Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 13
Gene Expression Profiling of Monozygotic Twins Affected by Psoriatic Arthritis
Authors Angioni MM , Floris A, Cangemi I , Congia M , Chessa E, Orrù S, Piga M , Cauli A
Received 10 November 2020
Accepted for publication 23 January 2021
Published 4 March 2021 Volume 2021:13 Pages 23—29
DOI https://doi.org/10.2147/OARRR.S291391
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Maria Maddalena Angioni,1 Alberto Floris,1 Ignazio Cangemi,1 Mattia Congia,1 Elisabetta Chessa,1 Sandro Orrù,2 Matteo Piga,1 Alberto Cauli1
1Rheumatology Unit, Department of Medical Sciences and Public Health, University of Cagliari, University Clinic AOU, Cagliari, 09042, Italy; 2Medical Genetics, Department of Medical Sciences and Public Health, University of Cagliari, Cagliari, 09042, Italy
Correspondence: Maria Maddalena Angioni
Rheumatology Unit, Department of Medical Sciences and Public Health, University of Cagliari, University Clinic AOU Cagliari, S.S. 554, Bivio per Sestu, Monserrato, Cagliari, 09042, Italy
Tel +39 07051093340
Email [email protected]
Introduction: Psoriatic Arthritis (PsA) is a multifactorial disease, where the relative burden of genetic, epigenetic and environmental factors in clinical course and damage accrual is not yet definitively clarified. In clinical practice, there is a real need for useful candidate biomarkers in PsA diagnosis and disease progression, by exploring its underlying transcriptomic and epigenomic mechanisms. This work aims to profile the transcriptome in monozygotic (MZ) twins with psoriatic arthritis (PsA) highly concordant for clinical presentation, but discordant for the radiographic outcomes’ severity.
Methods: We describe i) the clinical case of two MZ twins; ii) their comparative gene expression profiling (HTA 2.0 Affymetrix) and iii) signal pathways and pathophysiological processes in which differentially expressed genes are involved (in silico analysis by the IPA software, QIAGEN).
Results: One hundred sixty-three transcripts and 36 coding genes (28 up and 8 down) were differentially expressed between twins, and in the brother with the most erosive form, the transcriptomic profiling highlights the overexpression of genes known to be involved in immunomodulatory processes and on a broad spectrum of PsA manifestations.
Discussion: Twins’ clinical cases are still a gold mine in medical research: twin brothers are ideal experimental models in estimating the relative importance of genetic versus nongenetic components as determinants of complex phenotypes, non-Mendelian and multifactorial diseases as PsA.
Keywords: psoriatic arthritis, gene expression profiling, diseases in twin, genetic, environment–gene interaction, joint erosions
Introduction
Psoriatic Arthritis (PsA) is a multifactorial disease, where the relative burden of genetic, epigenetic and environmental factors in clinical course and damage accrual is not yet definitively clarified. Moll and Wright were the first to demonstrate familial aggregation of PsA estimating the recurrence risk ratio in first degree relatives,1 even more epidemiological evidences demonstrate a strong genetic basis to PsA.2
Genetic factors are evident from the high prevalence (7.6%) of PsA among first-degree relatives of PsA probands; furthermore, an excessive paternal transmission in PsA has been described. Using the CASPAR criteria, the probandwise concordance rates are approximately 11% and 5% in MZ and DZ twins, respectively.3–5 However, the role of genes in PsA development is still elusive.
The study of monozygotic twins may represent a very useful tool to understand the effects caused by genetic predisposition, epigenetic and the environmental factors in a disease, estimating the weight of these actors in determining the susceptibility and severity and to identify potential biomarkers in PsA.
Methods
Patients’ data regarding disease presentation and its clinical and radiological course were collected from medical records updated at each follow-up visit. Patients provided informed consent and ethical approval was granted by the local NHS authority (PROMISES/AC/2018 Prot. PG/2018/16313), in accordance with the 1964 Helsinki declaration.
The transcriptomic analysis was carried out using the mRNAs from whole blood (Qiagen RNA blood column kit) of the couple of MZ. Then, prior to the gene expression study, the RNA integrity and quality were checked out by Bioanalyzer (Agilent) and transcripts were analyzed by GeneChip Human Transcriptome Array 2.0 (HTA 2.0, Affymetrix); the full technical report is available in Supplementary Material. Transcripts differentially expressed between the two brothers were analyzed by the commercial software Partek Genomics Suite V 6.6, values are reported by a Fold Change cutoff ± 2.0 (further technical details and full transcripts’ list are available in Supplementary Data). Signal pathways and pathophysiological processes in which the abovementioned mRNAs are eventually involved, were in silico analyzed by the Ingenuity Pathway Analysis (IPA) software (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis).
Results
A pair of 47-year-old male MZ twins, A and B, affected by PsA according to 2006 CASPAR criteria, were studied. Twin A is a baker, while twin B is a surveyor, both were ex-smokers for about 30 years. None of the relevant was recorded in the past medical history while their family history revealed a paternal grandfather affected by cutaneous psoriasis.
Both patients presented the onset of their symptoms at the age of 17 years with symmetrical arthritis of ankles and knees. Furthermore, twin A experienced an episode of bilateral Achilles enthesitis and dactylitis of the 3rd finger of both hands, twin B presented arthritis of the left wrist: at that time, their general practitioner treated them with non-steroidal anti-inflammatory (NSAIDs) drugs only. Three years before being referred to our clinic, the two brothers were diagnosed with undifferentiated seronegative spondylarthritis and started treatment with Salazopyrine 2 gr/die and NSAIDs as needed.
When patients were firstly assessed in our tertiary Centre, they were 27 years old, the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were increased, rheumatoid factor and anti-citrullinated peptide antibodies (ACPA) were negative, family history was positive for psoriasis and HLA typing showed A03.11, B18-27, CW05.07, DRB1 03.16, DQB1 02.05. Standard X-ray of the pelvis showed bilateral subchondral sclerosis at the sacroiliac joints, predominantly on the iliac side, with irregular articular contours in both patients. Thus, both twins A and B were diagnosed with PsA sine psoriasis and treated with Etanercept 50mg/week in combination with Methotrexate.
Despite an overall good control of peripheral and axial symptoms, because of the occurrence of anterior uveitis at the age of 36 (twin A) and 37 years (twin B), both twins switched to Adalimumab, both achieving a persistent status of minimal disease activity:6 tender joint count ≤1, swollen joint count ≤1, enthesitis count ≤1, patient global visual analogue score VAS ≤ 20 mm, patient pain VAS ≤ 15 mm and health assessment questionnaire (HAQ) ≤ 0.5) (for 10 years).
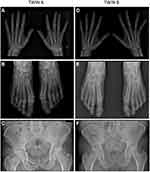
Hands and feet conventional radiography showed, in twin A, extensive and characteristic erosive and osteoproliferative alterations including typical pencil in cup lesions; tarsal and metatarsal joints of both feet were particularly involved (Figure 1A and b). In twin B, X-ray study showed less severe lesions, of note the erosive findings in the first PIP of the left foot (Figure 1D and E). Sacroiliitis was detectable in both patients (Figure 1C and F).
The gene expression analysis, the object of the present study, was performed at the age of 47. Covering 285,000 full-length transcripts (as well as non-coding transcripts, exon-exon junctions and over 245,000 coding sequences), the transcriptomic study highlighted a list of 163 transcripts and 36 coding genes (28 up and 8 down) differentially expressed in twin A versus B (FC ± 2.0). Looking for the physiopathologic role of coding transcripts by combining the bioinformatic analysis with the literature, we found that Differentially Expressed Genes (DEGs) list includes some coding genes involved in inflammatory and immunity-related pathologies, such as CEACAM8, CEACAM1, CLEC4D, IGK. Again, other DEGs in the brother A are related to the extracellular matrix system and ossification and therefore associated with pathologies affecting the joints, musculoskeletal and connective tissue, such as FMOD, DMD, MMP8; finally, genes that usually support cardiovascular and metabolic manifestations: ROR1, INPP5F, ACSM3, TTN and IGK (Table 1 List of selected coding DEGs between A and B twins).
Using IPA software, we examined the relationship between these DEGs to determine the most significant canonical pathways, diseases, and biological functions covered by these transcripts. The canonical pathways map representing from the top of the overrepresented network was made revealing shared biology among the identified genes (Figure 2 Overlapping Canonical Pathways map generated by IPA).
 |
Figure 2 Overlapping Canonical Pathways map generated by Ingenuity Pathway Analysis. A pathway network was generated representing from the top of overrepresented pathways determined by IPA, to reveal shared biology among the identified candidate genes. Notes: edge-connected canonical pathways share one or more genes in common. Nodes represent pathways and bright red represents more significant canonical pathways in the gene set. The canonical pathways map was generated by QIAGEN’s Ingenuity Pathway Analysis (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis). |
The top pathways enriched for DEGs included Myo-inositol Biosynthesis, Airway Pathology, Asthma, B Cell Receptor Signaling as shown in Figure 2. Between the top 5 diseases and biological functions, Cardiovascular, Hereditary, Skeletal and Muscular disorders, Inflammatory Response are included. Also, IPA identified significant networks associated with DEGs in twins A and B. Their associated functions were: cellular function and maintenance, cell death and survival, gastrointestinal disease, hereditary disorder, DNA replication and repair, and others represented on the picture in Supplementary Data.
Discussion
As first described by Moll and Wright in 1973, PsA can be divided into different clinical subsets and patients may demonstrate transitions between these subsets throughout the course of the disease. Furthermore, PsA has been associated with various comorbidities, including cardiovascular disease, metabolic syndrome, cancer and depression; thus, interventions that beneficially impact disease may also significantly affect morbidity and mortality.7
Monozygotic twins arise from a single cell and therefore share almost all their DNA sequence. However, the reason why the concordance rate of the autoimmune diseases in monozygotic twins is considerably below 100% is still unknown.8
With the emergence of epigenetics, researchers have begun to consider it as a critical contributing factor to discordant phenotypes. Notably, the environment plays a role in determining an individual’s phenotype, and the link between environment and genetics is mediated through epigenetic mechanisms.9–11
Considering the low concordance rate in twins already reported in the literature,4,5 here we report the case of extremely clinically concordant MZ twins which lived in a similar environment, showing a similar clinical course but different in terms of radiographic outcomes’ severity. This could be a situation in which not only the genetic components contribute to disease onset and course but also the environmental factors, eg intended as mechanical stress, perhaps played a role on the articular effort, evolving in a different severity outcome.
As reported in Table 1, the molecular profiling highlights in the twin A the overexpression of genes strictly related to inflammation and immunomodulatory processes. So, even with an almost identical genetic background and an outstanding parallel clinical course, in this clinical case, the erosive burden in PsA outcome is phenotypically varied between brothers, and it could depend on dysregulation of key genes as ROR1 and MMP8.
ROR1, a ROR2 closely related receptor, modulates some signaling pathways including the Hippo pathway. In particular, targets of the ROR-YAP cascade include WNT5A and connective tissue growth factor (CTGF).12 Notably, excessive activation of WNT/β-Catenin signaling promotes a form of terminal differentiation called chondrocyte hypertrophy,13 and during skeletal development, chondrocyte hypertrophy precedes cartilage calcification and ultimately its replacement by bone.
One characteristic feature of the cartilage degeneration is the breakdown of the extracellular matrix by different matrix metalloproteinases (MMPs). The type I, II, and III collagen-breaking MMP-8 seems to play some role in Osteoarthritis14 and the expression of MMP-8 is significantly increased in the joints of mice that have collagen-induced arthritis compared to healthy mice.15
Although the molecular data here described originate from a single pair of twins on chronic anti-TNFα treatment, which may represent a limitation and therefore needs further validations, nevertheless it is of note this clinical case of MZ twins surprisingly concordant for PsA onset and long-term course. In the brother (A) with the most erosive form, the molecular profiling highlights the overexpression of genes strictly related to inflammation and immunomodulatory processes but also associates with PsA comorbidities, such as cardiovascular and metabolic.16 Moreover, the presence of one disease (arthritis) within another (psoriasis, cardiovascular, metabolic, gastrointestinal) and relative severity degree may modify the molecular and clinical expression of either condition.
The present study confirms that even with an almost identical genetic background and an outstanding parallel clinical course, the erosive burden in PsA outcome may vary and could depend on a complex multi-hit model of disease, where a genetically predisposed individual encounters several environmental exposures (microbial, diet, alcohol, smoking, exercise and job) over a lifetime, which modulate the disease course.
Ethics Statement
Both patients provided informed consent for the case details and images to be published. Patients provided informed consent and ethical approval was granted by the local NHS authority (PROMISES/AC/2018 Prot. PG/2018/16313), in accordance with the 1964 Helsinki declaration.
Acknowledgments
We thank both the patients F.M. and F.M., who have participated in the study on a voluntary basis, and who have read and approved the manuscript.
Disclosure
Prof. Dr. Alberto Cauli reports personal fees from Abbvie, BMS, Celgene, Galapagos, Janssen, MSD, Sanofi, Alfa Sigma, UCB, and Lilly, grants and personal fees from Pfizer and Novartis, and non-financial support from Roche, outside the submitted work. The authors declare that they have no other potential conflicts of interest for this work.
References
1. Moll JM, Wright V. Familial occurrence of psoriatic arthritis.. Ann Rheum Dis. 1973;32(3):181–201. doi:10.1136/ard.32.3.181
2. Rahman P, Elder JT. Genetic epidemiology of psoriasis and psoriatic arthritis.. Ann Rheum Dis. 2005;64 Suppl 2(Suppl 2):ii37–ii41. doi:10.1136/ard.2004.030775
3. Rahman P, Gladman DD, Schentag CT, Petronis A. Excessive paternal transmission in psoriatic arthritis. Arthritis Rheum. 1999;42(6):1228–1231.
4. Pedersen OB, Svendsen AJ, Ejstrup L, Skytthe A, Junker P. On the heritability of psoriatic arthritis. Disease concordance among monozygotic and dizygotic twins. Ann Rheum Dis. 2008;67(10):1417–1421.
5. Chandran V, Schentag CT, Brockbank JE, et al. Familial aggregation of psoriatic arthritis. Ann Rheum Dis. 2009;68(5):664–667.
6. Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69(1):48–53.
7. Leung YY, Ogdie A, Orbai AM, et al. Classification and Outcome Measures for Psoriatic Arthritis. Front Med. 2018;5:246.
8. Selmi C, Lu Q, Humble MC. Heritability versus the role of the environment in autoimmunity. J Autoimmun. 2012;39(4):249–252.
9. Floreani A, Leung PS, Gershwin ME. Environmental Basis of Autoimmunity. Clin Rev Allergy Immunol. 2016;50(3):287–300.
10. Xiang Z, Yang Y, Chang C, Lu Q. The epigenetic mechanism for discordance of autoimmunity in monozygotic twins. J Autoimmun. 2017;83:43–50. doi:10.1016/j.jaut.2017.04.003
11. O’Rielly DD, Jani M, Rahman P, Elder JT. The Genetics of Psoriasis and Psoriatic Arthritis.. J Rheumatol Suppl. 2019;95:46–50. doi:10.3899/jrheum.190119
12. Park HW, Kim YC, Yu B, et al. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162(4):780–794. doi:10.1016/j.cell.2015.07.013
13. Enomoto-Iwamoto M, Kitagaki J, Koyama E, et al. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol. 2002;251(1):142–156. doi:10.1006/dbio.2002.0802
14. Davidson RK, Waters JG, Kevorkian L, et al. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res Ther. 2006;8(4):R124. doi:10.1186/ar2013
15. Booth G, Newham P, Barlow R, Raines S, Zheng B, Han S. Gene expression profiles at different stages of collagen-induced arthritis. Autoimmunity. 2008;41(7):512–521. doi:10.1080/08916930802095210
16. Lubrano E, Scriffignano S, Perrotta FM. Multimorbidity and comorbidity in psoriatic arthritis - a perspective. Exp Rev Clin Immunol. 2020;3:1–10.
17. De Preter V, Rutgeerts P, Schuit F, Verbeke K. Impaired Expression of Genes Involved in the Butyrate Oxidation Pathway in Crohnʼs Disease Patients. Inflamm Bowel Dis. 2013;19(3):E43–E44. doi:10.1002/ibd.22970
18. Walsh V, Somody L, Farrell A, et al. Analysis of the role of the SA gene in blood pressure regulation by gene targeting. Hypertension. 2003;41(6):1212–1218. doi:10.1161/01.HYP.0000069010.28143.5C
19. Gray-Owen S, Blumberg R. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6(6):433–446. doi:10.1038/nri1864
20. Zhao L, Furebring M, Xu S, Venge P. Subcellular localization and mobilization of carcinoembryonic antigen-related cell adhesion molecule 8 in human neutrophils. J Haematol. 2004;125(5):666–673. doi:10.1111/j.1365-2141.2004.04963.x
21. Seifert M, Przekopowitz M, Taudien S, et al. Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc Natl Acad Sci U S A. 2015;112(6):E546. doi:10.1073/pnas.1416276112
22. Lorentzen JC, Flornes L, Eklöw C, et al. Association of arthritis with a gene complex encoding C-type lectin–like receptors. Arthritis Rheum. 2007;56(8):2620–2632. doi:10.1002/art.22813
23. Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2003;2(12):731–740. doi:10.1016/S1474-4422(03)00585-4
24. Sjöberg A, Onnerfjord P, Mörgelin M, Heinegård D, Blom AM. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J Biol Chem. 2005;280(37):32301–32308. doi:10.1074/jbc.M504828200
25. Zheng Z, Zhang X, Dang C, et al. Fibromodulin Is essential for fetal-type scarless cutaneous wound healing. Am J Pathol. 2016;186(11):2824–2832. doi:10.1016/j.ajpath.2016.07.023
26. Zheng Z, Nguyen C, Zhang X, et al. Delayed wound closure in fibromodulin-deficient mice is associated with increased TGF-β3 Signaling. J Invest Dermatol. 2011;131(3):769–778. doi:10.1038/jid.2010.381
27. Streicher K, Morehouse CA, Groves CJ, et al. The plasma cell signature in autoimmune disease. Arthritis Rheumatol. 2014;66(1):173–184. doi:10.1002/art.38194
28. Ooms LM, Horan KA, Rahman P, et al. The role of the inositol polyphosphate 5-phosphatases in cellular function and human disease. Biochem J. 2009;419(1):29–49.
29. Zhu W, Trivedi CM, Zhou D, Yuan L, Lu MM, Epstein JA. Inpp5f is a polyphosphoinositide phosphatase that regulates cardiac hypertrophic responsiveness. Circ Res. 2009;105(12):1240–1247.
30. Saito T, Fukai A, Mabuchi A, et al. Transcriptional regulation of endochondral ossification by HIF-2 during skeletal growth and osteoarthritis development. Nat Med. 2010;16:678–686.
31. Yang S, Kim J, Ryu J-H, et al. Hypoxia-inducible factor-2 is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–693.
32. Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction Cardiovasc. Res. 2008;77(4):637–648.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.