Taylor MM, Aynalem G, Smith LV, Montoya J, Kerndt P. Methamphetamine use and sexual risk behaviours among men who have sex with men diagnose with early syphilis in Los Angeles County. Int J STD AIDS. 2017;18(2):93. doi:10.1258/095646207779949709
11. Mayer KH, Carballo-Diéguez A. Homosexual and bisexual behavior in men in relation to STDs and HIV infection. In: Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, editors. Sexually Transmitted Diseases.
12. Musher DM, Hamil RJ, Baughn RE. Effect of Human immunodeficiency virus (HIV) infection on the course of syphilis and on the response to treatment. Ann Intern Med. 1990;113(11):872–881. doi:10.7326/0003-4819-113-11-872
13. Yancheva N, Petrova E, Tchervenyakova T. Atypical secondary syphilis presentation in a patient with human immunodeficiency virus infection: a case report. J Med Case Rep. 2019;13(1):360–363. doi:10.1186/s13256-019-2291-5
14. Son JH, Park SY, Chung BY, Kim HO, Cho HJ, Park CW. Nodular secondary syphilis in an immunocompetent woman: case report and literature review. Dermatologica Sinica. 2018;36(1):36–41. doi:10.1016/j.dsi.2016.10.006
15. Tambe S, Zambare U, Nayak C. Nodulo-ulcerative and erythrodermic secondary syphilis in human immunodeficiency virus-infected individuals. Int J STD AIDS. 2019;30(5):505–508. doi:10.1177/0956462418815310
16. Burchak A, Rivera R, Douglas M. Nodular syphilis seen in an HIV-positive man: a case study. Mil Med. 2023;usad066. doi:10.1093/milmed/usad066
17. Shah D, Marfatia YS. Serological tests for syphilis. Indian J Sex Transm Dis AIDS. 2019;40(2):186–191. doi:10.4103/ijstd.IJSTD_86_19
18. Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236(2):145–150. doi:10.1159/000502641
19. Flamm A, Alcocer VN, Kazlouskaya V, Kwon EJ, Elston D. Histopathologic features distinguishing secondary syphilis from its mimickers. J Am Acad Dermatol. 2020;82(1):156–160. doi:10.1016/j.jaad.2019.07.011
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.